Abstract
Microparticles (MPs) resulting from vesiculation of platelets and other blood cells have been extensively documented in vitro and have been found in increased numbers in several vascular diseases, but little is known about MPs of endothelial origin. The aim of this study was to analyze morphological, immunological, and functional characteristics of MPs derived from human umbilical vein endothelial cells (HUVECs) stimulated by TNF, and to investigate whether these MPs are detectable in healthy individuals and in patients with a prothrombotic coagulation abnormality. Electron microscopy evidenced bleb formation on the membrane of TNF-stimulated HUVECs, leading to increased numbers of MPs released in the supernatant. These endothelial microparticles (EMPs) expressed the same antigenic determinants as the corresponding cell surface, both in resting and activated conditions. MPs derived from TNF-stimulated cells induced coagulation in vitro, via a tissue factor/factor VII–dependent pathway. The expression of E-selectin, ICAM-1, αvβ3, and PECAM-1 suggests that MPs have an adhesion potential in addition to their procoagulant activity. In patients, labeling with αvβ3 was selected to discriminate EMPs from those of other origins. We provide evidence that endothelial-derived MPs are detectable in normal human blood and are increased in patients with a coagulation abnormality characterized by the presence of lupus anticoagulant. Thus, MPs can be induced by TNF in vitro, and may participate in vivo in the dissemination of proadhesive and procoagulant activities in thrombotic disorders.
Introduction
Under physiological conditions, vascular endothelium represents a complex regulated surface maintaining an antithrombogenic potential. This pattern is shifted toward a prothrombotic state when endothelial cells (ECs) are activated by various agents such as proinflammatory cytokines (i.e., TNF and IL-1β), infectious agents, or their components (i.e., LPS) (1, 2). Endothelial activation is associated with a loss of anticoagulant surface molecules such as thrombomodulin (TM), expression of prothrombotic components such as tissue factor (TF) (3), or increased binding of coagulation factors (2, 4). This activation can also promote interactions of ECs with circulating cells by modulation of surface adhesion molecules (5).
A general feature of activated cells is that they can shed fragments of their plasma membranes into the extracellular space (6). Such fragments, resulting from an exocytotic budding process, are colloquially known as microparticles (MPs). They include cytoplasmic components and membrane-derived elements such as negatively charged phospholipids or cell-surface receptors. Most of the studies have focused on circulating cells and have stressed that MPs could have an impact in the development of procoagulant and immune responses (reviewed in ref. 6). For example, monocyte vesiculation has been presented as a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by LPS (7). Similarly, upon activation by a variety of agonists, including calcium ionophore, terminal complement complex, or autoantibodies, platelets release MPs from their plasma membrane (8). These MPs provide a catalytic phospholipid surface for assembly of factors Xa, Va, and Ca2+ (prothrombinase complex), thereby accelerating blood coagulation (8, 9). This procoagulant potential is corroborated by clinical studies showing elevated levels of platelet-derived MPs in patients with prothrombotic states (10–13).
Despite an increasing interest in membrane-shed MPs, little is known about MPs of endothelial origin. In vitro experiments have established that complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and exposure of the prothrombinase enzyme complex (14). However, characterization of major endothelial membrane antigens on these MPs has never been achieved. Furthermore, to the best of our knowledge, the existence of MPs derived from ECs has not been demonstrated in vivo. The purpose of this work was (a) to demonstrate morphologically the production of MPs by human ECs stimulated by TNF; (b) to assess the distribution of coagulation and adhesion molecules on MPs; (c) to provide evidence of endothelial MP procoagulant activity; and (d) to investigate whether these elements are detectable in healthy individuals and in patients with lupus anticoagulant (LA), an acquired coagulation abnormality known to be associated with a thrombotic tendency.
Methods
Reagents and mAb’s.
FITC-conjugated mAb to vitronectin receptor (anti-αvβ3 or anti-CD51, clone AMF7, IgG1) was from Immunotech (Marseille, France), and FITC-conjugated mAb to E-selectin (anti-CD62E, clone CI26CIOB7, IgG2a) was from Boehringer Ingelheim Bioproducts (Gagny, France). Unlabeled or phycoerythrin-conjugated (PE-conjugated) mAb to PECAM-1 (CD31, clone MBC782, IgG1) was from Boehringer Ingelheim. PE-conjugated anti-CD41 (clone P2, IgG1) and anti-CD14 (clone RMO52, IgG2a) were both from Immunotech. The other reagents were unlabeled mAb to ICAM-1 (CD54, IgG1; Biocytex, Marseille, France), mAb to TM (CD141, clone 3E2, IgG1; generous gift from C. Parry, Stago, Paris, France), and mAb to TF (CD142, clone TF9-9C3, IgG1; Ortho Diagnostics, Issy-Les-Moulineaux, France). Labeling FITC-F(ab′)2 goat anti-mouse IgG (FITC-Fab) was purchased from Silenus (Eurobio, Les Ullis, France). Isotype-matched mAb of irrelevant specificity included unlabeled IgG1 (Sigma, St. Quentin-Fallavier, France), FITC-IgG1, and FITC-IgG2a (Immunotech). FITC-conjugated annexin V was purchased from Bender MedSystems (Vienna, Austria). Calibrated latex beads (0.8 and 3 μm in diameter) were from Sigma. Cell culture reagents were from Sigma, except for FCS, which was from Dominique Dutscher SA (Brumath, France). Recombinant TNF was from TEBU (Le Perray en Yvelines, France), and neutralizing anti-human TNF polyclonal antibodies were from Genzyme Diagnostics (Cambridge, Massachusetts, USA).
Patients.
