Abstract
Expression of innate immune response proteins, including IL-1β, TNF, and the cytokine-inducible isoform of nitric oxide synthase (iNOS), have been documented in the hearts of humans and experimental animals with heart failure regardless of etiology, although the proximal events leading to their expression are unknown. Noting that expression of a human homologue of Drosophila Toll, a proximal innate immunity transmembrane signaling protein in the fly, now termed human Toll-like receptor 4 (hTLR4), appeared to be relatively high in the heart, we examined TLR4 mRNA and protein abundance in isolated cellular constituents of cardiac muscle and in normal and abnormal murine, rat, and human myocardium. TLR4 expression levels in cardiac myocytes and in coronary microvascular endothelial cells could be enhanced by either LPS or IL-1β, an effect inhibited by the oxygen radical scavenger PDTC. Transfection of a constitutively active TLR4 construct, CD4/hTLR4, resulted in activation of a nuclear factor-κB reporter construct, but not of an AP-1 or an iNOS reporter construct, in cardiac myocytes. In normal murine, rat, and human myocardium, TLR4 expression was diffuse, and presumably cytoplasmic, in cardiac myocytes. However, in remodeling murine myocardium remote from sites of ischemic injury and in heart tissue from patients with idiopathic dilated cardiomyopathy, focal areas of intense TLR4 staining were observed in juxtaposed regions of 2 or more adjacent myocytes; this staining was not observed in control myocardium. Increased expression and signaling by TLR4, and perhaps other Toll homologues, may contribute to the activation of innate immunity in injured myocardium.
J. Clin. Invest. 104:271–280 (1999).
Introduction
Evidence has accumulated over the last decade, documenting that levels of certain inflammatory cytokines, most notably TNF and IL-1β, as well as the cytokine-inducible isoform of nitric oxide synthase (iNOS), are increased in hearts of patients with heart failure regardless of etiology, as well as in the hearts of animals with experimental cardiac dysfunction (1–4). With the exception of documented infectious myocarditis, there is no evidence for a specific pathogen in the etiology of most heart failure syndromes. There is a link, however, among each of the inflammation-related proteins just listed: they are all principal effector proteins of innate immunity, an evolutionarily ancient, nonclonal immune recognition and effector system present in invertebrates as well as vertebrates, and distinct from adaptive immunity, which has evolved only in vertebrates (5–7). Triggering of innate immunity results in the amplification of many effector pathways, including iNOS activation, as well as providing essential costimulatory signals that permit an adaptive immune response.
Among the innate immunity signaling pathways identified to date, signal-transduction pathways triggered by the vertebrate homologue of Drosophila Toll may be relevant to the expression of innate immunity effector proteins in the heart. Toll, a type 1 transmembrane protein with an extracellular leucine-rich repeat domain and an intracellular Toll–IL-1 receptor (TIR) domain, is known to be essential for normal dorsal ventral patterning in Drosophila embryos (reviewed in ref. 8). In the adult fly, Toll and the highly homologous protein, 18 wheeler, are essential in mediating antifungal and antibacterial immune responses, respectively (9, 10). Recently, the first human homologue of Drosophila Toll, hToll, was cloned (11). Subsequently, 5 human Toll-like receptors (TLRs) were identified using computational analysis to scan a human expressed sequence tag (EST) database, and the initially described hToll sequence was termed hTLR4 (12). Activation of hTLR4 on human monocyte THP-1 cells was able to induce the expression of the cytokines IL-1, IL-6, and IL-8 and the surface receptor B7.1, i.e., cytokines and costimulatory molecules that are required for the activation of the adaptive immune response (11). Also, transfection of a constitutively active form of hTLR4 into Jurkat cells was shown to induce activation of nuclear factor-κB (NF-κB) (13).
The cytoplasmic TIR domain of Toll exhibits a high degree of homology with that of the mammalian receptor of IL-1 (IL-1R) and the IL-1R accessory protein (IL-1RAcP). Muzio et al. (14) and Medzhitov et al. (15) have shown recently that hTLR4 signaling occurs in a manner similar to that mediated by IL-1 (16). After recruiting the adapter protein MyD88 (similar to Drosophila tube) and interleukin receptor–associated kinase (IRAK), homologous to Drosophila pelle, the protein kinase NF-κB–inducing kinase (NIK) is activated through TNF coronary receptor–activated factor-6 (TRAF6). NIK subsequently activates an I-κB kinase that results in phosphorylation of I-κB (Drosophila cactus), thereby promoting NF-κB (Drosophila dorsal), translocation to the nucleus, and gene transcription. Indeed, a number of proteins involved in host defense with TIR domains and IRAK/pelle–like serine/threonine innate immunity kinases (SIIKs) have now been identified in plants, invertebrates, and vertebrates, attesting to their utility in roughly a billion years of evolution (17, 18).
Here, we examine the expression of TLR4 in human and rodent heart. Constitutive expression of this vertebrate Toll homologue was found in normal cardiac muscle, almost exclusively within cardiac myocytes. Dissociation of heart tissue, followed by isolation and primary culture of cardiac myocytes and coronary microvascular endothelial cells (CMEMs), was found to result in robust TLR4 expression in both cell types. Interestingly, in tissue sections from hearts of humans with cardiomyopathies and of rodents with experimental cardiac dysfunction, myocyte TLR4 expression becomes more focal and intense.
Methods
Chemicals.
Human recombinant IL-1β was purchased from Calbiochem-Novabiochem Corp. (San Diego, California, USA); murine IFN-γ (rmIFN-γ) was purchased from Life Technologies Inc. (Gaithersburg, Maryland, USA). All other chemicals, including LPS (Escherichia coli B05:55), were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA) unless noted otherwise.
Cell isolation and culture.
