Abstract
Reactive aldehydes derived from reducing sugars and peroxidation of lipids covalently modify proteins and may contribute to oxidative tissue damage. We recently described another mechanism for generating reactive aldehydes from free α-amino acids. The pathway begins with myeloperoxidase, a heme enzyme secreted by activated neutrophils. Conversion of α-amino acids to aldehydes requires hypochlorous acid (HOCl), formed from H2O2 and chloride by myeloperoxidase. When L-serine is the substrate, HOCl generates high yields of glycolaldehyde. We now demonstrate that a model protein, ribonuclease A (RNase A), exposed to free L-serine and HOCl exhibits the biochemical hallmarks of advanced glycation end (AGE) products — browning, increased fluorescence, and cross-linking. Furthermore, Nε-(carboxymethyl)lysine (CML), a chemically well-characterized AGE product, was generated on RNase A when it was exposed to reagent HOCl-serine, the myeloperoxidase-H2O2-chloride system plus L-serine, or activated human neutrophils plus L-serine. CML production by neutrophils was inhibited by the H2O2 scavenger catalase and the heme poison azide, implicating myeloperoxidase in the cell-mediated reaction. CML was also generated on RNase A by a myeloperoxidase-dependent pathway when neutrophils were activated in a mixture of amino acids. Under these conditions, we observed both L-serine–dependent and L-serine–independent pathways of CML formation. The in vivo production of glycolaldehyde and other reactive aldehydes by myeloperoxidase may thus play an important pathogenic role by generating AGE products and damaging tissues at sites of inflammation.
Introduction
Chemical modification of proteins may play a role in the pathogenesis of disorders ranging from diabetes to atherosclerosis and ischemia-reperfusion injury and, perhaps, to the aging process itself (1–8). Aldehydes are an important class of agents that covalently modify proteins (1–8), and they can be generated in the body by a variety of enzymatic and nonenzymatic mechanisms (1–8). One abundant aldehyde in the body is glucose, an α-hydroxyaldehyde that in its open-chain form modifies proteins; the amount of modification is increased during diabetic hyperglycemia (2, 6–8). Reactive aldehydes also may be produced through peroxidation of lipids and by oxidases and dehydrogenases (1–6).
Aldehydes react covalently with amino acid residues of proteins to form several abnormal products (1, 2, 4–10). One well-known class of adducts is the advanced glycation end (AGE) products, which make proteins insoluble, brown, and fluorescent (2, 3, 7, 8). Glucose-derived AGE products have been implicated in the pathogenesis of diabetic renal and vascular disease, and they become more abundant during aging in both diabetic and nondiabetic subjects (2, 6–14). Despite intense study, the mechanisms that generate AGE products are not fully understood. The first steps in the reaction — formation of a Schiff base between glucose and protein amino groups, followed by an Amadori rearrangement — are well documented (reviewed in refs. 2, 14). A complex series of poorly characterized reactions then converts the Amadori product, 1-(deoxyfructose)lysine, to AGE products (2, 6–14). Although AGE products were first described by Maillard more than 80 years ago (15), the structures of most are still not established. Two exceptions are Nε-(carboxymethyl)lysine (CML) and pentosidine, which have been well characterized chemically (9, 10).
Glomb and Monnier proposed that protein-bound glycolaldehyde may be an intermediate in the reactions that convert glucose into AGE products (16). They suggested that glucose binds to an amino acid residue and then is cleaved to glycolaldehyde, which remains protein bound. The resulting adduct is the equivalent of a Schiff base, and it can be further oxidized to form CML and perhaps other AGE products (16). Indeed, proteins exposed to high concentrations of reagent glycolaldehyde are alkylated (in the presence of a reducing agent) and undergo cross-linking, another characteristic of AGE products (17, 18).
We recently demonstrated another pathway for generating reactive aldehydes that potentially could be important in AGE product formation. It involves myeloperoxidase, a heme protein secreted by activated phagocytes (19). This enzyme uses H2O2, which phagocytes also secrete, to convert chloride ion to hypochlorous acid (HOCl) (19–21):
(equation 1)
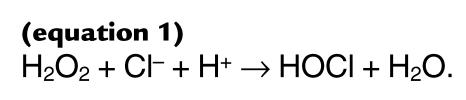 |
1 |
The initial product of the myeloperoxidase-H2O2-chloride system, HOCl, reacts with the amino group (RNH2) of free amino acids to form a chloramine (RNHCl) (19, 22–25):
(equation 2)
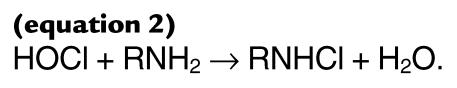 |
2 |
Amino acids [RCH(COOH)NH3] that have both a carboxyl group and amino group on the α-carbon (26) then decompose to form an aldehyde (RCHO) (24–28):
(equation 3)
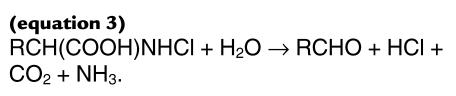 |
3 |
One excellent substrate for the reactions shown in eqs. 2 and 3 is L-serine, which is converted into glycolaldehyde in high yield by the myeloperoxidase-H2O2-chloride system (28).
In the current studies, we test the hypothesis that production of an α-hydroxy aldehyde by phagocytes might play a role in AGE product formation. Our observations suggest that glycolaldehyde generated by the myeloperoxidase-H2O2-chloride system might indeed lead to CML production and thereby play an important role in tissue damage at sites of inflammation.
Methods
Materials.
Crystalline catalase (from bovine liver, thymol-free) and glucose oxidase were purchased from Boehringer Mannheim Biochemicals (Indianapolis, Indiana, USA). Ribonuclease A (RNase A; lyophilized from bovine pancreas, code RAF) was obtained from Worthington Biochemical Corp. (Freehold, New Jersey, USA). Glycolaldehyde and H2O2 were purchased from Fluka Chemical Co. (Rononkoma, New York, USA) and Fisher Scientific Co. (Pittsburgh, Pennsylvania, USA), respectively. All other materials were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA) unless otherwise indicated.
Isolation of myeloperoxidase.
Myeloperoxidase (donor: hydrogen peroxide, oxidoreductase, EC 1.11.1.7) was extracted with cetyltrimethylammonium bromide from human leukocytes obtained by leukopheresis. Solubilized myeloperoxidase was purified by lectin affinity chromatography and size exclusion chromatography as described (29, 30). The enzyme (A430/A280 ratio of 0.6) was dialyzed against distilled water and stored in 50% glycerol at –20°C. Enzyme concentration was determined spectrophotometrically (ε = 170 mM–1cm–1; ref. 31).
Isolation of human neutrophils.
Neutrophils were isolated by buoyant density centrifugation as described previously (32). The cells were washed twice by centrifugation with medium A (magnesium-, calcium-, phenol-, and bicarbonate-free HBSS, supplemented with 100 μM DTPA [pH 7.2]). Residual red blood cells were removed by hypotonic lysis at 4°C. Neutrophils were pelleted by centrifugation, resuspended in medium A, and immediately used for experiments.