Thirty healthy donors and 30 patients with a diagnosis of LA were studied. The normal population was matched for age and sex with the patient population. All individuals were between 20 and 65 years old. LA patients were diagnosed according to the recommendations of the International Society of Thrombosis and Haemostasis subcommittee on antiphospholipid antibodies (15, 16). Clinical settings of the LA were as follows: primary (n = 5) or systemic lupus erythematosus–associated (SLE-associated) antiphospholipid syndrome (n = 8); SLE or other autoimmune diseases without thrombotic manifestations (n = 6); infections (n = 4); malignancy (n = 5); and idiopathic LA (n = 2). Among the 13 patients with a history of thrombosis, 5 were being treated with oral anticoagulants while they were being studied. All patients with thrombosis were studied at least 3 months after the last thrombotic event.
EC culture.
HUVECs were prepared as described previously (16, 17). HUVECs were incubated for 24 hours with either TNF (1–100 ng/mL) with or without neutralizing anti-TNF antibody added 1 hour before treatment, or with the anti-TNF antibody alone. After 3 washes, HUVECs were rapidly detached by trypsin-EDTA for 30 seconds at 37°C and analyzed by flow cytometry. In each experiment, EC viability was determined by trypan blue exclusion tests and propidium iodide (PI) staining.
Preparation of cell culture supernatant and platelet-free plasma samples for MP analysis.
Culture supernatants from flasks containing 12 × 106 cells were collected and cleared from cell fragments by centrifugation at 4,300 g for 5 minutes. The supernatant was then ultracentrifuged at 100,000 g for 90 minutes at 10°C. Pelleted endothelial microparticles (EMPs) were resuspended in 100 μL of cell culture PBS (DMEM; 0.01 M, pH 7.2, filtered) and used immediately.
For platelet-free plasma preparation, 5-mL blood samples were drawn by venipuncture into 0.129 M tri-sodium citrate. MPs were extracted from whole blood within 1 hour by 2 sequential centrifugations for 15 minutes at 1,500 g, followed by a 1-minute decantation at 13,000 g to remove all the residual platelets or cell fragments of a similar size, as described (17).
Cell and MP immunolabeling.
Suspensions of 105 ECs were incubated with relevant mAb’s (at saturating concentrations), washed twice, and incubated with FITC-Fab. Aliquots of 10 μL of the MP suspension were labeled at 4°C according to 3 different protocols: (a) by indirect immunofluorescence, after incubation with the first-layer mAb (10 μL at 200 μg/mL), followed by incubation with the second-layer FITC-Fab (100 μL, 1:200 dilution); (b) by single or double fluorescence, after incubation with PE- and/or FITC-conjugated mAb’s; and (c) by phosphatidylserine (PS) probing using 20 μL of annexin V-FITC diluted 1:50 in annexin buffer. In each experiment, control labeling was performed by incubating irrelevant mAb (conditions a and b) or annexin V in appropriate buffer (condition c). For confocal laser microscopy, samples were prepared according to condition b.
Flow cytometry analysis.
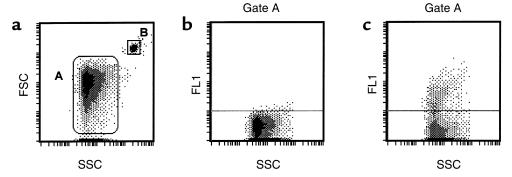
Suspensions containing 105 cells or the MPs produced by 106 cells were analyzed on a Coulter Epics XL (Coultronics France, Margency, France). The light scatters and fluorescence channels were set at logarithmic gain. Regions corresponding to cells or shed MPs were defined on separate protocols, using forward light scatter (FSC) versus side-angle light scatter (SSC) intensity dot plot representation. For HUVEC analysis, the cell region was defined, and mean fluorescence intensities (MFI) of the positive cell populations were measured for each antigen. MPs were defined as elements with a size less than 1.5 μm that were positively labeled by specific mAb’s. EMPs were enumerated using either specific mAb or FITC-annexin V labeling (Figure 1), as described previously (18). An internal standard corresponding to calibrated latex beads (Sigma) was added to samples before analysis. The diameter of the beads (3 μm, gate B) discriminated them from the MP population on the FSC-SSC cytogram. A known number of beads was added to each sample tube (e.g., 200,000), and the analysis was stopped when 20,000 beads were counted. For MP quantitation, SDs for inter- and intra-assays were 11% and 6%, respectively. Annexin V and PI were used to assess apoptosis, following manufacturer recommendations (Bender MedSystems).
Figure 1.
Flow cytometric quantitation of EMPs. MPs (Gate A) were discriminated by size on an FSC/SSC cytogram (a). Only events included within gate A were further analyzed for fluorescence associated with irrelevant (b) and specific (c) labeling. Gate A was defined by excluding the first FSC channel that contained most of the background noise and by using 0.8-μm latex beads. The gate was defined to include the beads in its upper 33%. In this example EMP quantitation was done using FITC-annexin V labeling, and EDTA buffer was used as a negative control. (a) Determination of forward scatter (FSC) and side-scatter (SSC) characteristics of MPs in suspension. A similar approach was used to analyze MPs in platelet-free plasma. The 2 gates represent the pattern of 0.8- and 3-μm latex beads (A and B, respectively). (b) Determination of the limit for negative fluorescence, performed in the presence of EDTA as a negative control for annexin V. (c) Detection of PS on MPs through annexin V-FITC binding (FL1), expressed in relation to structure (SSC).
Procoagulant activity of EMPs.
The procoagulant properties of EMPs were evaluated by a plasma recalcification time assay measuring the plasma clotting time. Pelleted EMPs suspended in Owren-Koller buffer (Stago) were added to normal or factor VII–deficient plasma (Stago). Control clotting time was performed by mixing plasma and either Owren-Koller buffer or ultracentrifugation supernatant (MP-free). Briefly, 50 μL of plasma was mixed with buffer, supernatant, or MP suspensions (25 μL, final volume) and incubated for 5 minutes in a 37°C warmed water bath. Then, 75 μL of 0.025 M CaCl2 was added and the clotting time was measured.
SDS-PAGE and Western blotting.