Neonatal rat ventricular myocytes (NRVMs) were isolated from 1-day-old Sprague-Dawley pups as described previously (19). Cells subsequently underwent 2 preplatings to minimize nonmyocyte contamination to less than 5% of the enriched myocyte population (17) and cultured in DMEM containing 10% FCS (Life Technologies Inc.). After 48 hours, the medium was changed to DMEM/F12 phenol red–free medium (Life Technologies Inc.) containing 1% insulin, transferrin, and selenium media supplement (ITS; Sigma Chemical Co.) with antibiotics; treatments were instituted 12 hours later.
Calcium-tolerant adult rat ventricular myocytes (ARVMs) were isolated from adult male Sprague-Dawley rats (225–275 g) as described previously (20). Primary myocyte cultures were plated on laminin-coated (1 μg/cm2) dishes and maintained at 37°C in 5% CO2. All experiments described were initiated within 24 hours of cell isolation.
CMECs from adult rat hearts were isolated as described previously (21). These primary isolates have been documented to contain more than 90% endothelial cells, with a phenotype at low passage number consistent with their microvascular origin, as described previously (21). After reaching confluency, CMECs were serum starved for 12 hours before treatment.
Myocardial infarction in mice.
C57BL6/C129 mice, 8–12 weeks old and 25–30 g, underwent coronary artery ligation for the production of myocardial infarction. The surgical procedure has been described in detail elsewhere (22). Because direct viewing of the left anterior descending artery is suboptimal in mice, paleness and enlargement of the left ventricle after suture placement suggested the presence of an infarction. In sham-operated animals, the suture was placed but not ligated. Antibiotics were not given during the procedure, but no apparent infection had developed by the time of autopsy. All mice were housed under identical conditions and given food and water ad libitum. The Standing Committee on Animal Research from our institution approved the animal study protocol.
Cloning of the rat Toll4 cDNA.
Total RNA was extracted from rat heart as described later here. Double-stranded cDNA was synthesized by the Marathon cDNA Amplification kit (CLONTECH Laboratories Inc., Palo Alto, California, USA) according to the manufacturer’s protocol and was ligated to the Marathon cDNA adapter. To clone rat Toll4 (rTLR4), PCR primers were designed based on the hTLR4 sequence (11), corresponding to bp’s 2,256–2,280 and 2,676–2,697. Based on the subsequent sequences that were generated, additional oligonucleotides were selected as primers in combination with AP1 for the rapid amplification of 3′ and 5′ cDNA ends (3′-RACE and 5′-RACE; CLONTECH Laboratories Inc.), and the entire sequence was cloned using repetitive nested primers according to the manufacturer’s instructions.
Northern and Western blots.
Total cellular RNA was isolated by a modification of the acid guanidinium/thiocyanate phenol/chloroform extraction method with Trizol LS (Life Technologies Inc.) according to the manufacturer’s protocol (23). RNA was size fractionated on 1.0% agarose, transferred to a nylon membrane, and ultraviolet cross-linked. A 555-bp rat Toll cDNA was radiolabeled by a random-primed labeling kit (Life Technologies Inc.) with [α-32P]dCTP and was hybridized with QuickHyb solution (Stratagene, La Jolla, California, USA) at 68°C for 2 hours. Membranes were washed at room temperature twice for 15 minutes with 2× SSC and 0.1% SDS and at 60°C for 30 minutes with 0.1× SSC and 0.1% SDS. Normalization of RNA for equal loading was carried out by rehybridizing with GAPDH cDNA probe. Autoradiograms were scanned using NIH Image version 1.61 (National Institutes of Health, Bethesda, Maryland, USA).
For Western blots, cells were harvested in an RIPA lysis buffer containing 1% Nonindet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease in PBS. After sonication, cell extracts were boiled for 5 minutes with sample buffer containing 0.062 M Tris-HCl, 2% SDS, 10% glycerol. Alternatively, membrane protein of NRVMs were harvested in a lysis buffer containing 50 mM Tris, 1 mM DTT, and protease inhibitors; after 30-minute centrifugation at 100,000 g, pellets were resuspended in lysis buffer. Equal amounts of the denatured protein per lane were loaded, separated on 7.5% SDS-PAGE, and transferred to PVDF membranes. After blocking with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST), membranes were incubated with an anti-hTLR4 immune serum for 90 minutes at a dilution of 1:2,500 in TBST with 1% nonfat dry milk. After 3 washes (15 minutes each), the membranes were incubated for 1 hour with an anti-rabbit IgG secondary antibody (1:10,000 dilution; Bio-Rad Laboratories Inc., Hercules, California, USA). After 6 additional washes, membranes were incubated with a chemiluminescent (Renaissance; Du Pont NEN Research Products, Boston, Massachusetts, USA) and autoradiographed.
Immunohistochemistry.
Cryostat sections of human, rat, and mouse heart tissue were prepared in the standard manner. Primary cultures of ARVM, NRVM, and CMEC cells were plated on tissue culture chamber slides (Nalge Nunc International, Naperville, Illinois, USA) and fixed with fresh 2% paraformaldehyde for 10 minutes and rinsed in PBS. Cells were labeled by the sequential application of the primary hTLR4 antibody serum (1:1,000), an anti-rabbit immunoglobulin, and a peroxidase/anti-peroxidase complex, followed by labeling with the chromogen diaminobenzidine (Sigma Chemical Co.) and H2O2. The slides were washed and counterstained with hematoxylin, dehydrated, and mounted for light microscopy. Evidence in favor of the specificity of the primary rabbit polyclonal anti-hTLR4 antibody (directed against an epitope in the COOH-terminal tail of the protein adjacent to the TIR domain) was generated by preincubating the antibody with the COOH-terminal hTLR peptide.
Measurement of nitrite concentration.
