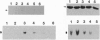Abstract
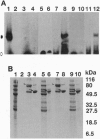
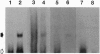
We have previously identified a testicular phosphoprotein that binds to highly conserved sequences (Y and H elements) in the 3' untranslated regions (UTRs) of testicular mRNAs and suppresses in vitro translation of mRNA constructs that contain these sequences. This protein, testis/brain RNA-binding protein (TB-RBP) also is abundant in brain and binds to brain mRNAs whose 3' UTRs contain similar sequences. Here we show that TB-RBP binds specific mRNAs to microtubules (MTs) in vitro. When TB-RBP is added to MTs reassembled from either crude brain extracts or from purified tubulin, most of the TB-RBP binds to MTs. The association of TB-RBP with MTs requires the assembly of MTs and is diminished by colcemid, cytochalasin D, and high levels of salt. Transcripts from the 3' UTRs of three mRNAs that contain the conserved sequence elements (transcripts for protamine 2, tau protein, and myelin basic protein) are linked by TB-RBP to MTs, whereas transcripts that lack the conserved sequences do not bind TB-RBP. We conclude that TB-RBP serves as an attachment protein for the MT association of specific mRNAs. Considering its ability to arrest translation in vitro, we propose that TB-RBP functions in the storage and transportation of mRNAs to specific intracellular sites where they are translated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainger K., Avossa D., Morgan F., Hill S. J., Barry C., Barbarese E., Carson J. H. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993 Oct;123(2):431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman J. B., Hall E. S., Eveleth J., Boekelheide K. Tau, the neuronal heat-stable microtubule-associated protein, is also present in the cross-linked microtubule network of the testicular spermatid manchette. Biol Reprod. 1992 Jan;46(1):120–129. doi: 10.1095/biolreprod46.1.120. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988 Jul 1;54(1):83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Ferrandon D., Elphick L., Nüsslein-Volhard C., St Johnston D. Staufen protein associates with the 3'UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell. 1994 Dec 30;79(7):1221–1232. doi: 10.1016/0092-8674(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Busciglio J., Cáceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Res Dev Brain Res. 1989 Oct 1;49(2):215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M., Penman S. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell. 1980 Jul;20(3):849–857. doi: 10.1016/0092-8674(80)90331-1. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992 Oct 16;71(2):301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. B., Steward O. Neurotoxic effects of colchicine: differential susceptibility of CNS neuronal populations. Neuroscience. 1982 Mar;7(3):695–714. doi: 10.1016/0306-4522(82)90075-6. [DOI] [PubMed] [Google Scholar]
- Grossi de Sa M. F., Martins de Sa C., Harper F., Olink-Coux M., Huesca M., Scherrer K. The association of prosomes with some of the intermediate filament networks of the animal cell. J Cell Biol. 1988 Oct;107(4):1517–1530. doi: 10.1083/jcb.107.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill D., Davis J., Drawbridge J., Suprenant K. A. Polyribosome targeting to microtubules: enrichment of specific mRNAs in a reconstituted microtubule preparation from sea urchin embryos. J Cell Biol. 1994 Nov;127(4):973–984. doi: 10.1083/jcb.127.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh J., Campbell G., Piechaczyk M., Blanchard J. M. Targeting of c-myc and beta-globin coding sequences to cytoskeletal-bound polysomes by c-myc 3' untranslated region. Biochem J. 1994 Feb 15;298(Pt 1):143–148. doi: 10.1042/bj2980143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Kwon Y. K., Hecht N. B. Binding of a phosphoprotein to the 3' untranslated region of the mouse protamine 2 mRNA temporally represses its translation. Mol Cell Biol. 1993 Oct;13(10):6547–6557. doi: 10.1128/mcb.13.10.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. K., Hecht N. B. Cytoplasmic protein binding to highly conserved sequences in the 3' untranslated region of mouse protamine 2 mRNA, a translationally regulated transcript of male germ cells. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3584–3588. doi: 10.1073/pnas.88.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman P., Barg J., Rindzoonski L., Ginzburg I. Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron. 1993 Apr;10(4):627–638. doi: 10.1016/0896-6273(93)90165-n. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Mowry K. L., Melton D. A. Vegetal messenger RNA localization directed by a 340-nt RNA sequence element in Xenopus oocytes. Science. 1992 Feb 21;255(5047):991–994. doi: 10.1126/science.1546297. [DOI] [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondel M. D., King M. L. Localized maternal mRNA related to transforming growth factor beta mRNA is concentrated in a cytokeratin-enriched fraction from Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7612–7616. doi: 10.1073/pnas.85.20.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Sadot E., Marx R., Barg J., Behar L., Ginzburg I. Complete sequence of 3'-untranslated region of Tau from rat central nervous system. Implications for mRNA heterogeneity. J Mol Biol. 1994 Aug 12;241(2):325–331. doi: 10.1006/jmbi.1994.1508. [DOI] [PubMed] [Google Scholar]
- Schwartz S. P., Aisenthal L., Elisha Z., Oberman F., Yisraeli J. K. A 69-kDa RNA-binding protein from Xenopus oocytes recognizes a common motif in two vegetally localized maternal mRNAs. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11895–11899. doi: 10.1073/pnas.89.24.11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C. R., Hecht N. B., Goldberg E., Schatten G. Tracing the incorporation of the sperm tail in the mouse zygote and early embryo using an anti-testicular alpha-tubulin antibody. Dev Biol. 1993 Aug;158(2):536–548. doi: 10.1006/dbio.1993.1211. [DOI] [PubMed] [Google Scholar]
- Singer R. H. The cytoskeleton and mRNA localization. Curr Opin Cell Biol. 1992 Feb;4(1):15–19. doi: 10.1016/0955-0674(92)90053-f. [DOI] [PubMed] [Google Scholar]
- Steward O., Banker G. A. Getting the message from the gene to the synapse: sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992 May;15(5):180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Thomsen G. H., Melton D. A. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993 Aug 13;74(3):433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Vedeler A., Pryme I. F., Hesketh J. E. The characterization of free, cytoskeletal and membrane-bound polysomes in Krebs II ascites and 3T3 cells. Mol Cell Biochem. 1991 Feb 2;100(2):183–193. doi: 10.1007/BF00234167. [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell. 1991 Nov 29;67(5):955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. E., Vale R. D. RNA on the move: the mRNA localization pathway. J Cell Biol. 1993 Oct;123(2):269–274. doi: 10.1083/jcb.123.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990 Feb;108(2):289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]