Confluent HUVECs and pelleted EMPs were solubilized by SDS (12%)-N-ethyl maleimide (30 mM) (1 vol EMPs per 4 vol reagent). After determination of protein content (Bio-Rad Laboratories GmbH, München, Germany) and electrophoresis, the samples were analyzed by Western blot with anti-CD31 or anti-CD51 (clone VMA 1920; Valbiotech, Paris, France) revealed by peroxidase-linked, species-specific anti-mouse Ig F(ab)′2 (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). Antibody binding was revealed using a chemiluminescence kit (Valbiotech).
Electron and confocal microscopy.
HUVECs were grown on 2% gelatin-coated glass slides and processed for scanning electron microscopy after a 6-hour incubation in RPMI/20% FCS with or without TNF (100 ng/mL). Cells were washed extensively in PBS and fixed in glutaraldehyde (2.5% in PBS) for 20 minutes at room temperature. Washed MPs were collected by gravity on 0.1-μm filters (4VMTP; Millipore SA, St. Quentin en Yvelines, France). Cells and MPs were then dehydrated in a graded series of ethanol (45–100%), critical-point dried in a CO2 system, mounted on specimen stubs, and gold-coated in a sputtering device. All samples were examined using an electron microscope (JEOL USA Inc., Peabody, Massachusetts, USA). For transmission electron microscopy, MPs were fixed 30 minutes in glutaraldehyde and postfixed in osmium for 30 minutes. Samples were then dehydrated in a series of acetone solutions (75–100%), soaked in araldite/acetone solution and in araldite alone for 90 minutes at 40°C, and allowed to polymerize overnight at 70°C.
For confocal laser microscopy, MPs were washed and resuspended in PBS, incubated with FITC-conjugated-mAb’s, and spotted on polylysine-coated slides. Samples were mounted in Mowiol and examined with a Leica confocal laser microscope (LEIKA Mikroskopie, Wetzlar, Germany).
Statistical analysis.
Differences between groups were evaluated using the nonparametric Mann-Whitney U test; P < 0.05 was considered to be significant.
Results
Release of MPs by HUVECs upon stimulation by TNF.
To view the process of MP blebbing and shedding on HUVEC surface, cells were stimulated with TNF for 6 hours and prepared for scanning electron microscopic analysis. Unstimulated HUVECs displayed a cobblestone shape and a smooth surface, with a relatively low number of vesicles, presenting a sparse distribution (Figure 2a and b). After stimulation (Figure 2c), the cells exhibited a more fusiform shape and a blebby surface — due to a marked increase in the number of vesicles, which nearly covered the cell surface. At a higher magnification (Figure 2d), these blebs showed different diameters ranging from 0.5 to 2.5 μm (arrows). Some of the blebs were almost detached from the surface (Figure 2d, arrowhead). Analysis of culture supernatants from TNF-stimulated cells by scanning electron microscopy showed MPs with diameters ranging from 0.1 to 1.5 μm (Figure 2, e and f).
Figure 2.
Morphology of HUVECs and EMPs. First-passage monolayers of resting (a and b) and TNF-stimulated (c and d) HUVECs were analyzed by scanning electron microscopy. (a and c) ×1,500; (b and d) ×3,000. (e and f) High-power magnification (×24,000 and ×36,000) of EMPs shed from TNF-stimulated HUVECs. Both resting and stimulated ECs showed surface blebs (arrows) or detached vesicles (arrowhead in d). Scale bars: 10 μm (a–d), 1 μm (e and f). P, filter pore.
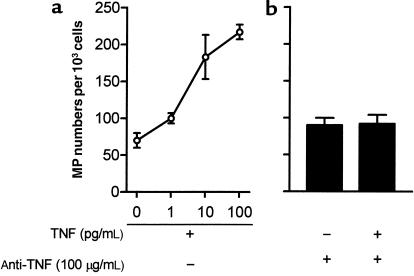
We therefore quantitated the effect of TNF on vesiculation (Figure 3). In resting conditions, HUVECs released 90 ± 8 MPs per 103 cells in the supernatant over a 24-hour period, as determined by annexin V binding. TNF stimulation induced, in a dose-dependent manner, an increase in the number of MPs released from HUVECs by a maximum of 2.5-fold for 100 ng/mL TNF stimulation. This effect was abrogated when neutralizing anti-TNF antibody was added 1 hour prior to TNF. The antibody pretreatment alone had no effect. Apoptosis was not detectable in ECs in our conditions of TNF activation, as shown by PI and annexin V staining (data not shown). Other EC agonists were investigated for their capacity to induce vesiculation, including IL-1β (10 U/mL, 24 hours at 37°C), PMA (100 ng/mL, 24 hours at 37°C), thrombin (0.1 U/mL, 24 hours at 37°C), and calcium ionophore (100 μmol/L, 10 minutes at 37°C). MP numbers released in these conditions were similar to those obtained after TNF stimulation.
Figure 3.
Effect of TNF on EMP shedding. HUVECs were incubated with either medium alone or varying concentrations of recombinant human TNF (a). A neutralizing anti-TNF antibody was added 1 hour before stimulation with the highest TNF concentration or was tested alone (b). Results are expressed in numbers of MPs labeled with annexin V-FITC, extracted from culture supernatants of 103 ECs. Bars represent SD of 3 determinations in 4 experiments.
Expression and modulation of surface molecules on ECs and EMPs.
We determined whether MPs displayed the same phenotype as the cells from which they came. The presence of endothelial membrane antigens involved in coagulation (TF and TM) and adhesion (E selectin, ICAM-1, αvβ3, and PECAM-1) were assessed on both HUVECs and their shed MPs using flow cytometry (Figure 4).
Figure 4.