Nitrite (NOx, an oxidative metabolite of NO) was measured by the Griess reaction as described previously (24). Briefly, the nitrite content in the supernatant was measured by combining 150 μL of medium with 900 μL of Griess reagent (0.75% sulfanilamide in 0.5 N HCl and 0.075% N-1-napthylethylenediamine) and by noting the OD (543 nm) of the resultant chromophore.
Electromobility shift assay.
Cells (i.e., CMECs or NRVMs) were lysed for 10 minutes on ice in a solution containing 10 mM HEPES (pH 7.6), 15 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 10 μg/mL leupeptin, and 0.2% Nonident P-40. Dishes were gently scraped, and nuclei were collected by centrifugation at 800 g for 30 seconds and then suspended in a solution of 50 mM HEPES, 400 mM KCl, 0.1 mM EDTA, 10% glycerol, 1 mM DTT, 0.5 mM PMSF, and 10 μg/mL leupeptin. The mixture was incubated on ice for 30 minutes, and the supernatant was collected after centrifugation for 15 minutes at 400 g. Binding reactions were performed with 5 or 10 μg of nuclear protein in 10 mM Tris, 1 mM DTT, 1 mM EDTA, 5% glycerol, 0.1% Triton X-100, 1 mg poly(dIdC), 5 μg BSA, and approximately 10,000 cpm of γ-32P–labeled oligonucleotide. The binding reactions for AP-1 activity also contained 5 mM MgCl2. Control reaction mixtures contained a 100-fold excess of unlabeled oligonucleotide and were incubated with nuclear extracts of IL-1β–treated cells. DNA complexes were separated on a 4% nondenaturing polyacrylamide gel in Tris-HCl (6.7 mM), EDTA (1 mM), and ammonium acetate (3.3 mM). The oligonucleotides contained the NF-κB binding site (sequence: 5′-AGT TGA GGG GAC TTT CCC AGG C-3′) or AP-1 (sequence: 5′-TTC CGG CTG ACT CAT CAA GCG-3′) and were labeled by standard procedures.
CD4/TLR4 myocyte transfection and NF-κB, AP-1, iNOS luciferase assay.
NRVMs or COS7 cells were transfected using Lipofectamine Plus (Life Technologies Inc.) with a constitutively active CD4/TLR4 construct at several concentrations (0.01, 0.1, and 0.5 μg per 6 wells), or with equal amounts of the same vector without the insert, as well as a luciferase reporter plasmid (1 μg per 6 wells) for NF-κB, AP-1, or iNOS (hiNOS 7.2; a gift of B. Taylor, University of Pittsburgh, Pittsburgh, Pennsylvania, USA) and with β-galactosidase (0.1 μg per 6 wells), according to the manufacturer’s protocol. Cells were lysed 48 hours after transfection and analyzed using a lumenometer.
Statistical analyses.
All replicate data are expressed as mean and ± SEM. In experiments with comparison of treatments, an unpaired Student’s t test was performed. In experiments with time courses, ordinary ANOVA was used, followed by Fisher’s post hoc test. Statistical significance was achieved when 2-tailed P values were less than 0.05. Statistical analyses were carried out using StatView statistics program (Abacus Concepts Inc., Berkeley, California, USA).
Results
Cloning of the full-length rat Toll4 cDNA.
Based on the hTLR4 sequence (11), the full-length rTLR4 cDNA was sequenced using RACE PCR from a rat heart cDNA library. The full-length cDNA contains 3,385 bp with an open reading frame of 2,508 bp, encoding a protein of 836 amino acids. The rTLR4 sequences exhibited an 84% homology with the hTLR4 DNA sequences and no similarities with TLR1-3 and 5. The extracellular domain has an identity of 57% or, taking conservative substitutions into account, a similarity of 72% with the amino acid sequence of hTLR4. As with Drosophila Toll (23–25), rTLR4 has an extracellular leucine-rich repeat domain with cysteine-rich flanking sequences; the tertiary structure is presumed to describe a horseshoe-like pattern analogous to the ribonuclease inhibitor (reviewed in ref. 26). The COOH-terminal TIR domain is highly conserved, with an identity of 84% and similarity of 91% to the primary structure of hTLR4 (overall primary structure identity with hTLR1 is 39%; with hTLR2, 37%; with hTLR3, 38%; and with hTLR5, 39%). The alignment of human and rat TLR4s is shown in Figure 1.
Figure 1.
Alignment of the hTLR4 and rTLR4 sequences. A full-length rTLR4 cDNA was sequenced by RACE PCR from a rat cDNA library using the hTLR4 sequence to design primers. The full-length rTLR4 contains 3,385 bp with an open reading frame of 2,508 bp, encoding a protein of 836 amino acids. The hTLR4 and rTLR4 sequences exhibited an overall 84% homology in DNA sequences (top, rTLR4; bottom, hTLR4; alignment by an algorithm: http://vega.igh.cnrs.fr; : = amino acid identity; . = conservative substitution).
TLR4 expression in rodent heart.
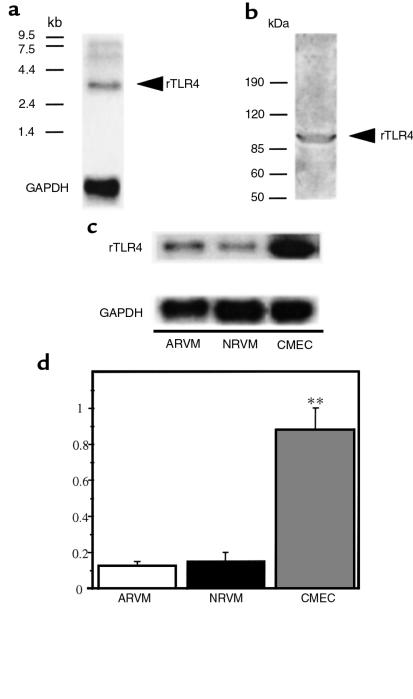
In their original description of the human homologue of Drosophila Toll, Medzhitov et al. (11) noted that hToll4 expression was relatively abundant in cardiac muscle, as well as in lung, peripheral blood leukocytes, and in spleen. Therefore, TLR4 mRNA expression was examined in fresh isolates of ARVMs and confluent primary cultures of NRVMs and CMECs. A 3.4-kb band consistent with the rTLR4 transcript was readily detectable by Northern blot in both adult and neonatal rat myocyte cultures, whereas an approximately 5-fold higher basal level of Toll mRNA abundance was present in the CMEC preparation (Figure 2).