Reaction conditions for protein modification by HOCl or myeloperoxidase.
For modification of RNase A with the HOCl-serine system, L-serine was incubated with HOCl (1.0:0.9 mol/mol) for 15 minutes at 37°C in buffer A (50 mM sodium phosphate buffer [pH 7.0]). RNase A (10 mg/mL final concentration) was then added to the reaction mixture and allowed to incubate at 37°C for the indicated amount of time. Preincubating L-serine with HOCl avoided competing reactions (e.g., with amino residues on the RNase A that also might consume HOCl; refs. 22, 23). For modification with the myeloperoxidase system, 60 nM myeloperoxidase, 1 mM L-serine, 1 mM H2O2, and 150 mM NaCl were incubated in buffer A at 37°C. The H2O2 was added in 2 equal aliquots 15 minutes apart to a final concentration of 1 mM. After a 90-minute incubation, RNase A was added to a final concentration of 1 mg/mL, and the incubation was continued at 37°C for the indicated amount of time. To determine whether CML formation occurred at plasma concentrations of free amino acids, experiments were carried out at 37°C in buffer A supplemented with physiological concentrations of the 7 most abundant free amino acids found in plasma (33). For these reactions, 1 mg/mL of RNase A was modified by a myeloperoxidase system consisting of 30 nM myeloperoxidase, 300 ng/mL glucose oxidase, and 100 μg/mL D-glucose. Reactions were stopped by freezing at –20°C.
Reaction conditions for protein modification by human neutrophils.
For modification of RNase A with human neutrophils, freshly harvested cells were added to medium B (50 mM sodium phosphate, 100 mM NaCl, 4 mM KCl, and 100 μM DTPA [pH 7.2]) supplemented with 1 mM L-serine at a final concentration of 106 cells/mL. Neutrophils were then activated with 250 nM PMA and incubated with intermittent inversion for 45 minutes at 37°C. Neutrophils were then removed by centrifugation (5 minutes × 5,220 g), and RNase A was added to the supernatant (1 mg/mL final concentration) and allowed to incubate for the indicated amount of time at 37°C. To determine whether neutrophils formed CML at plasma concentrations of free amino acids, experiments were carried out in medium B supplemented with 1 mg/mL of RNase A and the 7 most abundant free amino acids found in plasma (33). After a 1-hour incubation at 37°C, cells were removed by centrifugation, and the supernatant was incubated at 37°C for the indicated time. Reactions were terminated by freezing at –20°C.
SDS-PAGE analysis of proteins.
RNase A (20 μg protein in 50 μL of 50 mM Tris-HCl [pH 6.8] containing 1% β-mercaptoethanol and 2% SDS) was subjected to electrophoresis on a 10–20% gradient polyacrylamide gel containing 0.1% SDS in 375 mM Tris-HCl (pH 8.8) (34). The stacking gel was made with 5% polyacrylamide in 140 mM Tris-HCl (pH 6.8), 0.1% SDS. Running buffer consists of 25 mM Tris, 250 mM glycine (pH 8.3), with 1% SDS. Polyacrylamide gels were stained with Coomassie blue and destained with methanol/acetic acid/water (1:5:5 vol/vol/vol).
Analysis of protein-bound CML by gas chromatography/mass spectrometry.
Reaction mixtures were extensively dialyzed against H2O at 4°C to remove buffer salts and then concentrated to dryness by centrifugal evaporation. Internal standards (d8-lysine and d4-CML) were added to each sample, followed by hydrolysis in 6.0 N HCl for 24 hours at 110°C. The hydrolysates were then dried by centrifugal evaporation. For analysis by electron ionization gas chromatography/mass spectrometry (GC/MS), samples were derivatized for measurement of CML and lysine as their trifluoroacetyl methyl ester derivatives as described (35). CML, d4-CML, lysine, and d8-lysine were quantified using ions of mass-to-charge (m/z) ratio 392, 396, 180, and 187, respectively (35). Selected ion monitoring-GC/MS analysis of derivatized amino acids isolated was performed on an HP-6890 mass spectrometer (Hewlett-Packard, Palo Alto, California, USA equipped with a 30-m HP-5MS (5% phenyl, 95% methyl, siloxane) capillary column. Samples were injected in the splitless mode. The initial GC column temperature was 150°C for 2 minutes; the temperature was increased to 180°C at 5°/min, then to 300°C at 15°/min, and held for 5 minutes. The injector port was kept at 275°C (35).
For negative-ion electron capture GC/MS analyses, samples were dried by centrifugal evaporation following hydrolysis, resuspended in 1 mL of 1% trifluoroacetic acid, and immediately passed over a solid-phase C-18 extraction column (Supelclean LC-18 SPE tubes, 1 mL; Supelco Inc., Bellefonte, Pennsylvania, USA) that had been equilibrated with methanol and 1% trifluoroacetic acid. The column was then washed with two 1-mL aliquots of 1% trifluoroacetic acid. The column flow-through and 2 washes were pooled and dried by centrifugal evaporation, and the n-propyl pentafluoropropionyl derivatives of CML and lysine were prepared as described (36). CML, d4-CML, lysine, and d8-lysine were quantified using ions of m/z 560, 564, 460, and 468, respectively. Selected ion monitoring-GC/MS was carried out on a Finnigan SSQ equipped with a 12-m DB-1 capillary column (0.2-mm internal diameter, 0.33-μm film thickness; Hewlett-Packard). Samples were injected with a 20:1 split, with methane as the reagent gas. The injector port and detector were kept at 250°C, with the source at 200°C. For analysis of CML, the initial GC column temperature of 150°C was held for 5 minutes, and the temperature was then increased from 150°C to 270°C at 20°/min. For analysis of lysine, the initial GC temperature of 120°C was held for 5 minutes; the temperature was then ramped from 120°C to 275°C at 20°/min and held at 275°C for 1 minute.
Other procedures.
Fluorescence excitation and emission spectra were obtained at room temperature using an LS5B Perkin-Elmer Luminescent Spectrophotometer (Perkin-Elmer Corp., Norwalk, Connecticut, USA). Ultraviolet/visible absorption spectra were obtained at 37°C using a Beckman DU-7 spectrophotometer (Beckman Instruments Inc., Fullerton, California, USA) equipped with thermostatically controlled cells. Concentrations of H2O2 and HOCl were determined spectrophotometrically using ε240 = 43.6 M–1cm–1 and ε292 = 350 M–1cm–1, respectively (37, 38). Unless otherwise indicated, data are the mean of duplicate determinations and are representative of the results found in at least 3 independent experiments.
Results
The HOCl-serine system promotes the browning reaction with a model protein.
The classic biochemical characteristics associated with the formation of AGE products are a brown color, development of fluorescence, and protein cross-linking (2, 7, 8, 15). To explore the potential role of phagocyte-generated aldehydes in AGE product formation, we determined whether a model system consisting of HOCl and L-serine (0.9:1.0 mol/mol) in a physiological salt solution at neutral pH would induce such changes in a model protein, RNase A.
