Abstract
Polymorphonuclear neutrophil (PMN) activation is pivotal in acute inflammation and injury from reperfusion. To elucidate components controlling PMNs in vivo, we prepared novel transgenic mice with the human leukotriene (LT) B4 receptor (BLTR) for functional characterization. Overexpression of BLTR in leukocytes dramatically increased PMN trafficking to skin microabscesses and lungs after ischemia-reperfusion, whereas mice deficient in 5-lipoxygenase (5-LO) showed diminished PMN accumulation in reperfused lungs. Hence, both BLTR expression and LT biosynthesis are critical for PMN infiltration in reperfusion-initiated second-organ injury. Also, in BLTR transgenic mice, 5-LO expression and product formation were selectively increased in exudates, demonstrating that receptor overexpression amplifies proinflammatory circuits. Endogenous lipoxin (LX) A4 was produced in ischemic lungs and elevated by reperfusion. Because LXA4 and aspirin-triggered 15-epimeric LXA4 (ATL) selectively regulate leukocyte responses, they were tested in BLTR transgenic mice. Despite excessive PMN recruitment in BLTR transgenic mice, intravenous injection of ATL sharply diminished reperfusion-initiated PMN trafficking to remote organs, and topical application of LX was protective in acute dermal inflammation. These results demonstrate a direct role for BLTR with positive feedback, involving BLTR and 5-LO signaling in controlling PMNs. Moreover, LXA4 and ATL counter BLTR-amplified networks, revealing a novel protective role for LX and ATL in stress responses that has applications in perioperative medicine.
Introduction
Polymorphonuclear neutrophil (PMN) recruitment and sequestration to sites of inflammation and second-organ injury is initiated by proinflammatory mediators, among which leukotriene (LT) B4 is considered to be very important (1, 2). Recently, the LTB4 receptor (BLTR) was also reported to serve as a coreceptor for HIV-1 entry (3), which further emphasizes the crucial role of this system in host defense. Aspirin is widely used for its anti-inflammatory and analgesic properties and in several newly identified therapeutic actions, including preventing cardiovascular diseases and decreasing the incidence of lung, colon, and breast cancers (4), which raises the importance of obtaining complete knowledge of aspirin’s mechanism of actions. In addition to inhibiting prostanoid biosynthesis, administration of aspirin also triggers the endogenous transcellular production of 15-epimeric or 15R-LXA4, termed aspirin-triggered LXA4 (ATL), which appears to partly mediate some of aspirin’s therapeutic impact (5) and which is generated in vivo (6). LXA4 controls leukocyte responses via its own specific G protein–coupled receptor, denoted ALXR (5), which also engages 15-epi-LXA4 (5, 7). As with other local mediators, lipoxins (LXs) are rapidly generated, evoke responses, and are regulated by further metabolism (5). Thus, to explore potential anti-inflammatory actions of LXs and regulation of BLTR in vivo, LXA4 and ATL stable analogues were designed that resist rapid metabolic conversion and are ALXR agonists (5, 7). These designer compounds, modeled in view of the biochemical events initiated by aspirin, appear to act through similar mechanisms as endogenous LX to regulate leukocytes without aspirin’s unwanted side effects.
Recent cloning of BLTR in humans (8) and mice (9, 10) enabled the elucidation of this signaling pathway at the receptor and gene levels. Each of these BLTRs shows homology with ALXR and the chemoattractant peptide and chemokine family of receptors (∼30%) exemplified by FMLP, C5a, and IL-8 receptors (11), but does not show extensive homology with the prostanoid receptors, providing further evidence that the origin of receptors for LX and LT is distinct from that for prostanoids. Along these lines, we developed transgenic (TG) mice with human BLTR (hBLTR). By using these novel hBLTR TG mice in conjunction with mice deficient in 5-lipoxygenase (5-LO) (12), we demonstrate that LXA4 and ATL regulate PMN-initiated second-organ injury in reperfusion; we also uncover a novel positive circuit in 5-LO signaling.