Figure 2.
Expression of rTLR4 in cardiac myocytes and coronary microvascular endothelial cells. Representative Northern (a) and Western (b) blots for TLR4 in homogenized rat heart tissue. (c and d) Representative Northern blot and aggregate data from 5 independent experiments for basal levels of TLR4 mRNA abundance in ARVMs, NRVMs, and CMECs in primary cultures after normalization to GAPDH (**P ≤ 0.01). An approximately 5-fold higher basal level of TLR4 mRNA was present in the endothelial cell preparation compared with the cardiac myocytes.
Regulation of TLR4 expression by LPS, IL-1β, and IFN-γ.
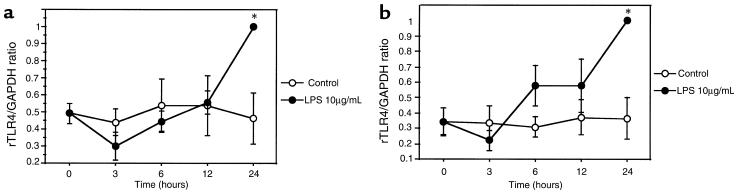
The innate immune system is known to recognize pathogen-associated molecular patterns (PAMPs) such as LPS. Therefore, we determined whether rTLR4 expression is regulated by LPS (10 μg/mL) in endothelial cells and cardiac myocytes. Confluent, serum-starved primary cultures of NRVMs and CMECs both exhibited significant increases in rTLR4 mRNA abundance after 24 hours, but not at 12 hours (Figure 3b). Similar results were observed at 100 ng/mL LPS (data not shown).
Figure 3.
Time course of rTLR4 expression in response to LPS. Primary cultures of confluent CMECs (a) and NRVMs (b) were exposed to 10 μg/mL LPS for the periods indicated. Cumulative data from 5 independent experiments for both cell types are shown (*P ≤ 0.05). Data have been normalized to GAPDH mRNA content. The rTLR4 mRNA content at 24 hours was defined as 1 arbitrary unit.
To investigate further the role of cytokines that play key effector roles in the innate immune response, primary cultures of neonatal myocytes and endothelial cells were treated with either 4 U/mL or 40 U/mL rhIL-1β or 50 U/mL rmIFN-γ for 24 hours. As shown in Figure 4, endothelial cells showed about a 2-fold increase in rTLR4 mRNA after IL-1β and IFN-γ, and neonatal myocytes, about a 5-fold higher level of TLR4 mRNA after IL-1β and about a 2-fold increase after IFN-γ treatment (average of 8 independent experiments). No additional significant increase in TLR4 expression was seen when LPS, IL-1β, and IFN-γ were combined when compared with LPS, IL-1β, or IFN-γ alone (data not shown). Despite these changes in mRNA abundance with LPS or cytokines, we did not detect a significant increase in TLR4 protein levels by Western blot for periods of up to 60 hours (data not shown).
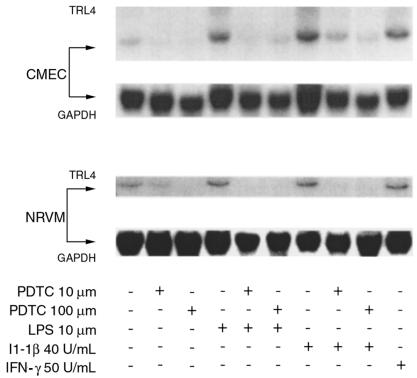
Figure 4.
Role of NF-κB in regulating the expression of rTLR4 mRNA. CMECs and NRVMs were treated as indicated in the text for 24 hours with either LPS or cytokines, with and without the NF-κB inhibitor PDTC. The Northern blots shown are representative of 4 independent experiments.
NF-κB and the regulation of TLR4 expression.
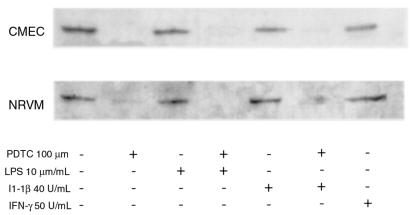
To investigate the role of NF-κB activity in the regulation of rTLR4 expression, CMEC and NRVM primary cultures were treated with 10 and 100 μmol PDTC, respectively, and the TLR4 mRNA and protein content was measured after 24 to 48 hours of treatment. In serum-starved confluent endothelial and cardiac myocyte cultures, the increase in TLR4 mRNA by either LPS or IL-1β described in Figure 4 could be suppressed by the reactive oxygen intermediate (ROI) scavenger PDTC in a dose-dependent manner. Moreover, in the presence of PDTC alone, particularly at 100 μM, TLR4 mRNA levels were depressed significantly (Figure 5). Similarly, TLR4 protein levels were downregulated by PDTC in endothelial cells after 24 hours and in cardiac myocytes after 48 hours (Figure 5).
Figure 5.
PDTC inhibits upregulation of rTLR4 expression in vitro in response to IL-1β or LPS. CMECs and NRVMs were exposed to LPS or cytokines in the presence or absence of PDTC as indicated. This Western blot is representative of 3 independent experiments and illustrates a 96-kDa band consistent with TLR4. CMECs were treated for 36 hours, and NRVMs for 48 hours, with LPS, cytokines, or the NF-κB inhibitor PDTC, as indicated.
