Abstract
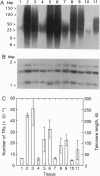
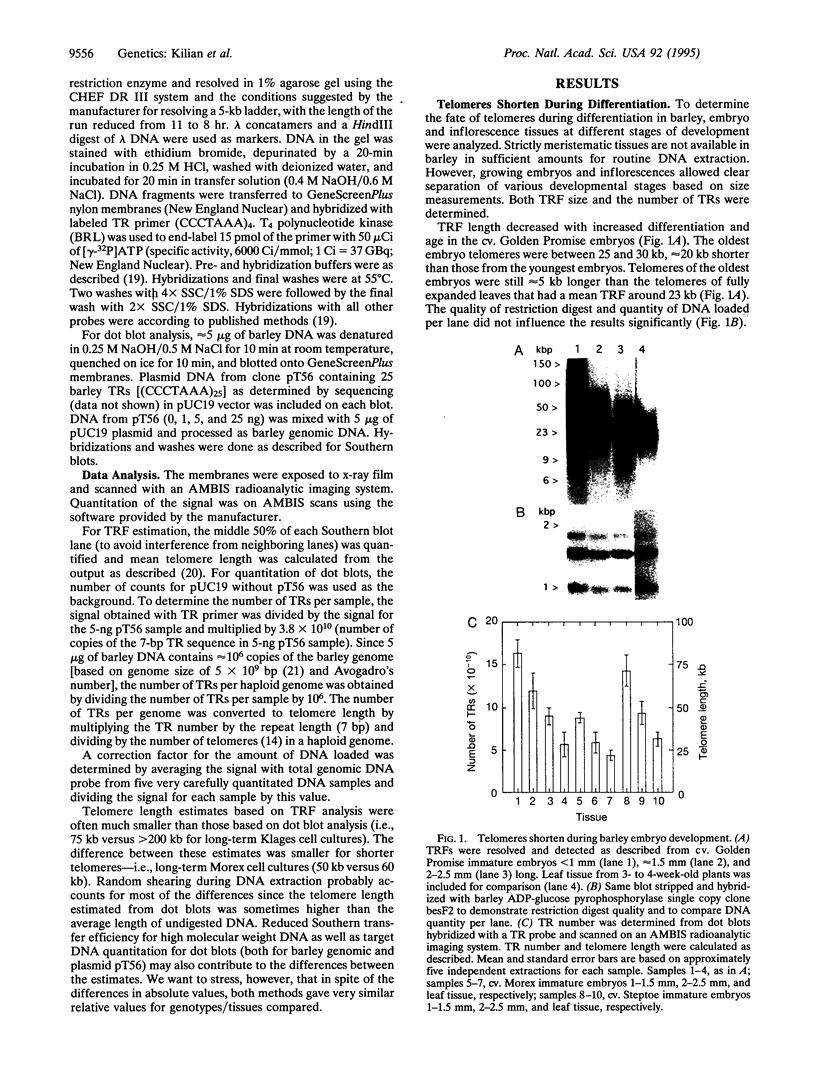
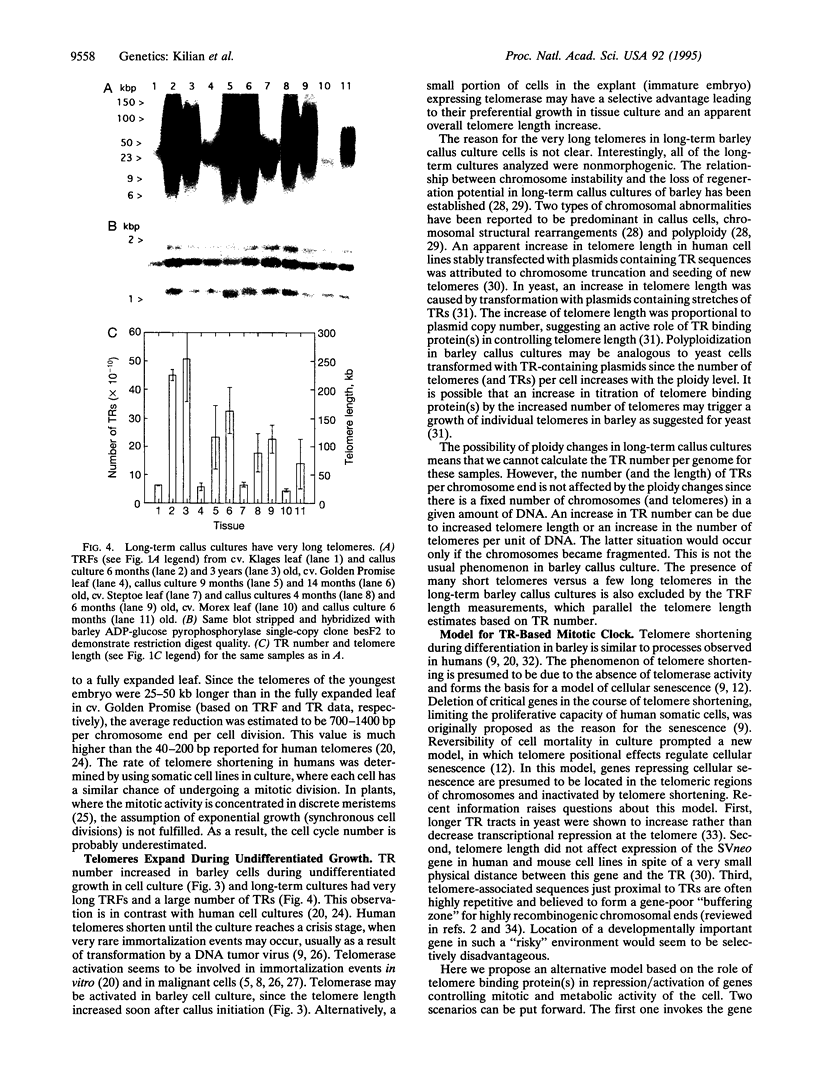
Eukaryotic chromosomes terminate with long stretches of short, guanine-rich repeats. These repeats are added de novo by a specialized enzyme, telomerase. In humans telomeres shorten during differentiation, presumably due to the absence of telomerase activity in somatic cells. This phenomenon forms the basis for several models of telomere role in cellular senescence. Barley (Hordeum vulgare L.) telomeres consist of thousands of TTTAGGG repeats, closely resembling other higher eukaryotes. In vivo differentiation and aging resulted in reduction of terminal restriction fragment length paralleled by a decrease of telomere repeat number. Dedifferentiation in callus culture resulted in an increase of the terminal restriction fragment length and in the number of telomere repeats. Long-term callus cultures had very long telomeres. Absolute telomere lengths were genotype dependent, but the relative changes due to differentiation, dedifferentiation, and long-term callus culture were consistent among genotypes. A model is presented to describe the potential role of the telomere length in regulation of a cell's mitotic activity and senescence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett M. A., Buckle V. J., Evans E. P., Porter A. C., Rout D., Smith A. G., Brown W. R. Telomere directed fragmentation of mammalian chromosomes. Nucleic Acids Res. 1993 Jan 11;21(1):27–36. doi: 10.1093/nar/21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Mason J. M. Genetics and molecular biology of telomeres. Adv Genet. 1992;30:185–249. doi: 10.1016/s0065-2660(08)60321-1. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Burr B., Burr F. A., Matz E. C., Romero-Severson J. Pinning down loose ends: mapping telomeres and factors affecting their length. Plant Cell. 1992 Aug;4(8):953–960. doi: 10.1105/tpc.4.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M. N., Wright J. H., Wolf A. J., Zakian V. A. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell. 1990 Nov 16;63(4):739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello N. P., Jazwinski S. M. Telomere length constancy during aging of Saccharomyces cerevisiae. J Bacteriol. 