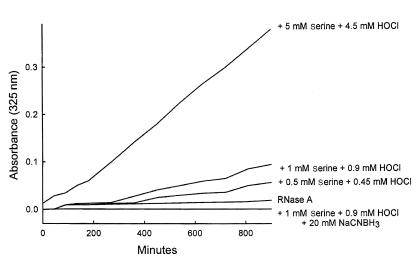
RNase A incubated with the HOCl-serine system exhibited a time-dependent browning, monitored as an increase in absorbance at 325 nm (Figure 1). The browning reaction required the presence of protein, L-serine, and HOCl; it was inhibited by the reducing agent NaCNBH3, which presumably reduced the initial Schiff base adduct, preventing subsequent reactions (14, 17). The material that absorbed light at 325 nm coeluted on size exclusion chromatography with RNase A modified by HOCl-serine, indicating that these moieties were protein bound (data not shown). Adding HOCl alone to RNase A also increased absorbance at 325 nm. However, the progress curve was much more rapid than that observed with the HOCl-serine system (data not shown), suggesting that RNase A was in this case modified by a different mechanism. Reagent glycolaldehyde also produced browning with RNase; the progress curve of the reaction was similar to that seen with the HOCl-serine system (data not shown). These results demonstrate that the HOCl-serine system causes browning of a model protein, and implicate glycolaldehyde in the reaction pathway.
Figure 1.
Progress curve of protein browning induced by the HOCl-serine system. HOCl and L-serine (0.9:1.0 mol/mol at the indicated final concentration) were incubated together in buffer A (50 mM sodium phosphate buffer [pH 7.0]) for 15 minutes at 37°C. RNase A (100 mg/mL in buffer A) was then added to a final concentration of 10 mg/mL, and the absorbance at 325 nm of the reaction mixture was monitored. When indicated, 20 mM NaCNBH3 was present during the reaction of HOCl and L-serine.
The HOCl-serine system increases the fluorescence of a model protein.
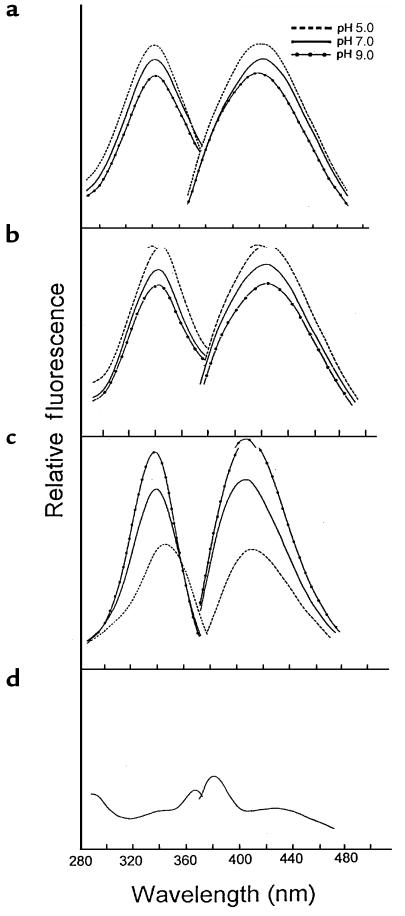
After RNase A was exposed to the HOCl-serine system, it also exhibited a marked increase in fluorescence (Figure 2a). The fluorescent material coeluted with RNase A on size exclusion chromatography, indicating that the fluorescence was protein bound (data not shown). Moreover, its fluorescence excitation (λmax 340 nm) and emission (λmax 425 nm) spectra were virtually identical to those observed after RNase A was modified with reagent glycolaldehyde. This suggests that glycolaldehyde derived from HOCl-serine was responsible for the reaction (Figure 2, a and b). The 2 systems also had a similar pH dependence: fluorescence intensity decreased with increasing pH in RNase A modified with either HOCl-serine or glycolaldehyde.
Figure 2.
Fluorescence excitation and emission spectra of RNase A modified by HOCl-serine. RNase A (10 mg/mL) was modified for 24 hours at 37°C in buffer A with the HOCl-serine system at 10 mM each (a), as described in the legend to Figure 1; with 10 mM glycolaldehyde (b); or with 9 mM HOCl alone (c). Reaction mixture was then diluted 1:10 with 50 mM sodium citrate-citric acid buffer (pH 5.0), 50 mM sodium phosphate buffer (pH 7.0), or 50 mM boric acid-borax buffer (pH 9.0). The excitation and emission spectra of each reaction mixture were then determined. (d) Excitation and emission spectra of 1 mg/mL RNase A in 50 mM sodium phosphate buffer (pH 7.0).
In contrast, the fluorescence intensity of RNase A modified with HOCl alone increased with increasing pH. The fluorescence excitation and emission spectra also differed slightly from those of RNase A modified by HOCl-serine (Figure 2c): the excitation maximum lay between 335 and 345 nm (depending on the pH), and the emission maximum was at 400 nm. Incubating RNase A with buffer A alone (Figure 2d) or L-serine alone in buffer A (data not shown) generated no fluorescence. Fluorescence was also undetectable in HOCl-serine reaction mixture that lacked protein (data not shown).
The HOCl-serine system promotes protein cross-linking.
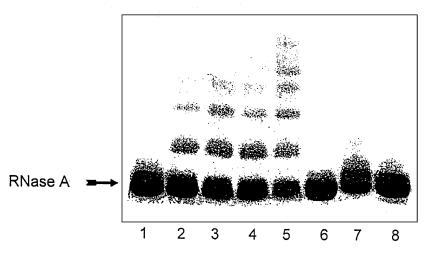
We also detected protein cross-linking after we incubated RNase A with HOCl-serine and then subjected the reaction mixture to SDS-PAGE under reducing conditions (Figure 3, lanes 2 and 3). The reaction depended on the concentration of HOCl-serine and was mimicked by reagent glycolaldehyde (Figure 3, lanes 4 and 5). At equal concentrations of reagents, we observed more cross-linking of RNase A by glycolaldehyde than by HOCl-serine. This suggests that glycolaldehyde levels were lower, owing to the presence of competing reactions that consumed L-serine and/or HOCl.
Figure 3.
SDS-PAGE analysis of RNase A modified by HOCl-serine. RNase A (10 mg/mL) was incubated with the HOCl-serine system in buffer A, as indicated in the legend to Figure 1, for 24 hours at 37°C. RNase A (20 μg of protein) was then subjected to electrophoresis on a 10–20% gradient polyacrylamide gel under denaturing (0.1% SDS) and reducing (1% β-mercaptoethanol) conditions. Reaction conditions were as follows: lane 1, RNase A alone; lane 2, RNase A + 5 mM L-serine + 4.5 mM HOCl; lane 3, RNase A + 10 mM L-serine + 9 mM HOCl; lane 4, RNase A + 5 mM glycolaldehyde; lane 5, RNase A + 10 mM glycolaldehyde; lane 6, RNase A + 10 mM L-serine; lane 7, RNase A + 9 mM HOCl; lane 8, RNase A + 10 mM L-serine + 9 mM HOCl + 20 mM NaCNBH3. NaCNBH3 was present during the incubation of HOCl and L-serine (lane 8).