Distribution of endothelial antigens on resting or TNF-stimulated HUVECs and their derived MPs. HUVECs were cultured for 24 hours in the presence or absence of TNF (100 ng/mL), detached and analyzed for mAb binding by flow cytometry (top). MPs derived from these ECs were labeled with the same mAb (bottom). For each antigen studied, the antibody binding was expressed as mean fluorescence intensity (MFI) of the positive population for HUVECs and as number of positive events for MPs, in view of the low intensity of labeling of the latter. For each mAb, MFI of cells and number of their derived MPs are shown under resting (left) and stimulated (right) conditions. Irrelevant mAb’s (both IgG1 and IgG2a) led to identical background staining.
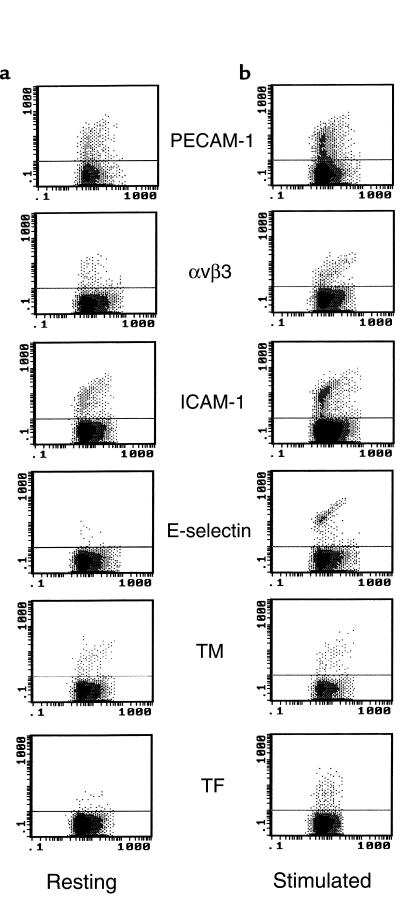
Constitutive antigens of ECs, such as PECAM-1, αvβ3, ICAM-1, and TM, were present on both unstimulated cells and derived MPs. In response to a 24-hour TNF stimulation, the expression of PECAM-1 was not significantly modified on cells, whereas a 1.5-fold increase in the number of MPs expressing the corresponding antigen was observed. TNF enhanced the surface expression of αvβ3 (1.5-fold) and ICAM-1 (50-fold) on ECs concomitantly with an increase in the number of αvβ3- and ICAM-1–positive MP (3- and 4-fold, respectively). As expected, TM expression was decreased on TNF-stimulated HUVECs, and this decrease was accompanied by a slight diminution of TM-positive MPs. Inducible antigens, such as E-selectin and TF, were not detectable on resting ECs, whereas both peaked 4–6 hours after stimulation (data not shown) and presented a residual level of expression at 24 hours. It is noteworthy that TF- and E-selectin–positive EMPs were detectable after 24 hours of stimulation, compared with resting conditions (Figure 5, b versus a).
Figure 5.
Flow cytometric analysis of EMPs under resting and stimulated conditions. MPs were obtained from EC supernatants and stained with mAb’s, as described in Methods. The cytograms (FL1/SSC) shown here are representative graphs of mAb binding on MPs, counted using 3-μm latex beads (gate B of Figure 1a) as an internal standard. In resting conditions (a), constitutive antigens such as PECAM-1, αvβ3, TM, and ICAM-1 were present on MPs, whereas inducible antigens such as TF and E-selectin were detectable only upon TNF stimulation (b). The horizontal bars represent the level of irrelevant mAb binding, used as control.
Representative staining patterns of MPs shed from HUVECs — stimulated by TNF or not — are illustrated in Figure 5, a and b, respectively. No direct comparison between detection by annexin V and mAb was made because surface distribution, affinity for the respective ligands, and incubation times were different. Nevertheless, these experiments indicate that particles found in the supernatant bore a substantial proportion of the antigens found on the corresponding cell.
TNF-generated procoagulant activity is associated with EMPs.
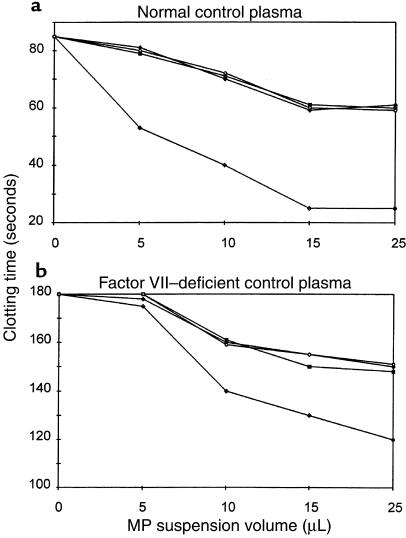
In view of the EC changes suggesting a switch toward a procoagulant activity, the functional consequences of TF induction and TM downregulation were investigated. Accordingly, the procoagulant activity of MPs shed from 24-hour TNF-stimulated HUVECs was assessed by a clotting assay. A dose-response curve was established by incubating dilutions of the MP suspensions (5, 10, 15, and 25 μL of MP in 25 μL [final volume] of Owren-Koller buffer). A 35–70% shortening of the control plasma clotting time was observed after addition of increasing numbers of TNF-stimulated, HUVEC-derived MPs (Figure 6). MPs shed from unstimulated HUVECs or MPs derived from the different control groups (TNF-neutralizing antibody with or without TNF) resulted in a slight shortening of the clotting time (5–20%).
Figure 6.
Plasma clotting time in the presence of EMPs. MPs were extracted by ultracentrifugation from the culture supernatant of unstimulated HUVECs (open circles), HUVECs incubated with anti-TNF antibody alone (filled triangles), anti-TNF antibody before TNF (100 ng/mL) (filled squares), and TNF (100 ng/mL) alone (filled diamonds). Various numbers of EMPs in suspension were added (10 μL corresponded to the number of MPs derived from 105 cells) to a chronometric test of normal (a) and factor VII–deficient (b) plasma clotting (Howell time). Data are expressed in absolute clotting times (measured in seconds).