Methods
Cloning and functional expression of hBLTR.
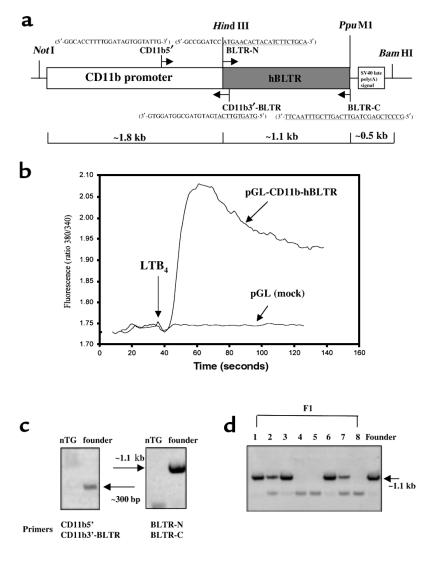
Total RNA was isolated from retinoic acid differentiated HL-60 cells (13) using Trizol reagent (GIBCO BRL, Grand Island, New York, USA), and was reverse transcribed for 30 minutes at 50°C followed by 40 cycles of PCR using Vent DNA polymerase (New England Biolabs Inc., Beverly, Massachusetts, USA) (98°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes) with specific primers for hBLTR (BLTR-N: 5′-GCCGGATCCATGAACACTACATCTTCTGCA-3′; and BLTR-C: 5′-GCCCTCGAGCTAGTTCAGTTCGTTTAACTT-3′) (Figure 1a). The hBLTR cDNA was inserted into pGL vector (Promega Corp., Madison, Wisconsin, USA) at the HindIII-PpuMI site downstream of human CD11b promoter (Figure 1a), which was subcloned from a CD11b genomic λ clone (a gift from D.G. Tenen, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA), as described (14). This CD11b promoter fragment extended from bp –1704 to +83, and therefore included 83 bp of the untranslated region extending up to the ATG start codon. Human embryonic kidney (HEK293) cells were transfected with this pGL-CD11b-hBLTR construct using SuperFect reagent (QIAGEN Inc., Valencia, California, USA). Ligand binding and intracellular calcium release were carried out as described (refs. 8 and 15, respectively). For stable transfection, hBLTR cDNA was inserted into pcDNA3 vector (Invitrogen Corp., Carlsbad, California, USA) carrying a neomycin-resistant gene. Human ALXR cDNA was inserted into pcDNA6 (Invitrogen Corp.) carrying a different selection marker (blasticidin-resistant gene). HEK293 cells were transfected with both constructs and selected with both neomycin and blasticidin. Cytosensor microphysiometry analysis of extracellular acidification rate (EAR) was carried out as described (16).
Figure 1.
Construction of hBLTR transgene and screening of hBLTR TG mice. (a) Illustration of the pGL-CD11b-hBLTR construct. Restriction sites used for cloning, and sense (right-facing arrows) and antisense (left-facing arrows) primers used for PCR, are indicated. Nucleotide sequences of the primers corresponding to the hBLTR are underlined. (b) pGL-CD11b-hBLTR and mock (pGL) plasmids were transiently transfected into HEK293 cells, and intracellular calcium was mobilized by addition of LTB4 (100 nM). Genomic DNA isolated from potential founder mice (c) and F1 generations (d) were analyzed by PCR using primers specific for the CD11b-hBLTR transgene construct. Molecular sizes of expected PCR products are indicated by arrows.
Preparation and identification of hBLTR TG mice.