Cross-linking was not observed when RNase A was incubated with either L-serine or HOCl alone (Figure 3, lanes 6 and 7). RNase A exposed to HOCl alone migrated as a slightly broader band with an apparent molecular weight similar to that of native RNase A, suggesting that it had undergone subtle structural changes. These observations again suggest that HOCl alone modifies the protein through a different mechanism than glycolaldehyde or HOCl-serine. Adding 20 mM NaCNBH3 to the reaction mixture completely abolished protein cross-linking by HOCl-serine (Figure 3, lane 8), consistent with a cross-linking mechanism involving the formation and rearrangement of a Schiff base (14, 17, 39).
The HOCl-serine system generates CML in proteins.
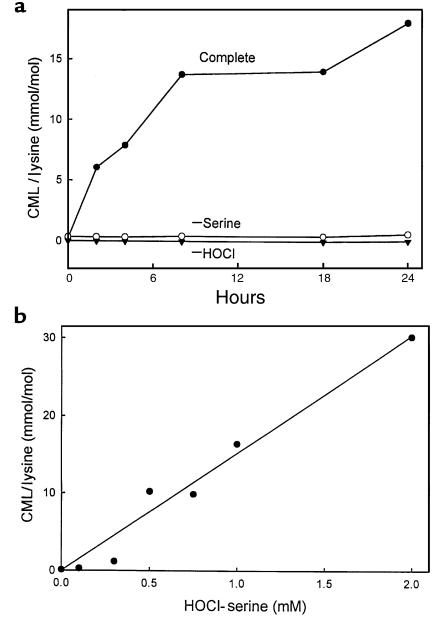
To explore the potential role of myeloperoxidase in the formation of CML, a chemically well-characterized AGE product, we used isotope dilution GC/MS to analyze RNase A added to an incubation mixture containing HOCl and L-serine. We observed a time-dependent increase in protein-bound CML (Figure 4a). The reaction required RNase A, L-serine, and HOCl; in the absence of any of these components, there was virtually no CML production. CML concentration also increased linearly with increasing concentrations of HOCl and L-serine (Figure 4b), indicating that protein-bound CML is generated by the HOCl-serine system.
Figure 4.
Progress curve and concentration dependence of CML formation on RNase A by HOCl-serine. (a) RNase A (1 mg/mL) was modified by the HOCl-serine system (0.9 mM and 1.0 mM, respectively) as described in the legend to Figure 1. At the indicated times, the reaction mixture was subjected to analysis for CML by electron ionization isotope dilution GC/MS with selected ion monitoring. (b) The indicated concentrations of HOCl and L-serine were incubated for 15 minutes at 37°C in buffer A, followed by addition of RNase A (final concentration 1 mg/mL) and incubation for 24 hours at 37°C.
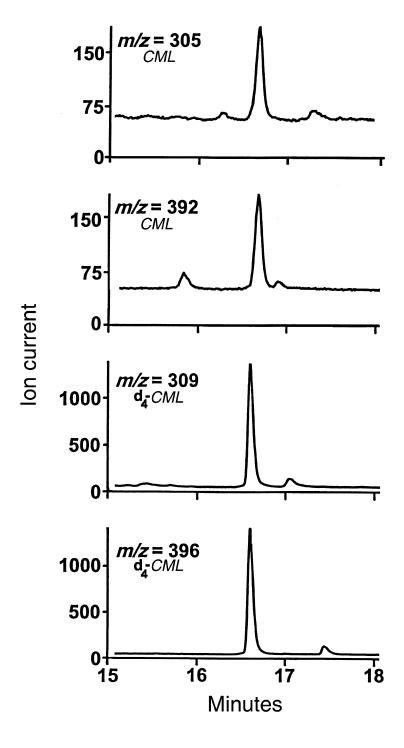
HOCl scavengers are potent inhibitors of CML formation in a model system.
To investigate the ability of different antioxidants to inhibit CML formation by the HOCl-serine system (40), we incubated L-serine together with equimolar concentrations of antioxidant and HOCl, added RNase A to the reaction mixture, and then quantified the levels of CML in the protein after a 24-hour incubation. The data in Table 1 show that the HOCl scavengers taurine and methionine both were inhibitory (41, 42). CML formation was moderately affected by taurine and almost completely abolished by methionine. These observations suggest relative reaction rates with HOCl of methionine > L-serine ≅ taurine.
Table 1.
Effect of HOCl scavengers, metal chelators, and antioxidants on the formation of protein-bound CML by the HOCl-serine system
The metal chelators EDTA and DTPA modestly inhibited CML production, suggesting a role for redox-active metal ions (43). Butylated hydroxytoluene, an inhibitor of lipid peroxidation (43), had virtually no effect, which is not surprising because RNase A contains no lipid moieties. Antioxidants such as the lipid-soluble compounds α-tocopherol and probucol were also ineffective as inhibitors (44). Thus, only agents that initially compete for HOCl appear to inhibit potently CML formation in this system.
The myeloperoxidase system generates CML through a pathway involving HOCl and l-serine.
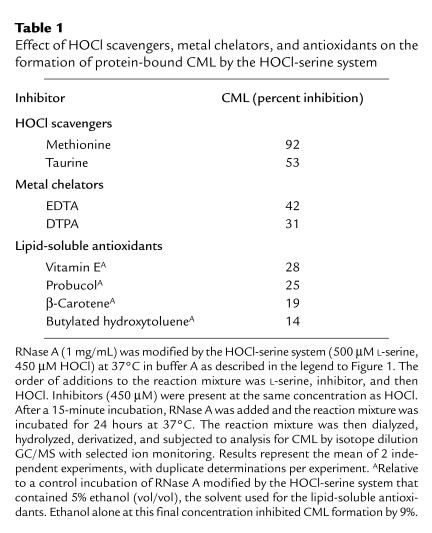
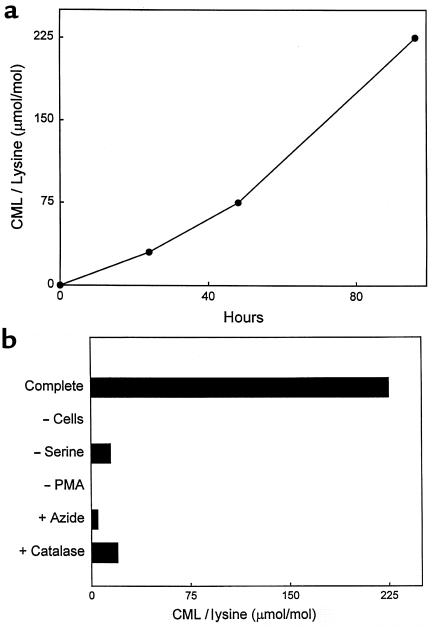
To confirm that the HOCl-serine system truly mimics the myeloperoxidase system, we exposed RNase A to isolated myeloperoxidase. First, purified myeloperoxidase was incubated in a physiological buffer supplemented with L-serine, H2O2, and Cl– (to generate HOCl) at neutral pH, and then RNase A was added to the reaction mixture. Under these conditions, CML was generated on RNase A in a time-dependent manner (Figure 5a). The reaction required enzyme, L-serine, peroxide, and halide (Figure 5b). It also was inhibited by the H2O2 scavenger catalase (400 nM) or the heme poison sodium azide (5 mM), as expected from a mechanism involving a heme peroxidase (data not shown).