Induction of TF on EMPs suggests that their procoagulant activity can be accounted for by activation of the extrinsic pathway of the coagulation system. To test this hypothesis, a factor VII–deficient plasma was used, allowing only intrinsic pathway involvement. In these conditions, a small decrease of the clotting time was observed after addition of MPs derived from TNF-stimulated HUVECs (a decrease of 5–30%). MPs obtained from control HUVECs or from HUVECs stimulated in the presence of anti-TNF antibody had a similar effect on the clotting time (5–15% decrease).
Identification of EMPs in peripheral blood.
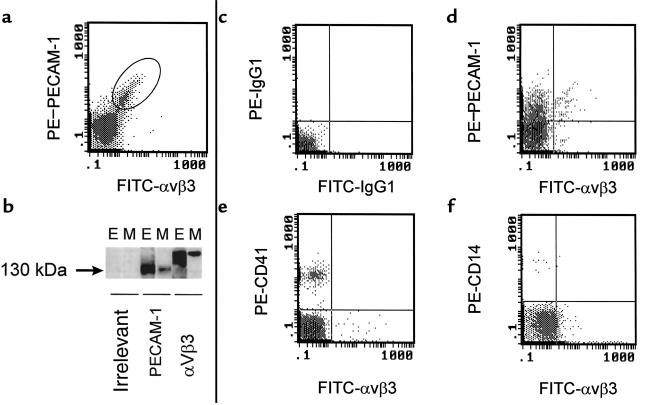
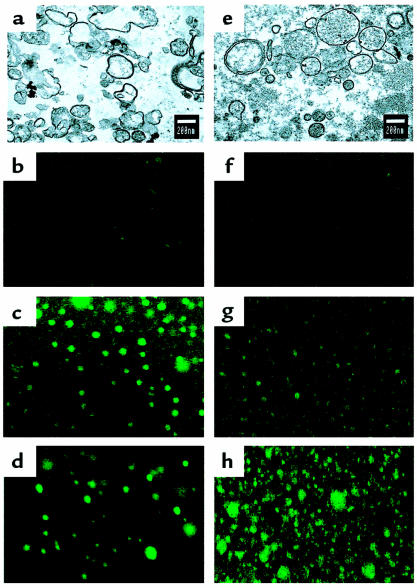
The demonstration that HUVECs and shed EMPs shared the same antigens was used to select a marker, or a combination of markers, that could allow the detection of EMPs in human plasma. We selected constitutive antigens that are highly expressed by ECs to ensure the most discriminative labeling of MPs. Accordingly, αvβ3 was used in combination with PECAM-1 to label MPs derived from HUVECs. As illustrated in Figure 7a, the whole MP population analyzed was double labeled. Biochemical evidence that EMPs express αvβ3 and PECAM-1 was provided by Western blot analysis on HUVEC and EMP lysates. Similar bands, at approximately 150 kDa for αvβ3 and 130 kDa for PECAM-1, were revealed on both HUVECs and EMPs (Figure 7b). Moreover, confocal laser microscopy showed αvβ3- and E-selectin–positive elements of less than 1.0 μm in diameter (Figure 8, c and d). Using transmission electron microscopy, TNF-induced EMPs appear as rounded vesicular structures with diameters ranging from 0.1 to 0.05 μm (Figure 8a).
Figure 7.
Use of PECAM-1 and αvβ3 coexpression to delineate the endothelial origin of MPs: in vitro setup and ex vivo detection. For in vitro studies, the release of MPs in EC supernatants was induced by TNF, as described. (a) Setup of PECAM-1 and αvβ3 double labeling of MPs generated in vitro. (b) SDS-PAGE and Western blot analysis of HUVECs (E) and MP lysates (M) under nonreducing conditions, revealed with control isotype mAb, PECAM-1, and αvβ3. Ex vivo, double labeling of MPs was performed in normal human plasma (c–f). (c) Labeling with isotype control mAb’s, allowing the setting of the background noise position on the subsequent cytograms. (d) Positive double labeling of plasma MPs with FITC–anti-αvβ3 and either PE–anti-PECAM-1, PE–anti-CD41 (e), or PE–anti-CD14 (f).
Figure 8.
Transmission electron and confocal laser microscopy of MPs. Transmission electron microscopy appearance of MPs generated in vitro (a) or in vivo (e), i.e., isolated from normal plasma (final magnification: ×25,000). In confocal laser microscopy, MPs appeared as elements with a diameter less than 1 μm and stained for αvβ3 (c) and E-selectin (d) in vitro. In vivo–generated MPs expressed αvβ3 (g) and CD41/GPIIb-IIIa (h). In this case, the majority of the elements were positive for CD41 (h), whereas only about 10% were positive for αvβ3 (g), confirming that they originate from ECs (final magnification: ×1,000). (b and f) Negative staining using isotope control IgG1.
In vivo–generated MPs revealed vesicular structures with diameters ranging from 0.1 to 1.0 μm (Figure 8e). Compared with those generated in vitro, these MPs appeared larger, more electron dense, and presented a more regular shape. The feasibility of MP detection using PECAM-1/αvβ3 double labeling was then investigated in normal human blood. Based on the control staining defined by irrelevant antibodies (Figure 7c), approximately 10% of MPs analyzed bound PECAM-1 and αvβ3 (Figure 7d), whereas about 90% were found to be positive only for PECAM-1, which is known to be expressed on blood cells. Because low levels of αvβ3 have been detected on platelets and monocytes, double-labeling experiments using anti-αvβ3 in combination with anti-CD41 or anti-CD14 were also performed. In both cases, no double-positive events were detectable; the proportion of αvβ3-labeled events was approximately 10%, whereas those positive for CD41 and CD14 represented 80% and 5%, respectively (Figure 7, e and f). These proportions of CD41-positive MPs versus αvβ3-positive elements were confirmed by confocal laser microscopy (Figure 8, g and h). Altogether, these experiments show that αvβ3 is not detectable on platelet- and monocyte-derived MPs and can be used to selectively discriminate EMPs in blood. Red blood cell, lymphocyte, and other leukocyte markers (glycophorin A, CD4, and CD45, respectively) were also found to be negative on αvβ3-labeled MPs (data not shown).