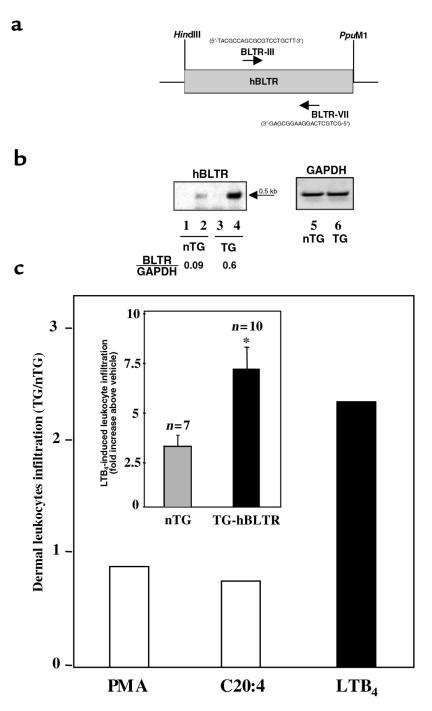
An approximately 3.4-kb hBLTR transgene was obtained by digestion of the plasmid (pGL-CD11b-hBLTR) with NotI and BamHI (Figure 1a) and was purified by Elutip (Schleicher & Schuell, Keene, New Hampshire, USA). Purified transgene construct (2 ng/μL) was injected into the male pronucleus of FVB homozygote oocytes that had been collected after hyperovulatory stimulation of donor females. The injected embryos were implanted in pseudopregnant Swiss females. Potential TG founder mice were screened by PCR analysis using genomic DNA isolated from mouse whole blood. Briefly, 15 μL of blood was collected from each mouse and resuspended in 250 μL of 10 mM Tris-HCl (pH 7.8) containing 1 mM EDTA and 0.5% NP-40. Cellular materials were obtained by centrifugation at approximately 9,500 g for 5 minutes and were resuspended in 40 μL of 0.1% Triton X-100 and 10 μL of 0.4 N NaOH to partially denature cell membranes and proteins. They were then heated at 95°C for 5 minutes, cooled on ice, and neutralized with 10 μL of 1 M Tris (pH 7.5), and each sample (1 μL) was used for 25 μL PCR. Hot-start PCR (98°C for 5 minutes before adding the Vent polymerase) was performed with 40 cycles of amplification (98°C for 1 minute, 60°C for 1 minute, and 72°C for 2 minutes) using primer sets (a) specific for hBLTR: BLTR-N and BLTR-C, which amplify the entire coding region of hBLTR; or (b) CD11b5′ (5′-GGCACCTTTTGGATAGTGGTATTG-3′) and CD11b3′-BLTR (5′-GTAGTGTTCATGATGTAGCGGTAGGTG-3′), which amplify 330 bp of the human CD11b promoter extending up to the bp +11 of hBLTR coding region (see Figure 1a). The positive founder obtained was bred with mice of the same strain. Potential positive litters (F1) from the TG founder were screened by PCR analysis as already described here. To verify BLTR and 5-LO expression, peritoneal leukocytes from mice with casein-induced peritonitis (2% casein for 4 hours) were collected as described (6), and total RNA was isolated. RT-PCR was performed using essentially the same conditions as described here and with primers that amplify both human and mouse BLTR: BLTR-III: 5′-TACGCCAGCGTCCTGCTT-3′; and BLTR-VII: 5′-GCTGCTCAGGAAGGCGAG-3′ (Figure 2a). For amplifying 5-LO, mouse-specific sense (5′-ATCAGGACGTTCACGGCCAGG-3′) and antisense (5′-CCAGGAACAGCTCGTTTTCCTG-3′) primers were used. Relative intensities of RT-PCR products were quantitated and normalized by GAPDH message levels using the NIH Image program (National Institutes of Health, Bethesda, Maryland, USA).
Figure 2.
