Abstract
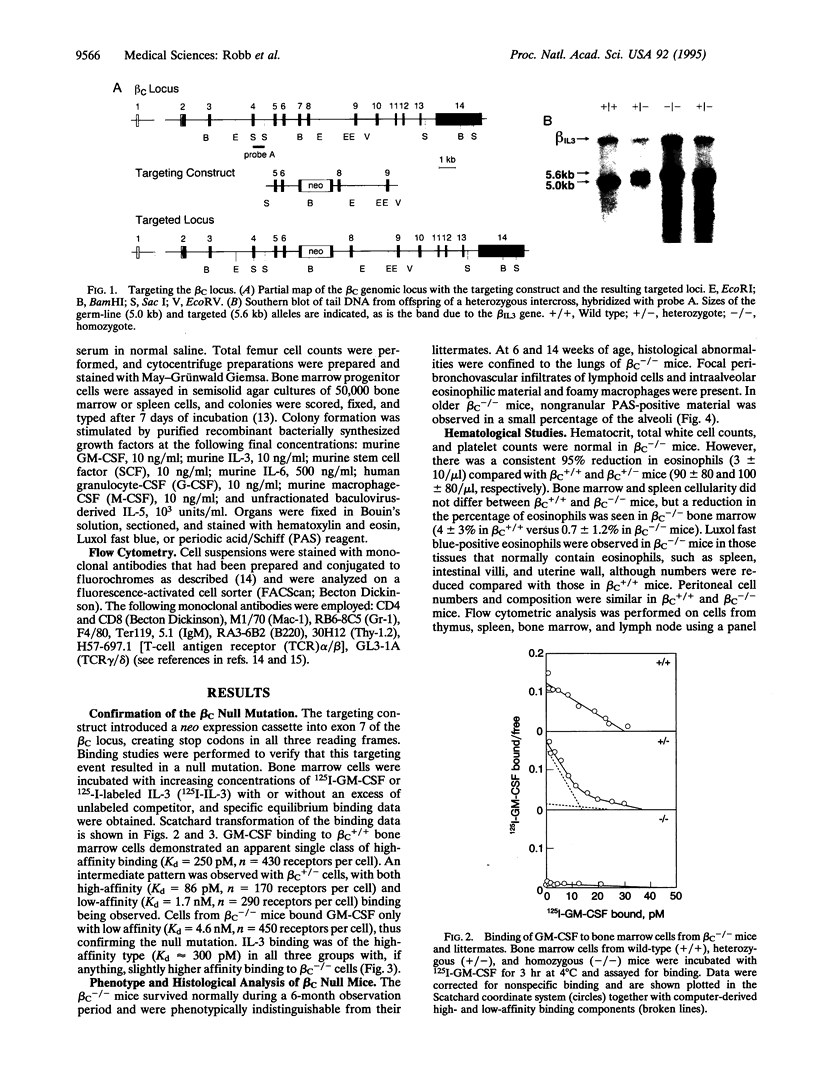
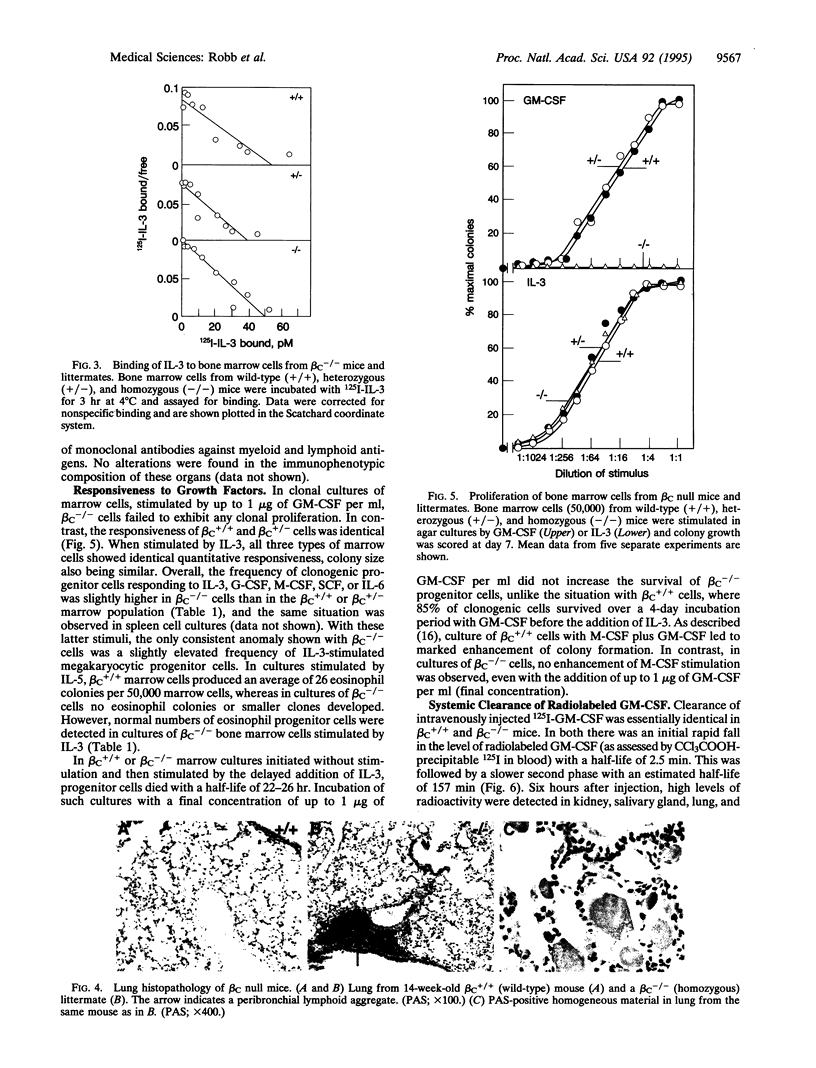
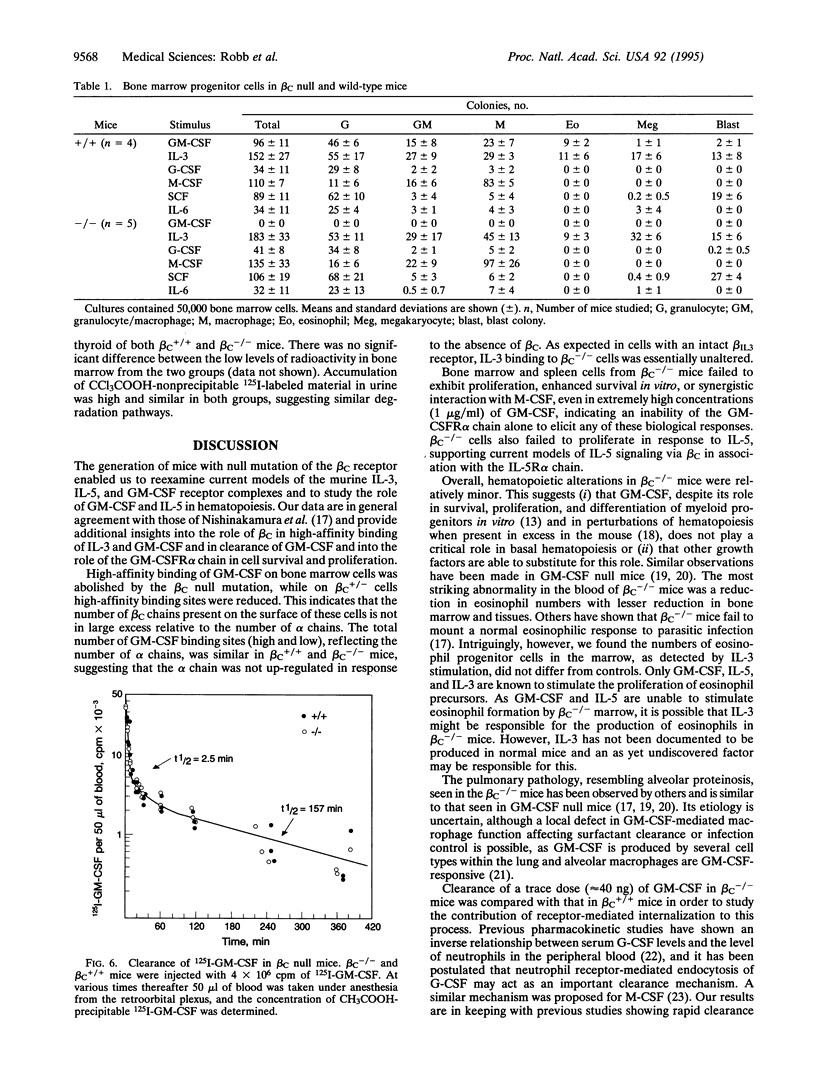
Gene targeting was used to create mice with a null mutation of the gene encoding the common beta subunit (beta C) of the granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3; multi-CSF), and interleukin 5 (IL-5) receptor complexes (beta C-/- mice). High-affinity binding of GM-CSF was abolished in beta C-/- bone marrow cells, while cells from heterozygous animals (beta C+/- mice) showed an intermediate number of high-affinity receptors. Binding of IL-3 was unaffected, confirming that the IL-3-specific beta chain remained intact. Eosinophil numbers in peripheral blood and bone marrow of beta C-/- animals were reduced, while other hematological parameters were normal. In clonal cultures of beta C-/- bone marrow cells, even high concentrations of GM-CSF and IL-5 failed to stimulate colony formation, but the cells exhibited normal quantitative responsiveness to stimulation by IL-3 and other growth factors. beta C-/- mice exhibited normal development and survived to young adult life, although they developed pulmonary peribronchovascular lymphoid infiltrates and areas resembling alveolar proteinosis. There was no detectable difference in the systemic clearance and distribution of GM-CSF between beta C-/- and wild-type littermates. The data establish that beta C is normally limiting for high-affinity binding of GM-CSF and demonstrate that systemic clearance of GM-CSF is not mediated via such high-affinity receptor complexes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartocci A., Mastrogiannis D. S., Migliorati G., Stockert R. J., Wolkoff A. W., Stanley E. R. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6179–6183. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyk N., Holt P. G. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993 Jun 1;177(6):1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994 Apr 29;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Strasser A., Metcalf D. Selective up-regulation of macrophage function in granulocyte-macrophage colony-stimulating factor transgenic mice. J Immunol. 1991 Nov 1;147(9):2957–2963. [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Layton J. E., Hockman H., Sheridan W. P., Morstyn G. Evidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factor. Blood. 1989 Sep;74(4):1303–1307. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Williamson D. J., Nice E. C., De Lamarter J., Mermod J. J., Thatcher D., Schmidt A. Hemopoietic responses in mice injected with purified recombinant murine GM-CSF. Exp Hematol. 1987 Jan;15(1):1–9. [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993 Dec 15;82(12):3515–3523. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. The clonal proliferation of normal mouse hematopoietic cells: enhancement and suppression by colony-stimulating factor combinations. Blood. 1992 Jun 1;79(11):2861–2866. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Tissue localization and fate in mice of injected multipotential colony-stimulating factor. Proc Natl Acad Sci U S A. 1988 May;85(9):3160–3164. doi: 10.1073/pnas.85.9.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of iodinated multipotential colony-stimulating factor (interleukin-3) to murine bone marrow cells. J Cell Physiol. 1986 Aug;128(2):180–188. doi: 10.1002/jcp.1041280207. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R., Nakayama N., Hirabayashi Y., Inoue T., Aud D., McNeil T., Azuma S., Yoshida S., Toyoda Y., Arai K. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995 Mar;2(3):211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Robb L., Lyons I., Li R., Hartley L., Köntgen F., Harvey R. P., Metcalf D., Begley C. G. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E., Lieschke G. J., Grail D., Metcalf D., Hodgson G., Gall J. A., Maher D. W., Cebon J., Sinickas V., Dunn A. R. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Szabó P., Mann J. R. Expression and methylation of imprinted genes during in vitro differentiation of mouse parthenogenetic and androgenetic embryonic stem cell lines. Development. 1994 Jun;120(6):1651–1660. doi: 10.1242/dev.120.6.1651. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Takaki S., Hitoshi Y. Interleukin-5 and its receptor system: implications in the immune system and inflammation. Adv Immunol. 1994;57:145–190. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]