Quantitation of EMPs in blood samples from LA-positive patients.
Because part of the MPs present in peripheral blood are of endothelial origin, we used αvβ3 to evaluate their number in 30 healthy individuals in comparison with a population presenting a thrombotic risk, such as LA-positive patients. Mean values of 37,000 ± 9,000 MPs/mL of plasma (± SD) were measured in healthy individuals, with no difference in EMP number linked to age and sex. The MP count was significantly higher in patients with LA, with mean values of 76,500 ± 64,000/mL of plasma (P = 0.001; Figure 9). Moreover, EMP count was significantly higher in patients who developed a thrombotic complication than in those who did not (102,000 ± 92,000 vs. 55,000 ± 19,000, respectively); results of the Mann-Whitney U test were P = 0.0074, even when the patient with the highest level of endothelial EMPs was excluded from the analysis. Interestingly, in the patients with thrombotic complications who received oral anticoagulants, the level of EMPs was not reduced when compared with untreated patients. Conversely, the highest EMP counts were found in 4 out of the 5 patients treated with coagulants.
Figure 9.
Flow cytometric analysis of EMPs in the plasma from healthy donors (n = 30; open triangles) and LA patients (n = 30; all circles) was performed using FITC–anti-αvβ3 labeling. EMPs were quantitated in plasma as described in Methods. Among the LA patients 15 had a history of thrombosis (filled circles). The difference between groups was analyzed by the Mann-Whitney U test.
Discussion
The present study led to 2 complementary in vitro and in vivo observations, allowing us to demonstrate that EMPs are present in human peripheral blood. In vitro, TNF induces vesiculation of the endothelial plasma membrane, which results in the release of MPs displaying the same coagulant and adhesive receptors as their cell of origin. TNF increased the amount and procoagulant activity of MP by way of a TF/factor VII–dependent pathway, indicating a link between inflammation and hemostasis. In normal individuals, we observed a basal level of EMPs, which was significantly enhanced in patients presenting a prothrombotic abnormality, namely the presence of antiphospholipid (aPL) antibodies with LA activity.
Scanning electron microscopy was used to show the release of MPs from ECs stimulated by TNF, whereas fewer of such membrane formations were observed on control cells. MPs were defined as the sedimented material obtained by ultracentrifugation of cell supernatants, with diameters between 0.1 and 1.5 μm, a size close to that defined for platelets (18, 19). Transmission electron microscopy shows MP structure consistent with previous reports (20).
Endothelial membrane vesiculation can be triggered by a variety of agonists, including C5b-9, thrombin, and calcium ionophore (14). In our study, TNF was selected because it can switch ECs toward a procoagulant and proadhesive endothelial phenotype (21) and can induce apoptosis (22). A functional link exists between this phenomenon and vesiculation, because it has been shown that the release of MPs is an early feature of apoptosis (8). However, in the present study, the generation of MPs occurs without the triggering of apoptosis itself.
The release of MPs by unstimulated endothelium implies an ongoing vesiculation, consistent with the basal level of MPs detected in the plasma of healthy subjects. It suggests that endothelial vesiculation does occur under physiological conditions, as demonstrated for platelets (12, 13, 23).
Comparative phenotypic analysis, determined in the presence and absence of TNF, demonstrated a strict relationship in the expression of coagulant and adhesive receptors between HUVECs and their derived particles. Moreover, when cells were stimulated by TNF, the number of MPs positive for each mAb was higher than that obtained with unstimulated cells, except for TM, in agreement with its downregulation induced by TNF stimulation (21). Following TNF stimulation, decreased expression of TM and persistence of TF strongly suggested the thrombin-generating capacity of MPs, as confirmed by the clotting assay. Our data indicate that MPs derived from TNF-stimulated HUVECs support thrombin generation by way of a TF/factor VII–mediated pathway, because these MPs generated thrombin more slowly in factor VII–deficient plasma than in normal plasma. However, because a remaining clotting effect was observed in the presence of this deficient plasma, it can be hypothesized that MP procoagulant activity was partly due to the presence of PS and other negatively charged phospholipids able to trigger the extrinsic pathway independently of TF. Also, it is likely that MPs from unstimulated ECs poorly support prothrombinase activity, as suggested by the weak clotting activity observed for unstimulated endothelium.
MPs derived from stimulated HUVECs also expressed adhesive molecules such as E-selectin, ICAM-1, and αvβ3, showing that, in addition to their procoagulant activity, they may have an adhesive potential. It is noteworthy that, like TF, E-selectin is still expressed on EMPs 24 hours after TNF stimulation. MPs derived from monocytes stimulated by LPS expressed both procoagulant and proadhesive phenotypes, as a result of the simultaneous expression of functional TF, PS, and CD11b/CD18 integrin (7). The adhesive potential of MPs shed from leukocytes was demonstrated by their capacity to adhere to and activate ECs (24). Similarly, MPs released by activated platelets aggregate neutrophils (19), modulate monocyte-EC interactions (25), and adhere to fibrinogen, thereby promoting platelet adhesion (26). In our observation, the presence of αvβ3 on MPs also suggests that they could mediate heterotypic cell adhesion in the presence of fibrinogen, in agreement with recent observations showing that αvβ3 is involved in platelet adhesion to the luminal side of activated ECs in the presence of fibrinogen (27).