Figure 5.
Progress curve and reaction requirements for generation of CML on RNase A by the myeloperoxidase-H2O2-chloride-serine system. (a) The complete myeloperoxidase system (60 nM myeloperoxidase, 1 mM H2O2, 1 mM L-serine, and 150 mM NaCl in buffer A) was incubated at 37°C for 90 minutes. RNase A (1 mg/mL final concentration) was added, and the reaction mixture was incubated for the indicated period at 37°C. The reaction mixture was then subjected to analysis for CML by electron ionization isotope dilution GC/MS with selected ion monitoring. (b) The complete myeloperoxidase system was incubated at 37°C as described for a. After the addition of 1 mg/mL RNase A, the reaction mixture was incubated for 24 hours. Reaction mixture was then subjected to analysis for CML by GC/MS. When indicated, chloride ion (Cl–), myeloperoxidase (MPO), L-serine, or H2O2 (peroxide) was omitted from the complete myeloperoxidase system.
After a 72-hour incubation at 37°C, RNase A exposed to myeloperoxidase, L-serine, and 1 mM H2O2 had acquired ∼5 mmol CML per mol of lysine. This level is similar to that observed in RNase A exposed to 250 mM glucose for 6 weeks — 1,000 hours (39). This calculation suggests that glycolaldehyde generated by myeloperoxidase is roughly 3,000-fold more effective than glucose at generating CML. RNase A has 10 ε-amino groups on lysine residues available for reaction. Assuming that 1 mol of H2O2 converted 1 mol of L-serine to glycolaldehyde and that 1 mol glycolaldehyde produced 1 mol of CML, each mol of H2O2 produced 2 mmol of CML.
In a mixture of amino acids, myeloperoxidase generates CML by pathways that are both l-serine–dependent and l-serine–independent.
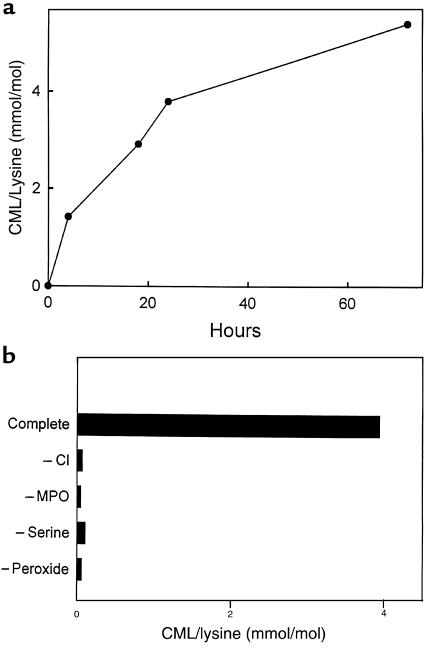
To determine whether myeloperoxidase would generate CML in the presence of other amino acids, we exposed RNase A to the enzymatic system in buffer supplemented with physiological concentrations of glucose and a mixture of the 7 most abundant free amino acids found in plasma (260 μM L-glutamate, 210 μM L-alanine, 200 μM L-serine, 175 μM glycine, 165 μM L-valine, 100 μM L-proline, and 100 μM L-lysine; ref. 33). In these experiments, we incubated all the components of the reaction system together. Peroxide was generated continuously at a low level using glucose oxidase and glucose to provide a more physiological level of oxidant. Under these conditions, we detected a marked increase in the level of CML in RNase A exposed to the complete myeloperoxidase system (Figure 6). CML generation required myeloperoxidase and glucose oxidase; it was inhibited by catalase and azide, implicating H2O2 and myeloperoxidase in the reaction. Omitting L-serine from the amino acid mixture reduced the yield of CML by ∼70%. These results indicate that myeloperoxidase generates CML on RNase A at plasma concentrations of amino acids by a reaction pathway that involves HOCl and L-serine. Because CML was also generated in the absence of L-serine, there are L-serine–independent pathways for CML formation by myeloperoxidase.
Figure 6.
Influence of plasma amino acids on CML formation by myeloperoxidase. The complete myeloperoxidase system (Complete; 30 nM myeloperoxidase, 300 ng/mL glucose oxidase, 100 μg/mL glucose) was incubated for 16 hours at 37°C in buffer A supplemented with 1 mg/ml RNase A, 260 μM L-glutamate, 210 μM L-alanine, 200 μM L-serine, 175 μM glycine, 165 μM L-valine, 100 μM L-proline, and 100 μM L-lysine. The reaction mixture was then subjected to analysis for CML by negative-ion electron capture GC/MS with selected ion monitoring. Sodium azide (Azide; 5 mM) or catalase (Cat; 400 nM) was added when indicated. The bottom 3 bars represent control reactions containing RNase A alone or supplemented with catalase or glucose oxidase. G, glucose; GO, glucose oxidase; MPO, myeloperoxidase.
When we incubated RNase A for 16 hours at 37°C in buffer supplemented with 100 μM glycolaldehyde and the mixture of amino acids, the yield of CML was ∼120 μmol per mol of lysine. Making the assumptions just outlined, this indicates that each mol of glycolaldehyde produced 0.9 mmol of CML. Thus, even in the presence of other competing amino acids, glycolaldehyde was roughly 6,000 times more reactive than glucose. These observations further support the hypothesis that conversion of L-serine into glycolaldehyde by myeloperoxidase may be a physiologically significant pathway for CML formation.
Human neutrophils generate CML on model proteins.