As IL-1β and LPS have been shown to activate NF-κB and induce iNOS in endothelial cells and cardiac myocytes, we also verified that iNOS-dependent production of NOx was decreased by PDTC. Activation of iNOS was monitored by measuring NOx accumulation in medium conditioned by either LPS or IL-1β–treated microvascular endothelial cells or cardiac myocytes. As expected, PDTC suppressed iNOS-dependent NOx expression in response to both LPS and IL-1β.
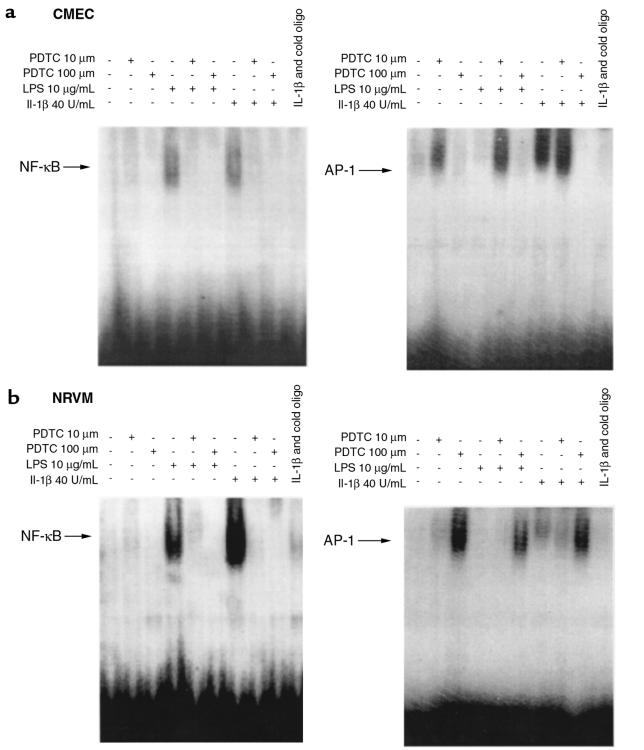
To determine whether this effect of the ROI scavenger PDTC on rTLR4 was due to its ability to block the activation of NF-κB induced by IL-1β or LPS, electrophoretic mobility shift assays (EMSAs) were performed using nuclear extracts from microvascular endothelial cell and neonatal cardiac myocyte cultures. As shown in Figure 6, a and b, PDTC prevented retardation of oligonucleotides on the gel of nuclear extracts from LPS or IL-1β–treated cells. In contrast, PDTC enhanced retardation of an AP-1 oligonucleotide by nuclear extracts from endothelial cells or cardiac myocytes after treatment with LPS or IL-1β.
Figure 6.
EMSAs of NF-κB and AP-1 in response to LPS and IL-1β. EMSAs were also performed on nuclear extracts from CMECs (a) and NRVMs (b) for NF-κB– and AP-1–mediated signaling. The data shown are representative of 3 experiments.
CD4/TLR4 activates NF-κB in cardiac myocytes, but not AP-1 or iNOS.
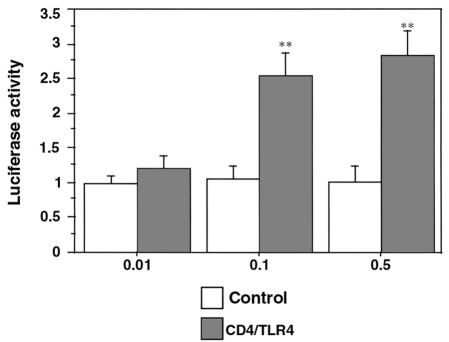
Because it was known that dominant constitutively activated, ventralizing Toll mutants were formed in Drosophila by removal of the extracellular LRR and cysteine-enriched flanking sequences, Medzhitov et al. (11) constructed a constitutively active Toll homologue consisting of a CD4 extracellular domain with the TLR4 transmembrane and TIR domains. NRVMs were cotransfected with a CD4/TLR4 construct and a luciferase reporter plasmid. As shown in Figure 7, NF-κB–sensitive luciferase activity in CD4/TLR4 transfected myocytes, but not in mock-transfected myocytes, is expressed as a function of the quantity of CD4/TLR4 transfected. In contrast, cotransfection with AP-1–sensitive and iNOS-sensitive (hiNOS 7.2; as described in ref. 27) luciferases could not show an increase of luciferase activity over mock-transfected myocytes. Also, cotreatment with LPS, IL-1β, or IFN-γ, or cotransfection of CD4/hTLR4 in COS7 cells, failed to show an additional iNOS promoter activation.
Figure 7.
CD4/TLR4 activates NF-κB in cardiac myocytes. A constitutively active TLR4 construct, consisting of CD4 linked to the hTLR4 transmembrane and TIR domains, was transfected into NRVM primary cultures at concentrations of 0.01, 0.1, and 0.5 μg/well, and the extent of activation of an NF-κB–luciferase construct was measured. Data are shown as fold induction of luciferase activity over that of mock-transfected (control) myocytes, with the average activity of mock-transfected cells arbitrarily given as 1. The data are from 32 independent experiments (**P < 0.01 vs. control).
Immunohistochemical analysis of TLR4 expression in rodent and human heart and TLR4 regulation in experimental myocardial infarction.