Overexpression of hBLTR selectively enhanced PMN infiltration in hBLTR TG mice. (a) BLTR-III (sense; right-facing arrow) and BLTR-VII (antisense; left-facing arrow) primers used for amplifying human and mouse BLTR messages are indicated. (b) BLTR (lanes 2 and 4) and GAPDH (lanes 5 and 6) expression in nTG (lanes 2 and 5) and hBLTR TG (lanes 4 and 6) mice was analyzed by RT-PCR. RT-PCR controls (minus RT) are shown in lane 1 (nTG) and lane 3 (TG). The expected molecular size for BLTR at approximately 0.5 kb is indicated by an arrow. The relative intensities of BLTR messages normalized by GAPDH (BLTR/GAPDH) are reported. (c) LTB4 (1 μg), arachidonic acid (10 μg), or PMA (100 ng) was applied topically to the right ears of nTG and TG mice. Left ears received vehicle alone (acetone). Ear skin biopsy samples were taken for MPO analysis. Data were expressed as ratio (TG/nTG) of dermal leukocyte infiltration and (inset) fold increase above vehicle control (*P < 0.01; n = 7 for nTG and n = 10 for TG-hBLTR).
Ear skin inflammation model.
hBLTR TG and nontransgenic (nTG) littermates were anesthetized, and 16-phenoxy-LXA4 [15(R)-16-phenoxy-LXA4 methyl ester] was prepared by total synthesis by N.A. Petasis and colleagues (Department of Chemistry, University of Southern California, Los Angeles, California, USA) following procedures used in ref. 7. Each LX (∼26 nmol/10 μL acetone) was applied to the inner side of right ears. Acetone alone was applied to the left ear for a vehicle control. Five minutes later, LTB4 (5S,12R-dihydroxy-6,8,10,14-eicosatetraenoic acid; 1 μg) in 10 μL acetone was applied to both ears. Ear skin punch biopsies were collected for leukocyte myeloperoxidase (MPO) activity (7).
Hindlimb ischemia-reperfusion–induced second-organ injury.
Mice were anesthetized, and ATL analogue 15-epi-16-(para-fluoro)-phenoxy-LXA4 [15(S)-16-(para-fluoro)-phenoxy-LXA4 methyl ester; as in ref. 7] or vehicle was administered intravenously to the TG mice. Approximately 5 minutes later, a tourniquet was placed proximally around each hindlimb and secured with a metal clamp (17). Vascular occlusion was verified by engorgement and discoloration of the feet. After 3 hours of ischemia, the tourniquets were removed and followed by 3 hours of reperfusion. The 5-LO+/+ and 5-LO–/– mice (The Jackson Laboratory, Bar Harbor, Maine, USA) (18), as well as hBLTR TG mice, were then euthanized with an overdose of pentobarbital by intraperitoneal injection, in accordance with the Harvard Medical Area Standing Committee on Animals (protocol no. 02570-R98). The left lungs were harvested, homogenized in 1.5 mL of potassium phosphate buffer (pH 7.4), and then centrifuged at approximately 15,000 g (5 minutes). The precipitates were extracted for leukocyte MPO analysis (7), and the supernatants were stored (–80°C) for eicosanoid analysis.
Liquid chromatography-tandem mass spectrometry.
Murine peritoneal exudates were obtained from casein-induced peritonitis and incubated with A23187 (5 μM) at 37°C for 30 minutes. The samples were prepared by solid-phase extraction (7), with 10 ng of d4-LTB4 (6,7,14,15-tetradeuterium [d4]-LTB4) added to each as internal standard to calculate recovery. Liquid chromatography-tandem mass spectrometry (LC/MS/MS) was performed with an LCQ (Finnigan Corp., San Jose, California, USA) ion trap mass spectrometer system equipped with an electrospray ionization probe. Samples were suspended in mobile phase and injected into the HPLC component (Thermo Separation Products, San Jose, California, USA), which consisted of a quaternary gradient pump, a LUNA C18-2 column (150 mm × 2 mm, 5 μm; Phenomenex, Torrance, California, USA), and a scanning ultraviolet/visible absorbance detector. The column was eluted at 0.2 mL/min for 20 minutes with isocratic methanol/water/acetic acid (65:34.99:0.01 vol/vol/vol), followed by a 20-minute linear gradient to 99.99:0.01 methanol/acetic acid (vol/vol). Mass spectra were recorded in the negative ion mode with the spray voltage set to 5 kV, the heated capillary to 250°C, and a maximum ion injection time of 350 milliseconds. Selected ion monitoring (SIM) mass spectra were measured between m/z 315 and 360 throughout the elution with product ion spectra (MS/MS) recorded for molecular anions ([M–H]–).