1991 Nov;173(21):6709–6713. doi: 10.1128/jb.173.21.6709-6713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D., Blackburn E. H. Lack of telomere shortening during senescence in Paramecium. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1955–1958. doi: 10.1073/pnas.91.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I. R., Chambers A. Use of a selection technique to identify the diversity of binding sites for the yeast RAP1 transcription factor. Nucleic Acids Res. 1994 Jan 25;22(2):124–130. doi: 10.1093/nar/22.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Huet J., Cottrelle P., Cool M., Vignais M. L., Thiele D., Marck C., Buhler J. M., Sentenac A., Fromageot P. A general upstream binding factor for genes of the yeast translational apparatus. EMBO J. 1985 Dec 16;4(13A):3539–3547. doi: 10.1002/j.1460-2075.1985.tb04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs T. Control of the cell cycle. Dev Biol. 1992 Sep;153(1):1–15. doi: 10.1016/0012-1606(92)90087-w. [DOI] [PubMed] [Google Scholar]
- Kilian A., Kleinhofs A. Cloning and mapping of telomere-associated sequences from Hordeum vulgare L. Mol Gen Genet. 1992 Oct;235(1):153–156. doi: 10.1007/BF00286193. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kipling D., Cooke H. J. Hypervariable ultra-long telomeres in mice. Nature. 1990 Sep 27;347(6291):400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Klein C., Struhl K. Protein kinase A mediates growth-regulated expression of yeast ribosomal protein genes by modulating RAP1 transcriptional activity. Mol Cell Biol. 1994 Mar;14(3):1920–1928. doi: 10.1128/mcb.14.3.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrion G., Liu K., Liu C., Lustig A. J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993 Jul;7(7A):1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- Leer R. J., Van Raamsdonk-Duin M. M., Mager W. H., Planta R. J. Conserved sequences upstream of yeast ribosomal protein genes. Curr Genet. 1985;9(4):273–277. doi: 10.1007/BF00419955. [DOI] [PubMed] [Google Scholar]