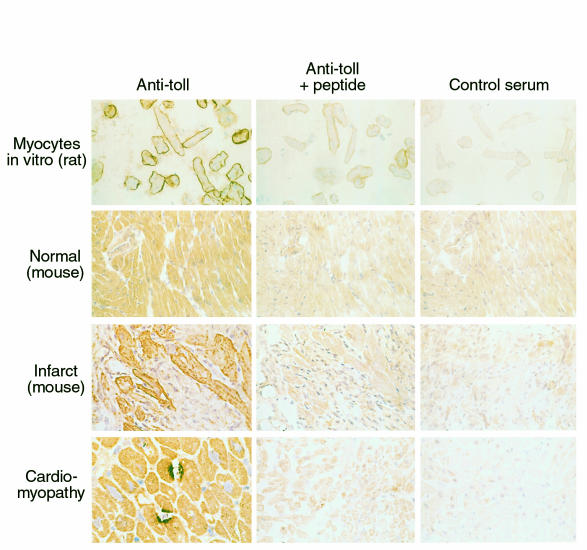
Immunohistochemical staining confirmed the expression of rTLR4 in myocytes and endothelial cells (Figure 8). Primary isolates of ARVMs in vitro exhibited intense sarcolemmal membrane staining. In contrast, myocytes in situ in normal ventricular muscle exhibited diffuse staining that was inhibited by preincubation with the COOH-terminal hTLR4 peptide used to generate the primary antibody or when a control antiserum was used. In contrast to results by mRNA and Western blot analysis, immunohistochemical staining for TLR4 was not observed in CMEMs in primary culture or in vivo in normal murine, rat, and human heart muscle.
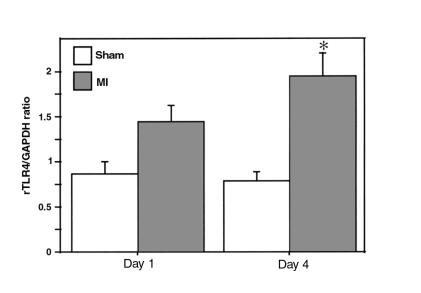
Figure 8.
rTLR4 mRNA expression in murine ventricular muscle after myocardial infarction. rTLR4 mRNA levels in apical ventricular muscle of sham-operated and infarcted animals are shown after normalization to GAPDH (*P < 0.05). Approximately 2.5-fold higher mRNA was present in infarcted animals compared with sham-operated animals at day 4 after the procedure.
In specimens obtained from murine hearts 4 days after experimental myocardial infarction induced by ligation of the left coronary artery, a significant 2.5-fold increase in TLR4 mRNA was detected on the apical level compared with sham-operated animals (Figure 8). On immunohistochemical analysis, the diffuse pattern of TLR4 expression seen in sham-operated animals was noted to change 4 days after experimental myocardial infarction. In cardiac muscle sections adjacent to the site of ischemic injury, enhanced and predominantly sarcolemmal staining was observed. Scattered foci of intense TLR4 staining involving 2 or more contiguous myocytes were noted, a feature not observed in sections from hearts of normal, control animals. This focal staining could also be inhibited by preincubation with the hTLR4-peptide and was not observed when a control antiserum was used. A similar intense focal staining of TLR4 could be observed in cardiac specimens taken from humans with idiopathic dilated cardiomyopathy (Figure 9). This intense focal staining for TLR4 was not observed in sham-operated animals or on immunohistochemical analysis of sections of normal human myocardium (data not shown). Surprisingly, when compared with the intense focal expression of TLR4 in cardiac muscle remote from the infarcted muscle in mouse, cells comprising the inflammatory infiltrates in the peri-infarct area exhibited no labeling for TLR4.
Figure 9.
Immunohistochemical analysis of TLR4 in rat, murine, and human cardiac muscle. Photomicrographs are shown for primary isolates of ventricular myocytes isolated from adult rat hearts (×400) 24 hours after isolation, stained with a polyclonal Toll antibody targeted to a TLR4-specific epitope adjacent to the cytoplasmic TIR domain of hTLR4. Absorption with the peptide used to generate the primary antibody (Anti-Toll + peptide) and substitution of the primary antibody with a control rabbit antiserum (Control serum) were used as controls. Both normal murine cardiac muscle (×200), as shown here, and sham-operated murine cardiac muscle and normal rat cardiac muscle (not shown) exhibited diffuse, homogeneous myocyte staining. However, cardiac myocytes adjacent to an area of ischemic injury (Infarct) in the mouse exhibited intense sarcolemmal staining for TLR4. Note the absence of significant staining of infiltrating inflammatory cells (×400). Finally, in samples from both humans with a dilated cardiomyopathy (bottom left panel) and in remodeling murine ventricular muscle remote from the site of ischemic injury (not shown), intensely stained focal expression of TLR4 was observed in adjacent regions of 2 or more juxtaposed cardiac myocytes (×600). This intense focal staining pattern for TLR4 was not observed in sections of normal human myocardium (not shown).
Discussion
The specific cytokines and cytokine receptors most intensively studied to date with regard to the pathophysiology of heart failure, specifically TNF, IL-1β, IL-6, chemokines, and IFN-γ, along with iNOS, are now recognized as being principal effector proteins of innate immunity, a phylogenetically ancient and essential component of the immune response. Unlike adaptive immunity, for which specific antigen receptors are generated by somatic hypermutation and selection “in the field,” innate immune systems use hardwired germ-line–encoded proteins that act as pattern recognition receptors (PRRs), which recognize largely invariant PAMPs (reviewed in refs. 5–7 and 18).