Statistical analysis.
Results were expressed as the mean ± SEM. Student’s t test was performed, with P values less than 0.05 taken as statistically significant.
Results
TG mice were prepared for these experiments with hBLTR cloned in our laboratory from retinoic acid differentiated HL-60 cells that display LTB4-specific binding and signaling (13), using reported sequences (8). This BLTR transgene was placed in control of a human CD11b promoter (Figure 1a) capable of driving high levels of transgene expression in mature murine myeloid cells (14). To verify our clone, HEK293 cells were transfected with hBLTR cDNA (pGL-CD11b-hBLTR). These cells displayed both specific [3H]LTB4 binding (data not shown) and ligand-stimulated mobilization of intracellular calcium (Figure 1b), which together qualified its use for preparing hBLTR TG mice. Genomic DNA from founder mice was isolated and screened by PCR using primer sets (a) denoted as BLTR-N and BLTR-C (Figure 1a), which were specific to the hBLTR; or (b) CD11b5′ and CD11b3′-BLTR, which amplified the nucleotide segment corresponding to the CD11b promoter-hBLTR junction that is unique to our transgene. Both sets of primers yielded PCR products with genomic DNA from 1 potential founder of 12, whereas no apparent bands were observed with genomic DNA from nTG mice of the same strain (Figure 1c). Primers that amplified the entire coding region of hBLTR (e.g., BLTR-N and BLTR-C) yielded more prominent PCR signals, and thus were selected for future screening of the F1 generations. Twenty-four positive F1 mice were obtained (out of 36), with representative results shown in Figure 1d.
Peritoneal leukocyte exudates (from casein-induced peritonitis) from positive hBLTR TG mice were collected, and displayed an approximately 7-fold increase in BLTR message levels compared with nTG littermates (Figure 2b, lanes 2 and 4) that were age and strain matched (relative intensities of BLTR messages were ∼0.09 for nTG and ∼0.60 for TG mice, normalized by GAPDH as shown in Figure 2b). Primers BLTR-III and BLTR-VII (Figure 2a), which amplified both human and mouse BLTR, were used in RT-PCR to compare the message levels of endogenous mouse BLTR with those of exogenous hBLTR transgene. No apparent bands were observed in RT-PCR controls (minus RT; Figure 2b, lanes 1 and 3), indicating no genomic DNA contamination in our total RNA preparations. Thus, our results showed that the hBLTR transgene was indeed expressed and total BLTR expression was dramatically increased. Of interest, these hBLTR TG mice appeared healthy and without apparent gross pathological findings in the absence of a specific challenge (see below).
LTB4 applied topically to mouse ear skin induces PMN influx and microabscess in an acute dermal inflammatory response (7). Our TG mice showed a pronounced increase (7.4 ± 0.9–fold) in PMN infiltration into skin (Figure 2c, inset), whereas the nTG mice response was increased 3.2 ± 0.5–fold compared with the vehicle control. In sharp contrast, PMN infiltration into ear skin with either the native precursor of LTB4, namely arachidonic acid (C20:4), or the protein kinase C direct agonist PMA was not augmented in hBLTR TG mice (Figure 2c). Arachidonic acid (10 μg/ear) produced 3.0- and 2.5-fold increases in PMN infiltration in nTG and TG mice, respectively. Application of PMA (100 ng/ear) evoked 12.0- and 11.5-fold increases in nTG and TG mice, respectively. It should be noted that both C20:4 and PMA were added at levels that did not destroy tissue architecture of the ear skin. These findings indicate that hBLTR was functionally expressed and directly correlated with profound amplification of LTB4-initiated PMN microabscess formation in skin.