- Lustig A. J., Kurtz S., Shore D. Involvement of the silencer and UAS binding protein RAP1 in regulation of telomere length. Science. 1990 Oct 26;250(4980):549–553. doi: 10.1126/science.2237406. [DOI] [PubMed] [Google Scholar]
- Mantell L. L., Greider C. W. Telomerase activity in germline and embryonic cells of Xenopus. EMBO J. 1994 Jul 1;13(13):3211–3217. doi: 10.1002/j.1460-2075.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Olovnikov A. M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973 Sep 14;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- Palladino F., Laroche T., Gilson E., Axelrod A., Pillus L., Gasser S. M. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993 Nov 5;75(3):543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- Prowse K. R., Avilion A. A., Greider C. W. Identification of a nonprocessive telomerase activity from mouse cells. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1493–1497. doi: 10.1073/pnas.90.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regad F., Lebas M., Lescure B. Interstitial telomeric repeats within the Arabidopsis thaliana genome. J Mol Biol. 1994 Jun 3;239(2):163–169. doi: 10.1006/jmbi.1994.1360. [DOI] [PubMed] [Google Scholar]
- Richards E. J., Ausubel F. M. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988 Apr 8;53(1):127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Runge K. W., Zakian V. A. Introduction of extra telomeric DNA sequences into Saccharomyces cerevisiae results in telomere elongation. Mol Cell Biol. 1989 Apr;9(4):1488–1497. doi: 10.1128/mcb.9.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay J. W., Pereira-Smith O. M., Wright W. E. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991 Sep;196(1):33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Shore D. RAP1: a protean regulator in yeast. Trends Genet. 1994 Nov;10(11):408–412. doi: 10.1016/0168-9525(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Starling J. A., Maule J., Hastie N. D., Allshire R. C. Extensive telomere repeat arrays in mouse are hypervariable. Nucleic Acids Res. 1990 Dec 11;18(23):6881–6888. doi: 10.1093/nar/18.23.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]