Considerable data now exist linking innate immunity effector proteins to the pathophysiology of myocardial dysfunction in heart failure. For example, both serum and intramyocardial levels of IL-1β and TNF-α are activated in patients with myocardial dysfunction resulting from several disease processes, and both cytokines have been shown to have direct cardiodepressant effects (reviewed in refs. 2, 4, and 25). TNF is produced by cardiac myocytes in response to increased strain in vitro and in failing cardiac muscle in vivo (26), and genetically engineered mice that were designed to overexpress TNF selectively in cardiac myocytes exhibited progressive cardiac hypertrophy and, ultimately, progressive chamber dilation and heart failure (28, 29). Also, increased expression of iNOS has also been demonstrated in experimental animals and in humans with heart failure due to various etiologies (30–33). Finally, in addition to the effector proteins of innate immunity implicated in the pathophysiology of heart failure already noted here, it has been demonstrated recently that several innate immunity PRRs, including pentraxin 3 (34) and a number of complement components, including C3 and C9 (35, 36) and possibly others, are synthesized in the heart. Indeed, as demonstrated by McGeer and colleagues, myocardial levels of C3 and C9, after ischemia followed by reperfusion, exceed levels of production of these complement components in the liver in a rabbit model (35, 36).
We have demonstrated here that TLR4 is expressed in nonprofessional immunocyte cell types: cardiac myocytes and microvascular endothelial cells. In agreement with these findings, it has been recently published that human dermal endothelial cells do express TLR4 (37). TLR4 mRNA abundance in vitro could be enhanced by LPS and the cytokines IL-1β and IFN-γ. This is not surprising, as PAMPs such as LPS, and innate immunity effector cytokines such as IL-1β, are known to amplify expression of a number of innate immunity cytokines and signaling proteins. Furthermore, a “basal” level NF-κB activity, at least under the in vitro conditions we used here, was necessary for maintenance of TLR4 expression because PDTC could reproducibly suppress TLR4 mRNA and protein abundance in both cell types, even in the absence of IL-1 or LPS. Unlike the diffuse, homogeneous immunohistochemical staining for TLR4 in normal cardiac muscle, myocytes in vitro exhibited intense circumferential staining, as did myocytes within inflammatory infiltrates in areas of ischemic injury. Finally, in sections obtained from both rodent and human hearts undergoing progressive remodeling and dilatation that were remote from areas of injury or discrete inflammatory infiltrates, intense focal staining for TLR4 was observed in portions of membranes of 2 or more juxtaposed cells.
These data, however, beg the question that if there is no evidence of infection in the hearts of animals or patients with cardiac dysfunction, what is driving Toll expression and signaling under these conditions? Unlike the rapid clarification to date of the intracellular signaling pathways used by Toll, the ligand for Toll activation in vertebrates is unknown. In the Drosophila embryo, the Toll ligand is Spätzle, a cysteine-knot superfamily member that is generated from an inactive precursor by a serine protease cascade that includes Nudel, Snake, and Easter (8). While Spätzle remains essential for innate immunity–mediated Toll signaling in the adult fly, the cleavage of pro-Spätzle is mediated by a different sequence of proteases (reviewed in ref. 38). Although currently unknown, it is likely that this protease is also activated by a serine protease cascade (perhaps analogous to the complement or clotting cascades) and will likely have been highly conserved, like Toll, throughout evolution (17). Finally, there are now 2 reports that TLR2, which is highly expressed in peripheral blood leukocytes, spleen, and bone marrow, acts as a receptor for LPS (39, 40). TLR2 “sensing” of LPS was also dependent on soluble LPS-binding protein, and the presence of CD14, along with TLR2, markedly enhanced the sensitivity of cells expressing both proteins to LPS.
This interaction with CD14 is now known not to be unique to TLR2. A more recent report identifying the gene responsible for LPS resistance in 2 inbred strains of mice (C3H/HeJ and C57BL/10ScCr) was found to be TLR4 (41, 42). C3H/HeJ mice have a single point mutation in the cytosolic TIR domain, resulting in a proline→histidine substitution, whereas C57BL/ 10ScCr mice express no TLR4 mRNA. The most economical explanation using the information available as of this writing is that TLR4 is required for LPS-mediated hypotension, and that the LPS-CD14 interaction accentuates this response. However, although we could show NF-κB activation by the constitutively active CD4/TLR4 construct, we have not seen increases in iNOS expression whose promoter contains several functional NF-κB binding sites or of AP-1 activation, although both are activated by LPS in cardiac myocytes as in other cell types, implicating a TLR4-independent pathway for LPS signaling in these cells.
In the absence of evidence for infection, there is another explanation for the involvement of Tolls and perhaps other innate immunity PRRs and effector proteins in injured tissues. Over the past 2 years, a number of reports have revealed the role of innate immunity pattern recognition receptors and effector proteins in facilitating engulfment of apoptotic cells by both professional phagocytes and parenchymal cells (43, 44). Perhaps best understood is the role of class B scavenger receptors, such as CD36 and the homologous Drosophila protein croquemort (“catcher of death”) (45). Recently, Masaki and colleagues (46) have demonstrated that class A scavenger receptors, such as the lectin-like OxLDL receptor/(LOX-1) on endothelial cells, also recognizes apoptotic cells. This is presumably due to interactions with anionic phospholipids (e.g., phosphatidylserine), prompting engulfment of dying cells by these “amateur” phagocytes.
Perhaps the most intriguing recent observation linking innate immunity and apoptosis was the discovery that CD14, a leucine-rich repeat PRR attached to the plasma membrane of a number of cell types by a GPI tether, participates in recognition and engulfment of apoptotic cells (43). An antibody directed against CD14 was sufficient to inhibit engulfment of apoptotic lymphoma cells by macrophages, whereas transfection of COS cells with a CD14 construct mediated docking and engulfment of apoptotic cells by COS cells (albeit not as efficiently as in a professional phagocyte). These authors also noted that although LPS-initiated CD14 signaling in phagocytes is well known to initiate multiple proinflammatory signals, this did not occur with CD14’s interaction with apoptotic cells (44).