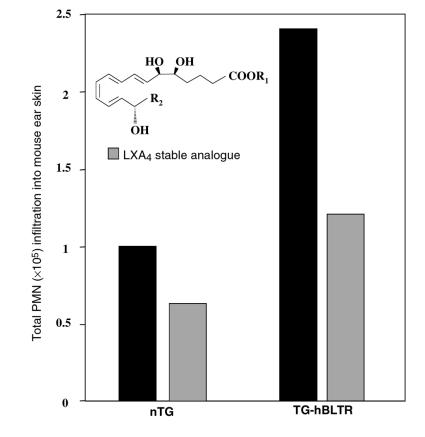
To determine whether LXA4 regulates this amplified LTB4-BLTR signaling pathway, and if these BLTR TG mice respond to LXA4 as wild-type mice, a stable LX analogue that resists rapid conversion and mimics LXA4 actions (5, 7) (Figure 3, inset) was examined for its ability to modulate PMN trafficking. In spite of excessive PMN infiltration in hBLTR TG mice, topical application of the LXA4 stable analogue was clearly able to block PMN infiltration (Figure 3). The percent inhibition in hBLTR TG versus nTG mice (∼45% vs. ∼37% inhibition) did not prove to be significantly different. But in magnitude, the LXA4 stable analogue inhibited infiltration of approximately 3 times as many PMNs in hBLTR TG mice as in nTG mice (Figure 3), which was evident when leukocyte MPO activities from these punch biopsies were calibrated and converted to total number of PMNs. No apparent increases in ALXR message levels were observed (data not shown). Hence, LXA4 and ALXR constitute a potent system for BLTR regulation in vivo.
Figure 3.
Topical application of LXA4 inhibits PMN infiltration in hBLTR TG mice. The LXA4 stable analogue (10 μg, 26 nmol) 16-phenoxy-LXA4 (inset: LXA4 analogue template R2= phenoxy) was applied topically to right ears of nTG and hBLTR TG mice (hatched bars). Left ears received vehicle alone (filled bars). LTB4 (1 μg) was then applied to both ears of each mouse 5 minutes later. Skin biopsies were obtained as just described. Values represent total PMN infiltration into ear skin (representative data from 3 experiments).
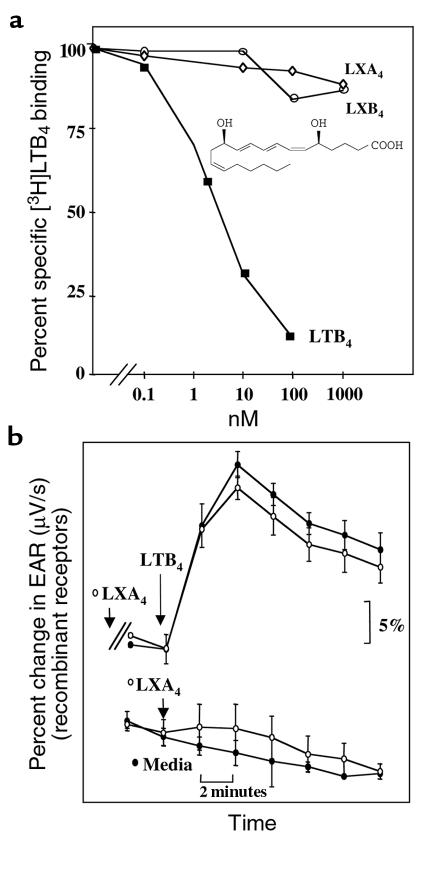
The question arises as to whether LXA4 directly interacts with hBLTR. To address this, we examined specific binding and signaling in HEK293 cells expressing BLTR or BLTR together with ALXR as stable transfectants. LXA4 did not compete with [3H]LTB4 binding (Figure 4a), nor did LXA4 or its analogues affect the LTB4-evoked EAR (16) (Figure 4b). Human BLTR couples to pertussis toxin–sensitive G proteins in HL-60 cells (8). Along these lines, Martin et al. (10) recently reported that transfected mouse BLTR functionally couples with Gi in Xenopus laevis and in Chinese hamster ovary cells. These signal transduction components of BLTR demonstrated in in vitro systems may also be involved in vivo, and are likely to mediate LTB4’s proinflammatory actions. It is thus likely that ALXR activation inhibits signal transduction components that occur downstream from LTB4-BLTR recognition.