The fact that clinical studies have extensively documented MPs from platelets and other blood cells, but not from endothelium, is presumably related to the low number of EMPs in circulating blood and to the limited number of endothelial-specific markers. Among the molecules selected here for EMP immunophenotyping, only E-selectin is specific for the endothelium (28). However, in normal individuals, as well as in patients, MPs were not detectable with this marker (not shown). This may be because these MPs come from resting ECs, which do not express E-selectin. Thus, we used an mAb directed against the αv subunit of αvβ3, the predominant integrin expressed by the endothelium (300,000 sites per cell) (29). Using αvβ3/PECAM-1 double staining, we observed a major αvβ3-negative population, defined as “nonendothelial,” and a minor αvβ3-positive population, defined as “endothelial,” representing about 10% of the MPs analyzed. Although αvβ3 was also described on platelets and monocytes, it was as a minor component, because we determined its levels on these cells to be less than 800 sites on platelets and 5,000 sites on monocytes (not shown). In addition, αvβ3 is mostly present in the surface-connected canalicular system, including that of α granules (30). We ruled out the fact that αvβ3 could be detectable on MPs derived from platelets or monocytes by double-labeling experiments that showed the lack of coexpression with CD41 or CD14. Because the numbers of αvβ3- and αvβ3/PECAM1–positive events were comparable in our samples, we inferred that αvβ3 was selective enough to discriminate EMPs in whole blood. For the first time, we believe, EMPs are in evidence in normal human plasma and are found to be increased in patients selected according to a biological abnormality, namely being positive for LA. This coagulation abnormality is well recognized to be strongly associated with a thrombotic tendency (31, 32). This association has been termed antiphospholipid syndrome (APS). Moreover, among the LA-positive patient population, those who experienced thrombotic events had higher numbers of EMPs than the thrombosis-free patients. It is noteworthy that SLE patients without LA manifested EMP levels similar to those of controls. Although the hypothesis of antibody-mediated alteration of the vascular endothelium predominates (33), the pathogenic basis of the prothrombotic effect of aPL remains unclear. Various endothelial-dependent mechanisms have been proposed, such as imbalance of prostacyclin/thromboxane biosynthesis (34), interference with the protein C/TM anticoagulant pathway (35), impairment of the heparin sulfate/antithrombin–dependent inhibition of coagulation proteases (36), alterations of fibrinolysis (37), induction of cell-adhesive receptors for leukocytes (38), or modification of annexin V exposure (39). Recent studies have focused on the role of aPL in the induction of the TF-dependent procoagulant pathway — immunoglobulin fractions containing aPL (40) or mAb’s raised from patients with APS (41) — bound to cultured ECs and induced TF activity and mRNA, which may lead to a prothrombotic state. Moreover, in a thrombosis model, plasma aPL antibodies from patients induced procoagulant activity, in synergy with suboptimal concentrations of TNF (42). Because EMPs detected in patients were not analyzed functionally, their pathophysiological significance in vivo remains to be determined. However, the multiple-parameter characterization performed in vitro suggests that their release into the circulation could disseminate procoagulant and proadhesive activities, and thereby may contribute to the prothrombotic tendency of these patients. Moreover, the sustained presence of TF and E-selectin on EMPs may confer to these elements a crucial role in localizing the hemostatic process at a distance from the site of endothelium vesiculation. This generation of EMPs could represent a novel control mechanism of the coagulation system by ECs seen in inflammatory and thrombotic syndromes.
Acknowledgments
We thank C. Alasia, M. Fraterno, and J.L. Ansaldi for electron microscopy; C. Parry for his gift of anti-thrombomodulin mAb; Immunotech and Biocytex for providing antibodies; C. Guilianelli, M. Nonotte, and P. Stellmann for HUVEC preparation; and A. Boyer for technical assistance.
References
- 1.Bombeli T, Mueller M, Haeberli A. Anticoagulant properties of the vascular endothelium. Thromb Haemost. 1997;77:408–423. [PubMed] [Google Scholar]
- 2.Rodgers GM. Hemostatic properties of normal and perturbed vascular cells. FASEB J. 1992;2:116–125. doi: 10.1096/fasebj.2.2.3277885. [DOI] [PubMed] [Google Scholar]
- 3.Crossman DC, Carr DP, Tuddenham EG, Pearson D, McVey JH. The regulation of tissue factor mRNA in human endothelial cells in response to endotoxin or phorbol ester. J Biol Chem. 1990;265:9782–9787. [PubMed] [Google Scholar]
- 4.Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to internalisation and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989;73:159–165. [PubMed] [Google Scholar]
- 5.Bevilacqua MP. Endothelial leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 6.Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 7.Satta N, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 8.Pasquet JM, Toti F, Nurden AT, Dachary-Prigent J. Procoagulant activity and active calpain in platelet-derived microparticles. Blood. 1996;82:509–522. doi: 10.1016/0049-3848(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 9.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. J Biol Chem. 1989;264:17049–17057. [PubMed] [Google Scholar]
- 10.Nieuwland R, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 11.Gawaz M, Neumann FJ, Ott I, Schiessler A, Sschömig A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation. 1996;93:229–237. doi: 10.1161/01.cir.93.2.229. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin E, et al. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–3699. [PubMed] [Google Scholar]