In summary, the vertebrate Toll homologue TLR4 is abundantly expressed in the heart. Although the role of Toll-mediated signaling in the injured or remodeling heart remains to be defined, the evidence reported suggests that TLR4 expression and signaling could be participating in a response to tissue injury.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft and by the National Institutes of Health (grants HL-52320 and HL-36141). The authors thank C.A. Janeway for the gift of reagents and the critique of the manuscript, and Bradley Taylor for the gift of the iNOS-luciferase-reporter construct.
References
- 1.Kelly RA, Balligand J-L, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- 2.Kelly RA, Smith TW. Cytokines and cardiac contractile function [editorial] Circulation. 1997;95:778–781. doi: 10.1161/01.cir.95.4.778. [DOI] [PubMed] [Google Scholar]
- 3.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR. Tumor necrosis factor-α and cardiomyopathy. Circulation. 1998;97:1340–1341. doi: 10.1161/01.cir.97.14.1340. [DOI] [PubMed] [Google Scholar]
- 5.Fearon DT. Seeking wisdom in innate immunity. Nature. 1997;388:323–324. doi: 10.1038/40967. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 7.Ezekowitz RAB, Hoffman J. Innate immunity. The blossoming of innate immunity. Curr Opin Immunol. 1998;10:9–11. [Google Scholar]
- 8.Anderson KV. Pinning down positional information: dorsal-ventral polarity in the Drosophila embryo. Cell. 1998;95:439–442. doi: 10.1016/s0092-8674(00)81610-4. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 10.Williams MJ, Rodriguez A, Kimbrell DA, Eldon ED. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 1997;16:6120–6130. doi: 10.1093/emboj/16.20.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 12.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 14.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor–associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medzhitov R, et al. MyD88 is an adapter protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 16.Burns K, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 17.Janeway CA., Jr Presidential Address to the American Association of Immunologists. The road less traveled by: the role of innate immunity in the adaptive immune response. J Immunol. 1998;161:539–544. [PubMed] [Google Scholar]
- 18.Medzhitov R, Janeway CA., Jr Self-defense: the fruit fly style. Proc Natl Acad Sci USA. 1998;95:429–430. doi: 10.1073/pnas.95.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springhorn JP, Ellingsen O, Berger H-J, Kelly RA, Smith TW. Transcriptional regulation in cardiac muscle. Coordinate expression of Id with a neonatal phenotype during development and following a hypertrophic stimulus in adult rat ventricular myocytes in vitro. J Biol Chem. 1992;267:14360–14365. [PubMed] [Google Scholar]
- 20.Ellingsen O, et al. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol. 1993;265:H747–H754. doi: 10.1152/ajpheart.1993.265.2.H747. [DOI] [PubMed] [Google Scholar]
- 21.Nishida M, et al. Isolation and characterization of human and rat cardiac microvascular endothelial cells. Am J Physiol. 1993;264:H639–H652. doi: 10.1152/ajpheart.1993.264.2.H639. [DOI] [PubMed] [Google Scholar]
- 22.Michael LH, et al. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Balligand J-L, et al. Induction of NO synthase in rat cardiac microvascular endothelial cells by IL-1 beta and IFN-gamma. Am J Physiol. 1995;268:H1293–H1303. doi: 10.1152/ajpheart.1995.268.3.H1293. [DOI] [PubMed] [Google Scholar]
- 25.Arstall MA, Kelly RA. The role of nitric oxide in ventricular dysfunction. J Card Fail. 1998;3:249–260. doi: 10.1016/s1071-9164(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 26.Kapadia SR, et al. Hemodynamic regulation of tumor necrosis factor-α gene and protein expression in adult feline myocardium. Circ Res. 1997;81:187–195. doi: 10.1161/01.res.81.2.187. [DOI] [PubMed] [Google Scholar]
- 27.Taylor BS, et al. Multiple NF-κB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 28.Bryant D, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 29.Kubota T, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 30.de Belder AJ, et al. Nitric oxide synthase activities in human myocardium. Lancet. 1993;341:84–85. doi: 10.1016/0140-6736(93)92559-c. [DOI] [PubMed] [Google Scholar]
- 31.Haywood GA, et al. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1094. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 32.Habib FM, et al. Tumor necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet. 1996;347:1151–1155. doi: 10.1016/s0140-6736(96)90610-8. [DOI] [PubMed] [Google Scholar]
- 33.Satoh M, et al. Inducible nitric oxide synthase and tumor necrosis factor-α in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol. 1997;29:716–724. doi: 10.1016/s0735-1097(96)00567-0. [DOI] [PubMed] [Google Scholar]
- 34.Bottazzi B, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 35.Yasojima K, Schwab C, McGeer EG, McGeer PL. Human heart generates complement proteins that are upregulated and activated after myocardial infarction. Circ Res. 1998;83:860–869. doi: 10.1161/01.res.83.8.860. [DOI] [PubMed] [Google Scholar]
- 36.Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart. Upregulation by ischemia and reperfusion. Circ Res. 1998;82:1224–1230. doi: 10.1161/01.res.82.11.1224. [DOI] [PubMed] [Google Scholar]
- 37.Zhang FX, et al. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 38.Mizuguchi K, Parker JS, Blundell TL, Gay NJ. Getting knotted: a model for the structure and activation of Spätzle. Trends Biochem Sci. 1998;23:239–242. doi: 10.1016/s0968-0004(98)01216-x. [DOI] [PubMed] [Google Scholar]
- 39.Yang R-B, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 40.Kirschning CJ, Wesche H, Ayres TM, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 42.Hoshino K, et al. Cutting edge: toll-like receptor 4 (TLR4)–deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 43.Savill J. Phagocytic docking without shocking. Nature. 1998;392:442–443. doi: 10.1038/33025. [DOI] [PubMed] [Google Scholar]
- 44.Devitt A, et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 45.Franc NC, Dimarz J-L, Lagueux M, Hoffmann J, Ezekowitz RAB. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- 46.Oka K, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]