Figure 4.
Recombinant BLTR recognition and signaling. (a) Displacement of specific [3H]LTB4 binding by LTB4 (filled squares; structure shown), LXA4 (open diamonds), or LXB4 (open circles) was determined in HEK293 cells stably transfected with BLTR. (b) EARs in response to LXA4 and LTB4 were analyzed by cytosensor microphysiometry in HEK293 cells with BLTR and ALXR (stable transfectants). Cells were exposed to LXA4 (1 μM; open circles) or media alone (filled circles) 20 minutes before exposure to LTB4 (1 μM). Values are expressed as EAR (μV/s) normalized to baseline (100%), and ligand additions are indicated by arrows.
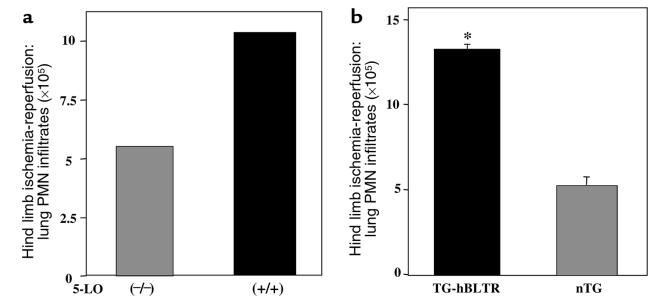
Ischemia-reperfusion is an event with major clinical importance. In humans, surgically based clamping procedures are well known to lead to aberrant PMN activation, giving rise to second-organ injury that contributes to longer hospitalization (19). To evaluate the contribution of both BLTR and LT formation in reperfusion injury, we used a hindlimb tourniquet model of second-organ injury (17) with both 5-LO–deficient and hBLTR TG mice (Figure 5, a and b). 5-LO+/+ mice demonstrated twice as much leukocyte accumulation in lungs compared with 5-LO–/– mice (10.5 × 105 vs. 5.4 × 105 PMNs greater than that of control animals) (Figure 5a). These 5-LO–deficient mice are unable to synthesize LTs (18). Thus, our data indicate that 5-LO–derived products (e.g., LTB4; see Figure 7, inset) are major mediators of PMN recruitment into lungs after hindlimb ischemia-reperfusion. Pharmacological evidence also implicated LTB4 as a key component in ischemia-reperfusion–induced second-organ injury in mice (17) and rats (20). Results with PMN recruitment in 5-LO–/– mice also point to the contribution of other mediators, such as C5a, IL-8, and platelet-activating factor (17), as PMN recruitment occurs in the absence of endogenous LTB4 formation (Figure 5a). The hBLTR TG mice also showed dramatic increases in PMN infiltration into lungs after reperfusion, compared with nTG mice (Figure 5b). When taken together, results from these genetically manipulated mice (namely, 5-LO–deficient and hBLTR TG mice) demonstrate that both the enhanced appearance of BLTR and the LT formation are important events in ischemia-reperfusion–induced second-organ injury, and therefore are potential therapeutic targets for further consideration in perioperative treatment.
Figure 5.
5-LO pathway and BLTR are major determinants of PMN infiltration into lungs after hindlimb ischemia-reperfusion. 5-LO–/– and 5-LO+/+ (a) and hBLTR TG and nTG (b) mice were subjected to hindlimb ischemia-reperfusion. The left lungs were collected. MPO activities were determined, and data are expressed as (a) increase in lung PMN infiltrates (×105) compared with control animals (ischemia alone) from 2 separate experiments, and (b) total PMN infiltration into lungs after ischemia-reperfusion. Total PMN numbers obtained from hBLTR TG and nTG mice were significantly different (*P = 0.02; n = 3).