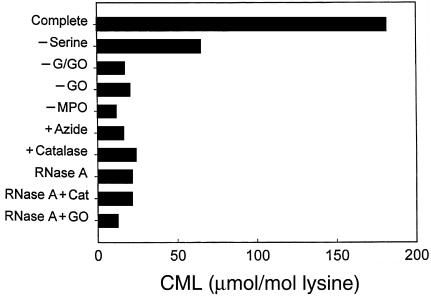
To determine whether neutrophils would use myeloperoxidase to produce CML, we incubated the cells with L-serine in a physiological salt solution at neutral pH and then added RNase A and incubated the reaction mixture for various periods at 37°C. Reverse-phase HPLC revealed that the neutrophils initially converted L-serine into glycolaldehyde (data not shown; ref. 28), suggesting that the aldehyde was an intermediate in the reaction pathway. Amino acids from the acid hydrolysate of RNase A exposed to activated neutrophils were derivatized and then analyzed by electron ionization GC/MS (Figure 7). The trifluoroacetyl methyl ester derivative of CML, monitored as the ions at m/z 392 (M•+ – CH3OH) and m/z 305 (M•+ – 2 COOCH3 – H), was readily detected in the amino acid mixture by selected ion monitoring. The relative abundance and retention time of the ions derived from the compound generated by activated neutrophils were identical to those of authentic CML. By selected ion monitoring, the ions derived from d4-labeled CML elute slightly earlier than those of CML, as has been reported for other deuterated internal standards (45). The progress curve of the reaction showed a time-dependent increase in CML over 96 hours (Figure 8a). CML formation required activation of the neutrophils with PMA and the presence of L-serine (Figure 8b). It was inhibited by catalase and heme poisons (Figure 8b), again consistent with a mechanism depending on myeloperoxidase.
Figure 7.
Detection of the trifluoroacetyl methyl ester derivative of CML generated by activation human neutrophils by electron ionization GC/MS with selected ion monitoring. Freshly harvested human neutrophils (106/mL) were incubated at 37°C in medium B supplemented with 1 mM L-serine. Cells were stimulated with 250 nM PMA and maintained in suspension by intermittent inversion. After a 45-minute incubation, cells were removed by centrifugation, and RNase A (1 mg/mL final concentration) was added. After a 96-hour incubation at 37°C, protein-bound CML in the incubation medium was detected by electron ionization GC/MS with selected ion monitoring. The ions at m/z 392 (M•+ – CH3OH) and m/z 305 (M•+ – 2 COOCH3 –H) represent the most abundant ions in the full-scan mass spectrum of CML. Note that the ions derived from d4-labeled CML at m/z 309 and m/z 396 elute slightly earlier than the corresponding ions of CML, as has been shown for many other deuterated compounds (45).
Figure 8.
Progress curve and reaction requirements for generation of CML on RNase A by activated human neutrophils. (a) Freshly harvested human neutrophils (106/mL) were incubated at 37°C in medium B supplemented with 1 mM L-serine as described in the legend to Figure 6. After incubation for the indicated period at 37°C, the reaction was stopped by freezing at –20°C. The incubation medium was then subjected to analysis for CML by electron ionization isotope dilution GC/MS with selected ion monitoring. (b) Freshly harvested human neutrophils (106/mL) were incubated at 37°C in medium B supplemented with 1 mM L-serine (Complete) as in a. After the addition of 1 mg/mL RNase A, the reaction mixture was incubated for 96 hours. Reactions were then subjected to analysis for CML by electron ionization selected ion monitoring GC/MS. When indicated, cells, L-serine,or PMA was omitted, or azide (5 mM) or catalase (500 nM) was included in the complete cellular system. Values are corrected for the endogenous CML content (12.2 μmol/mol lysine) of RNase A.
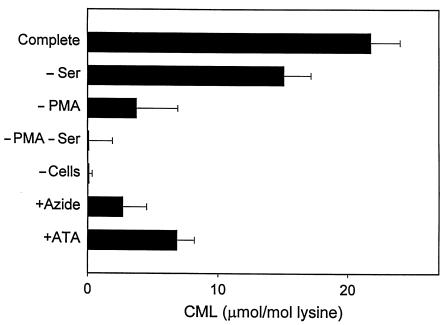
To determine whether activated human neutrophils would generate CML in the presence of other amino acids, we exposed RNase A to PMA-stimulated cells in medium supplemented with plasma concentrations of the 7 most abundant amino acids. Under these conditions, we observed a marked increase in the level of CML in RNase A (Figure 9). Generation of CML required activation of the cells with PMA and was inhibited by 3-aminotriazole and azide, implicating oxidant generation and myeloperoxidase in the reaction. Omitting L-serine from the amino acid mixture significantly reduced but did not abolish CML formation (P < 0.02). These results indicate that activated human neutrophils use the myeloperoxidase system to generate CML in a physiological mixture of amino acids, but that the enzyme also can generate this abnormal amino acid by pathways that do not involve L-serine.
Figure 9.
Reaction requirements for generation of CML on RNase A by activated human neutrophils in medium supplemented with plasma concentrations of free amino acids. Isolated neutrophils (106/mL) were activated with 200 nM PMA in medium B supplemented with 1 mg/mL RNase A, 260 μM L-glutamate, 210 μM L-alanine, 200μm L-serine, 175 μM glycine, 165 μM L-valine, 100 μM L-proline, and 100 μM L-lysine. When indicated, L-serine was omitted (–Ser) from the medium, and sodium azide (Azide; 5 mM) or 3-aminotriazole (ATA; 10 mM) was included. After a 1-hour incubation at 37°C, cells were removed by centrifugation, and the medium was incubated for another 16 hours at 37°C. Reaction mixtures were then subjected to analysis for CML by negative-ion electron capture isotope dilution GC/MS with selected ion monitoring. Values are corrected for the endogenous CML content (21.0 μmol/mol lysine) of RNase A and represent the mean ± SEM of 6 determinations.
Discussion
We previously showed that HOCl, which is generated by the myeloperoxidase system of activated phagocytes, converts L-serine to glycolaldehyde in high yield (28). Because glucose, another α-hydroxyaldehyde, is implicated in the formation of AGE products in proteins, we wanted to determine whether reactive aldehydes generated by myeloperoxidase could also covalently modify protein residues.
The biochemical hallmarks of protein-bound AGE products are browning, increased fluorescence, and cross-linking (2, 7, 8, 15). When we exposed RNase A to a mixture of HOCl and L-serine, which generates glycolaldehyde in high yield, we observed protein browning (monitored as increased absorbance at 325 nm), development of fluorescence, and protein cross-linking (monitored by SDS-PAGE under reducing conditions). Reagent glycolaldehyde produced similar changes in RNase A, strongly supporting the hypothesis that the myeloperoxidase reaction is mediated by glycolaldehyde generated from L-serine and HOCl.
When used alone, HOCl produced browning and fluorescence in RNase A. However, 3 lines of evidence indicate that this reaction may be distinct from that mediated by the HOCl-serine system. First, the progress curves of the 2 reactions differed. Second, the excitation and emission fluorescence spectra of HOCl-modified RNase A were optimal at shorter wavelengths, compared with those of RNase A modified by HOCl-serine. Moreover, the fluorescence of HOCl-modified RNase A decreased with decreasing pH, whereas that of HOCl-serine–modified RNase A increased. Finally, both HOCl-serine and glycolaldehyde induced cross-linking in RNase A, but HOCl alone did not promote cross-linking. Collectively, these results indicate that RNase A modified by HOCl-serine exhibits all of the biochemical features of protein-bound Maillard and AGE products (2, 7, 8, 15). Proteins exposed to HOCl alone possess some of these features, but the reactions that induce browning and fluorescence probably are different.
