Gene expression is tightly regulated in response to both extracellular and intracellular signals, which control virtually every biological process. Intracellular signaling molecules such as steroid hormone, thyroid hormone, retinoids, and vitamin D exert their function through direct binding to their cognate nuclear receptors (1). Nuclear receptors are transcription factors that recognize specific sequences in target genes via a centrally located DNA-binding domain (DBD). Some receptors bind DNA as monomers, some as homodimers, and some as heterodimers with a common partner, the retinoid X receptor (RXR). Both the DBD and the COOH-terminal ligand-binding domain (LBD) contribute to dimerization. Many nuclear receptors repress transcription in the absence of ligand, due to a repression function in the LBD. Interaction of the LBD with ligand abolishes repression and activates transcription via a COOH-terminal activation domain (activation function 2, or AF2), which in some receptors works in tandem with an additional activation domain (AF1) in the NH2-terminus, or A/B region. There are over 150 mammalian members of the nuclear receptor superfamily. Many of these are "orphan" receptors, whose ligands are yet to be discovered.
In contrast to the small lipophilic molecules that signal via nuclear receptors, extracellular signaling molecules such as peptide hormones and cytokines communicate with their intracellular targets through surface receptors, which activate signal transduction pathways that ultimately lead to regulation of gene expression mediated by transcription factors such as fos, jun, cAMP-response element binding protein (CREB), and others. Most often the mechanism involves direct phosphorylation of the transcription factor by a kinase that is activated as a result of the ligand-receptor interaction at the cell surface. Nuclear receptors are also targets of kinases involved in signal transduction.
In this issue of the JCI, Kremer and colleagues report that activation of mitogen-activated protein kinase (MAPK) attenuates the ligand-dependent transactivation function of the vitamin D receptor (VDR) and its heterodimer partner RXR (2). This effect appears to be mediated by phosphorylation of human RXRα on a serine residue at amino acid 260 in the protein. This report not only provides a potential mechanism for ras-induced transformation of human keratinocytes, but also has broader implications because RXR heterodimerizes with several other nuclear receptors including thyroid hormone receptor, retinoic acid receptor, and peroxisome proliferator–activated receptor. Thus, it will be interesting to determine in future studies whether phosphorylation of RXR alters the biological activity of these receptors.
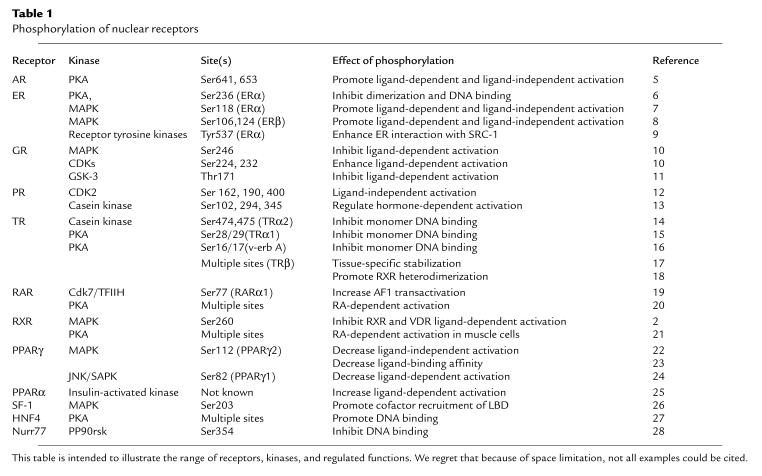
Phosphorylation of nuclear receptors provides an important mechanism for crosstalk between signaling pathways. Phosphorylation has been shown to modulate the activity of many nuclear receptors (Table 1). Multiple kinase pathways have been implicated, including cAMP-dependent protein kinase, casein kinase, glycogen synthase kinase (GSK), jun kinase, cyclin-dependent kinases (Cdks), and MAPKs. All aspects of receptor function can be regulated, including DNA binding and dimerization, transcriptional activity, interaction with cofactors, and ligand-binding affinity.
Table 1.
Phosphorylation of nuclear receptors
The mechanism by which phosphorylation of RXR inhibits vitamin D signaling is not clear. Phosphorylated RXR was not recognized by an antibody that recognized the nonphosphorylated protein, suggesting that phosphorylation resulted in a conformational change in the protein. Such a change in tertiary structure might alter affinity of the VDR/RXR heterodimer for transcriptional coregulators. This is an attractive hypothesis because ligand activation of nuclear receptors is clearly related to a conformational change that favors coactivator interaction (3). An alternative possibility is that the phosphate group has a more direct role in a critical function such as coregulator interaction, as has been observed for the interaction between phosphorylated CREB and its main coactivator CBP (4). Although the structures of the DBD and LBD have been solved for numerous receptors, no full-length receptor has been crystallized, nor has the structure of any phosphorylated nuclear receptor been determined. Thus, it remains to be determined which effects of phosphorylation listed in Table 1 are due to conformational changes and which are due to more localized effects of the phosphate group on receptor function. Moreover, the combination of phosphorylation and ligand binding may in some cases lead to an entirely novel structure.