- 13.Wiedmer T, et al. Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelet of paroxysmal nocturne hemoglobinuria. Blood. 1993;82:1192–1196. [PubMed] [Google Scholar]
- 14.Hamilton KK, Hattori R, Esmon CT, Sims PJ. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990;5:3809–3814. [PubMed] [Google Scholar]
- 15.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. Thromb Haemost. 1995;74:1185–1190. [PubMed] [Google Scholar]
- 16.Mutin M, George F, Lesaule G, Sampol J. Reevaluation of trypsin-EDTA for endothelial cell detachment before flow cytometry analysis. Endothelium. 1996;4:289–295. [Google Scholar]
- 17.Combes V, Dignat-George F, Mutin M, Sampol J. A new flow cytometry method of platelet-derived microvesicle quantification in plasma. [letter] Thromb Haemost. 1997;77:220. [PubMed] [Google Scholar]
- 18.Jy W, Horstman LL, Arce M, Ahn YS. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med. 1992;119:334–345. [PubMed] [Google Scholar]
- 19.Jy W, Mao WW, Horstman LL, Tao J, Ahn YS. Platelet microparticles bind, activate and aggregate neutrophils in vitro. Blood Cells Mol Dis. 1995;30:217–231. doi: 10.1006/bcmd.1995.0025. [DOI] [PubMed] [Google Scholar]
- 20.Holme PA, et al. Glycoprotein IIb-IIIa on platelet-derived microparticles structures studied by electron microscopy, confocal laser microscopy and crossed radio-immunoelectrophoresis. Platelets. 1996;7:207–214. doi: 10.3109/09537109609023580. [DOI] [PubMed] [Google Scholar]
- 21.Nawroth PP, Stern DM. Modulation of endothelial cells by TNF haemostatic properties. J Exp Med. 1986;163:740–756. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slowik MR, et al. Evidence that tumor necrosis factor triggers apoptosis in human endothelial cells by interleukin-1-converting enzyme-like protease-dependent and -independent pathways. Lab Invest. 1997;77:257–267. [PubMed] [Google Scholar]
- 23.Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelets-derived microparticles in humans. Blood. 1990;1:128–138. [PubMed] [Google Scholar]
- 24.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 25.Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holme PA, Solum NO, Brosstad F, Pedersen T, Kveine M. Microvesicles bind soluble fibrinogen, adhere to immobilized fibrinogen and coaggregate with platelets. Thromb Haemost. 1998;79:389–394. [PubMed] [Google Scholar]
- 27.Gawaz M, et al. Vitronectin receptor (αvβ3) mediates platelet adhesion to the luminal aspect of endothelial cells. Circulation. 1997;96:1809–1818. doi: 10.1161/01.cir.96.6.1809. [DOI] [PubMed] [Google Scholar]
- 28.Bevilacqua ME, et al. Selectins: a family of adhesion receptors. [letter] Cell. 1991;67:233. doi: 10.1016/0092-8674(91)90174-w. [DOI] [PubMed] [Google Scholar]
- 29.Mutin M, Dignat-George F, Sampol J. Immunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface molecules. Tissue Antigens. 1997;50:449–458. doi: 10.1111/j.1399-0039.1997.tb02899.x. [DOI] [PubMed] [Google Scholar]
- 30.Poujol C, Nurden AT, Nurden P. Ultrastructural analysis of the distribution of the vitronectin receptor (αvβ3) in human platelets and megakaryocytes reveals an intracellular pool and labelling of the α-granule membrane. Br J Haematol. 1997;97:823–835. doi: 10.1046/j.1365-2141.1997.d01-2109.x. [DOI] [PubMed] [Google Scholar]
- 31.Harris EN, Pierangeli SS, Gharavi AE. Diagnosis of the antiphospholipid syndrome: a proposal for use of laboratory tests. Lupus. 1998;7(Suppl. 2):S144–S148. doi: 10.1177/096120339800700232. [DOI] [PubMed] [Google Scholar]
- 32.Wahl DG, et al. Risk for venous thrombosis related to antiphospholipid antibodies in systemic lupus erythematosus: a meta-analysis. Lupus. 1997;6:467–473. doi: 10.1177/096120339700600510. [DOI] [PubMed] [Google Scholar]
- 33.Arnout J. The pathogenesis of the antiphospholipid syndrome: a hypothesis based on parallelisms with heparin-induced thrombocytopenia. Thromb Haemost. 1996;75:536–541. [PubMed] [Google Scholar]
- 34.Lellouche F, Martinuzzo M, Said P, Maclouf J, Carreras LO. Imbalance of thromboxane/prostacyclin biosynthesis in patients with lupus anticoagulant. Blood. 1991;78:2894–2899. [PubMed] [Google Scholar]
- 35.Oosting JD, et al. Antiphospholipid antibodies directed against a combination of phospholipids with prothrombin, protein C, or protein S: an explanation for their pathogenic mechanism? Blood. 1993;81:2618–2625. [PubMed] [Google Scholar]
- 36.Shibata S, Harpel PC, Gharavi A, Rand J, Fillit H. Autoantibodies to heparin from patients with antiphospholipid antibody syndrome inhibit formation of antithrombin III-thrombin complexes. Blood. 1994;83:2532–2540. [PubMed] [Google Scholar]
- 37.Tsakiris DA, Marbet GA, Makris PE, Settas L, Duckert F. Impaired fibrinolysis as an essential contribution to thrombosis in patients with lupus anticoagulant. Thromb Haemost. 1989;61:175–177. [PubMed] [Google Scholar]
- 38.Simantov R, et al. Activation of cultured vascular endothelial cells by endothelial antibodies. J Clin Invest. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rand JH. Antiphospholipid antibody syndrome: new insights on thrombogenic mechanisms. Am J Med Sci. 1998;16:142–151. doi: 10.1097/00000441-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Branch DW, Rodgers GM. Induction of endothelial cell tissue factor activity by sera from patients with antiphospholipid syndrome: a possible mechanism of thrombosis. Am J Obstet Gynecol. 1993;168:206–210. doi: 10.1016/s0002-9378(12)90915-1. [DOI] [PubMed] [Google Scholar]
- 41.Amengual O, Atsumi T, Khamashta MA, Koike T, Hughes GRV. Specificity of ELISA for antibody to β2-glycoprotein I in patients with antiphospholipid syndrome. Br J Rheumatol. 1996;35:1239–1243. doi: 10.1093/rheumatology/35.12.1239. [DOI] [PubMed] [Google Scholar]
- 42.Oosting JD, Derksen RH, Blokzijl L, Sixma JJ, de Groot PG. Antiphospholipid antibody positive sera enhance endothelial cell procoagulant activity studies in a thrombosis model. Thromb Haemost. 1992;68:278–284. [PubMed] [Google Scholar]