One of the best-characterized AGE products is CML (9, 11, 12), a colorless and nonfluorescent compound. CML is formed in post-Amadori reactions and is a true end product that always accompanies the formation of brown, fluorescent, and cross-linked species in the Maillard reaction (2, 9, 16). CML is formed in higher yield than other known AGE products and can be quantified by isotope dilution GC/MS, a sensitive and specific assay (35).
Glycolaldehyde derived from protein-bound glucose may play a role in CML formation during glycoxidation reactions and AGE formation (16). Because myeloperoxidase also can generate glycolaldehyde, we determined whether CML would appear in RNase A exposed to HOCl-serine, the myeloperoxidase-H2O2-chloride system plus L-serine, or activated neutrophils. Mass spectrometric analysis revealed that all 3 systems produced CML. Importantly, the cell-mediated reaction required L-serine and neutrophil activation, and it was inhibited by catalase (a scavenger of H2O2) and azide (a heme poison), implicating myeloperoxidase in the reaction pathway. These results provide strong chemical evidence that oxidation of L-serine by myeloperoxidase promotes AGE product formation in vitro.
We have previously shown that HOCl generated by myeloperoxidase reacts with a wide variety of α-amino acids to yield a battery of reactive aldehydes (28, 46). An important issue then becomes whether myeloperoxidase will generate CML from L-serine in the presence of competing amino acids. CML levels increased markedly in RNase A exposed to myeloperoxidase and peroxide in buffer supplemented with physiological concentrations of the 7 most abundant free amino acids found in plasma. Formation of CML was reduced by ∼70% when L-serine was omitted from the reaction mixture. Human neutrophils likewise generated CML on RNase A in the presence of the amino acid mixture. CML production required activation of the cells with PMA and was inhibited by heme poisons, implicating oxidant generation and myeloperoxidase in the cell-mediated reaction. Importantly, CML generation by the cells was only partially reduced when L-serine was omitted from the medium.
These results indicate that activated human neutrophils use myeloperoxidase to generate CML on a model protein at plasma concentrations of free amino acids. Under these conditions, we observed both L-serine–dependent and L-serine–independent pathways of CML formation. The nature of the latter reaction(s) is unknown, but it may involve the conversion of other amino acids into reactive aldehydes by myeloperoxidase-generated HOCl (28, 46). Collectively, these observations indicate that myeloperoxidase promotes the formation of CML at physiological concentrations of free amino acids, raising the possibility that the enzyme promotes AGE formation in vivo.
Although many oxidation reactions are inhibited by water-soluble or lipid-soluble antioxidants (40), we found that classic antioxidants such as α-tocopherol (44) were poor inhibitors of CML formation by the HOCl-serine system. In contrast, the HOCl scavenger methionine (41, 42) potently inhibited this reaction. The effect of an antioxidant on myeloperoxidase-catalyzed reactions is critically dependent on both the target for oxidation and the reaction pathway. For example, α-tocopherol almost completely inhibits cholesterol chlorination by myeloperoxidase under acidic conditions (47). In future studies, it will be of interest to explore the effects of antioxidants and HOCl scavengers on AGE product formation in vivo.
A key question is whether activated phagocytes, via myeloperoxidase, generate AGE products at sites of inflammation such as vascular lesions. Active myeloperoxidase is present in human atherosclerotic lesions, where it partly colocalizes with lipid-laden macrophages (48). Recent studies using antibodies specific for CML demonstrated intense immunostaining of macrophages in such lesions (49). Moreover, AGE products have been detected by immunohistochemical techniques in atherosclerotic lesions of euglycemic animals, suggesting that elevated blood glucose may not be the only factor responsible for the generation of CML and other AGE products (50). In studies using isotope dilution GC/MS analysis, we found that CML and other AGE products were up to 8–9 times more abundant in LDL isolated from human atherosclerotic lesions than in LDL isolated from blood (our unpublished observations). These results suggest that a portion of the CML seen in atherosclerotic lesions may result from the oxidative activity of myeloperoxidase. Other pathways that could contribute CML to vascular lesions include (a) ascorbate-dependent reactions and (b) glyoxal generated during lipid peroxidation or glucose autoxidation (6, 51, 52).
Cross-linking of long-lived tissue proteins and extracellular matrix by the formation of AGE products is thought to accelerate vascular disease in diabetics (2, 8). AGE products bind to specific cell-surface receptors, triggering cytokine secretion and oxidant generation (53, 54). Moreover, Amadori products undergo a complex series of rearrangement and cleavage reactions that produce reactive oxygen species and dicarbonyl compounds (2, 14, 55–57); such chemicals could promote additional damage (2, 8). Indeed, CML and pentosidine have been detected in nondiabetic atherosclerotic lesions (50) and in the serum of patients with rheumatoid arthritis (58, 59), and have been localized to focal lesions in diabetic kidneys (60), suggesting that pathways independent of glycoxidation may promote AGE formation in vivo. Therefore, if myeloperoxidase mediates AGE product formation in vivo, as it does in vitro, many of the mechanisms implicated in tissue dysfunction in diabetes and aging also may operate at locations where the enzyme is produced — at sites of inflammation (48–50, 60, 61). Myeloperoxidase therefore has the potential to play key roles in inflammatory conditions, which contribute to a large number of clinical disorders.
Acknowledgments
We thank J. Moreno and S. Smith for expert technical assistance. GS/MC experiments were performed in part at the Washington University School of Medicine Mass Spectrometry Resource. This work was supported by the Monsanto-Searle/Washington University Biomedical Program and by grants DK-02456, AG-15013, AG-12293, HL-07275, RR-00954, and AG-11472 from the National Institutes of Health. J.R. Requena is the recipient of a Juvenile Diabetes Foundation postdoctoral fellowship. M.M. Anderson was supported by the Department of Pharmacology and Molecular Biology cardiovascular training grant. J.W. Heinecke is an Established Investigator of the American Heart Association.