The concept that nuclear hormone receptor function is determined by rapid, reversible, and combinatorial structural changes governing interactions with other cellular proteins provides a framework for understanding how the receptor can integrate a variety of signals in a physiological context. This level of understanding puts us light years ahead of where we were just a short time ago.
Acknowledgments
We thank the National Institute for Diabetes Digestive and Kidney Diseases (NIDDK) for grants in support of research in the lab of M.A. Lazar.
References
- 1.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon C, White JH, Kremer R. MAP kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylation of human RXRα on serine 260. J Clin Invest. 1999;103:1729–1735. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolte RT, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 4.Radhakrishnan I, et al. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- 5.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Pace PE, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–1015. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato S, Kitamoto T, Masuhiro Y, Yanagisawa J. Molecular mechanism of a cross-talk between estrogen and growth-factor signaling pathways. Oncology. 1998;55(Suppl 1.):5–10. doi: 10.1159/000055253. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 9.Auricchio F, et al. Protein tyrosine phosphorylation and estradiol action. Ann NY Acad Sci. 1996;784:149–172. doi: 10.1111/j.1749-6632.1996.tb16234.x. [DOI] [PubMed] [Google Scholar]
- 10.Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogatsky I, Waase CL, Garabedian MJ. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem. 1998;273:14315–14321. doi: 10.1074/jbc.273.23.14315. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–832. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J Biol Chem. 1994;269: 31034–31040. [PubMed] [Google Scholar]
- 14.Katz D, Reginato MJ, Lazar MA. Functional regulation of thyroid hormone receptor variant TR alpha 2 by phosphorylation. Mol Cell Biol. 1995;15:2341–2348. doi: 10.1128/mcb.15.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzagarakis-Foster C, Privalsky ML. Phosphorylation of thyroid hormone receptors by protein kinase A regulates DNA recognition by specific inhibition of receptor monomer binding. J Biol Chem. 1998;273:10926–10932. doi: 10.1074/jbc.273.18.10926. [DOI] [PubMed] [Google Scholar]
- 16.Glineur C, Zenke M, Beug H, Ghysdael J. Phosphorylation of the v-erbA protein is required for its function as an oncogene. Genes Dev. 1990;4:1663–1676. doi: 10.1101/gad.4.10.1663. [DOI] [PubMed] [Google Scholar]
- 17.Ting YT, Bhat MK, Wong R, Cheng S. Tissue-specific stabilization of the thyroid hormone beta1 nuclear receptor by phosphorylation. J Biol Chem. 1997;272:4129–4134. doi: 10.1074/jbc.272.7.4129. [DOI] [PubMed] [Google Scholar]
- 18.Bhat MK, Ashizawa K, Cheng SY. Phosphorylation enhances the target gene sequence-dependent dimerization of thyroid hormone receptor with retinoid X receptor. Proc Natl Acad Sci USA. 1994;91:7927–7931. doi: 10.1073/pnas.91.17.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochette-Egly C, et al. Phosphorylation of the retinoic acid receptor-alpha by protein kinase A. Mol Endocrinol. 1995;9:860–871. doi: 10.1210/mend.9.7.7476969. [DOI] [PubMed] [Google Scholar]
- 20.Taneja R, et al. Phosphorylation of activation functions AF-1 and AF-2 of RAR alpha and RAR gamma is indispensable for differentiation of F9 cells upon retinoic acid and cAMP treatment. EMBO J. 1997;16:6452–6465. doi: 10.1093/emboj/16.21.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowhan DH, Muscat GE. Characterization of the AB (AF-1) region in the muscle-specific retinoid X receptor-gamma: evidence that the AF-1 region functions in a cell-specific manner. Nucleic Acids Res. 1996;24:264–271. doi: 10.1093/nar/24.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen- activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 23.Shao D, et al. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 24.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor γ1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 25.Shalev A, et al. The peroxisome proliferator-activated receptor α is a phosphoprotein: regulation by insulin. Endocrinology. 1996;137:4499–4502. doi: 10.1210/endo.137.10.8828512. [DOI] [PubMed] [Google Scholar]
- 26.Hammer GD, et al. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 27.Jiang G, Nepomuceno L, Yang Q, Sladek FM. Serine/threonine phosphorylation of orphan receptor hepatocyte nuclear factor 4. Arch Biochem Biophys. 1997;340:1–9. doi: 10.1006/abbi.1997.9914. [DOI] [PubMed] [Google Scholar]
- 28.Davis IJ, Hazel TG, Chen RH, Blenis J, Lau LF. Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol. 1993;7:953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]