References
- 1.Esterbauer HR, Schaur J, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Monnier VM, Sell DR, Nagaraj RH, Miyata S. Mechanisms of protection against damage mediated by the Maillard reaction in aging. Gerontology. 1991;37:152–165. doi: 10.1159/000213256. [DOI] [PubMed] [Google Scholar]
- 4.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 5.Witz G. Biological interactions of alpha, beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 6.Thornally PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification—a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 7.Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible processes for aging of long-lived proteins. Science. 1981;211:491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M, Thorpe SR, Baynes JW. Identification of Nε-(carboxymethyl)lysine as a degradation product of fructoselysine in glycated proteins. J Biol Chem. 1986;61:4889–4894. [PubMed] [Google Scholar]
- 10.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix: implications of pentoses in the aging process. J Biol Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- 11.Dyer DG, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCance DR, et al. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monnier VM, Glomb M, Elgawish A, Sell DR. The mechanism of collagen cross-linking in diabetes: a puzzle nearing resolution. Diabetes. 1996;45(Suppl. 3):S67–S72. doi: 10.2337/diab.45.3.s67. [DOI] [PubMed] [Google Scholar]
- 14.Ledl F, Schleicher E. New aspects of the Maillard reaction in foods and in the human body. Angew Chem Int Ed Engl. 1990;29:565–594. [Google Scholar]
- 15.Maillard LC. Action des acides amines sur les sucres: formation des melaniodines par voie methodique. CR Acad Sci. 1912;154:66–68. [Google Scholar]
- 16.Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- 17.Geoghegan KF, Ybarra DM, Feeney RE. Reversible reductive alkylation of amino groups in proteins. Biochemistry. 1979;18:5392–5399. doi: 10.1021/bi00591a021. [DOI] [PubMed] [Google Scholar]
- 18.Acharya AS, Manning JM. Reaction of glycolaldehyde with proteins: latent crosslinking potential of α-hydroxyaldehydes. Proc Natl Acad Sci USA. 1983;80:3590–3594. doi: 10.1073/pnas.80.12.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 20.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 21.Foote CS, Goyne TE, Lehrer RI. Assessment of chlorination by human neutrophils. Nature. 1981;301:715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- 22.Test ST, Lampert MB, Ossanna PJ, Thoene JG, Weiss SJ. Generation of nitrogen-chloride oxidants by human phagocytes. J Clin Invest. 1984;74:1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas EL, Grisham MB, Jefferson MM. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 24.Zgliczynski JM, Stelmaszynska T, Domanski J, Ostrowski W. Chloramines as intermediates of oxidation reactions of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971;235:419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- 25.Strauss RR, Paul BB, Jacobs AA, Sbarra AJ. Role of the phagocyte in host-parasite interactions. Myeloperoxidase-H2O2-Cl–-mediated aldehyde formation and its relationship to antimicrobial activity. Infect Immun. 1971;3:595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazen SL, Hsu FF, Heinecke JW. p-Hydroxyphenylacetaldehyde is the major product of L-tyrosine oxidation by activated human phagocytes: a chloride-dependent mechanism for the conversion of free amino acids into reactive aldehydes by myeloperoxidase. J Biol Chem. 1996;271:1861–1867. doi: 10.1074/jbc.271.4.1861. [DOI] [PubMed] [Google Scholar]
- 27.Hazen SL, d’Avignon A, Anderson MM, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-H2O2-Cl– system to oxidize α-amino acids to a family of reactive aldehydes: mechanistic studies identifying labile intermediates along the reaction pathway. J Biol Chem. 1998;273:4997–5005. doi: 10.1074/jbc.273.9.4997. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinecke JW, Li W, Daehnke HL, III, Goldstein JA. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J Biol Chem. 1993;268:4069–4077. [PubMed] [Google Scholar]
- 30.Rakita RM, Michel BR, Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990;29:1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- 31.Morita Y, Iwamoto H, Aibara S, Kobayashi T, Hasegawa E. Crystallization and properties of myeloperoxidase from normal leukocytes. J Biochem. 1986;99:761–770. doi: 10.1093/oxfordjournals.jbchem.a135535. [DOI] [PubMed] [Google Scholar]
- 32.Heinecke JW, Li W, Francis GA, Goldstein JA. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91:2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linder, M.C. 1992. Nutritional biochemistry and metabolism. Elsevier Science. New York, NY. 98.
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Knecht KJ, et al. Effect of diabetes and aging on carboxymethyllysine levels in human urine. Diabetes. 1991;40:190–196. doi: 10.2337/diab.40.2.190. [DOI] [PubMed] [Google Scholar]
- 36.Leeuwenburgh CL, et al. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 37.Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C. Anal Biochem. 1972;49:474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- 38.Morris JC. The acid ionization constant of HOC1 from 5–35 degrees. J Phys Chem. 1966;70:3798–3805. [Google Scholar]
- 39.Wells-Knecht MC, Thorpe SR, Baynes JW. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry. 1995;34:15134–15141. doi: 10.1021/bi00046a020. [DOI] [PubMed] [Google Scholar]
- 40.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 41.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 43.Heinecke JW, Baker L, Rosen H, Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986;77:757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niki E, Saito T, Kawakami A, Kamiya Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J Biol Chem. 1984;259:4177–4182. [PubMed] [Google Scholar]
- 45.Watson JT. Selected-ion measurements. Methods Enzymol. 1990;193:86–106. [Google Scholar]
- 46.Hazen SL, Hsu FF, d’Avignon A, Heinecke JW. Human neutrophils employ myeloperoxidase to convert alpha-amino acids to a battery of reactive aldehydes: a pathway for aldehyde generation at sites of inflammation. Biochemistry. 1998;37:6864–6873. doi: 10.1021/bi972449j. [DOI] [PubMed] [Google Scholar]
- 47.Heinecke JW, Li W, Mueller DM, Bohrer A, Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide system: potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33:10127–10136. doi: 10.1021/bi00199a041. [DOI] [PubMed] [Google Scholar]
- 48.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palinski W, et al. Immunological evidence for the presence of advanced glycation end products in atherosclerotic lesions of euglycemic rabbits. Arterioscler Thromb Vasc Biol. 1995;15:571–582. doi: 10.1161/01.atv.15.5.571. [DOI] [PubMed] [Google Scholar]
- 51.Fu M, et al. The advanced glycation product Nε-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 52.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt AM, et al. RAGE: a novel cellular receptor for advanced glycation end products. Diabetes. 1996;45:S77–S80. doi: 10.2337/diab.45.3.s77. [DOI] [PubMed] [Google Scholar]
- 54.Yan SD, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 55.Kato H, Cho RK, Okitani A, Hayase F. Responsibility of 3-deoxyglucosone for the glucose-induced polymerization of proteins. Agric Biol Chem. 1987;51:683–689. [Google Scholar]
- 56.Knecht KJ, Feather MS, Baynes JW. Detection of 3-deoxyfructose and 3-deoxyglucose in human urine and plasma: evidence for intermediate stages of the Maillard reaction in vivo. Arch Biochem Biophys. 1992;294:130–137. doi: 10.1016/0003-9861(92)90146-n. [DOI] [PubMed] [Google Scholar]
- 57.Liggins J, Furth AJ. Role of protein-bound carbonyl groups in the formation of advanced glycation end products. Biochim Biophys Acta. 1997;1361:123–130. doi: 10.1016/s0925-4439(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 58.Rodríguez-García J, Requena JR, Rodríguez-Segade S. Increased concentrations of serum pentosidine in rheumatoid arthritis. Clin Chem. 1998;44:250–255. [PubMed] [Google Scholar]
- 59.Takahashi M, Suzuki M, Miyamoto S, Inoue T. Relationship between pentosidine levels in serum and urine and activity in rheumatoid arthritis. Br J Rheumatol. 1997;36:637–642. doi: 10.1093/rheumatology/36.6.637. [DOI] [PubMed] [Google Scholar]
- 60.Katsunori H, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. J Clin Invest. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia induced formation of intracellular advanced glycation end products in bovine endothelial cells. J Clin Invest. 1996;97:1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]