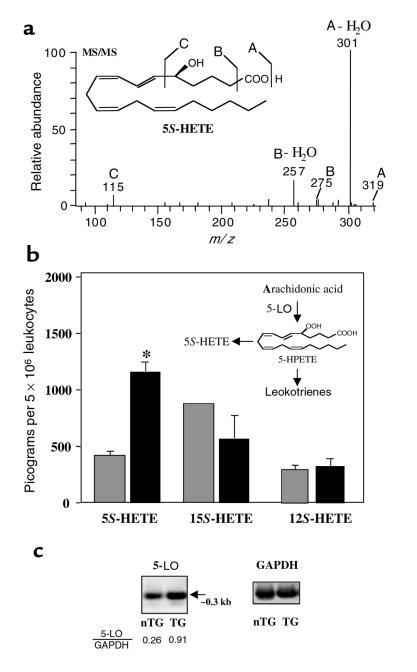
Figure 7.
Positive-feedback loop in hBLTR TG mice. 5-LO transcripts and product (5-HETE) are upregulated in peritonitis. (a) MS/MS spectrum of 5S-HETE. Peritoneal leukocytes from casein-induced peritonitis were collected and analyzed by LC/MS/MS. SIM chromatograms at m/z 319, as well as MS/MS spectra of 5-HETE, were acquired with a Finnigan LCQ LC/MS/MS system. 5-HETE was identified by its diagnostic MS/MS product ions at m/z 319 (A), 301 (A-H2O), 275 (B), 257 (B-H2O), and 115 (C). (b) Levels of 5-HETE, 15-HETE, and 12-HETE in nTG (hatched bars) and hBLTR TG mice (filled bars) were quantitated by LC/MS/MS and expressed in picograms per 5 × 106 peritoneal exudate leukocytes (*P < 0.01; n = 3). (c) Total RNA from isolated exudate leukocytes of nTG and hBLTR TG mice was isolated and analyzed for 5-LO and GAPDH expression by RT-PCR. The expected PCR product for 5-LO at approximately 0.3 kb was indicated by an arrow, and the relative intensities of RT-PCR products (5-LO/GAPDH) were determined.
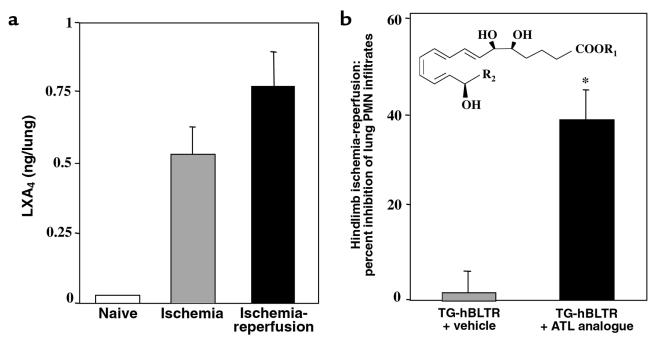
In view of the ability of LXA4 to modulate PMN-directed responses (5), it was of interest to determine whether LXA4 is generated during ischemia-reperfusion and whether it has an impact in the TG amplified BLTR signaling. Hindlimb ischemic mice showed increased amounts of endogenous LXA4 in lungs, and tourniquet release (reperfusion) further increased these levels (Figure 6a). Therefore, an analogue of ATL (Figure 6b, inset) was administered via intravenous injection in hBLTR TG mice before hindlimb ischemia-reperfusion. The administration of ATL significantly diminished PMN infiltration into lungs when compared with hBLTR TG mice injected with vehicle alone (Figure 6b). Thus, LXA4 formation within ischemic tissue, and its elevation by reperfusion, might represent an endogenous compensatory or protective role to limit PMN trafficking and PMN-mediated damage. This is supported by results showing that intravascular injection of the ALXR agonist (e.g., ATL stable analogues that display increased biostability compared with the endogenous LXA4; compare refs. 5 and 7) before ischemia-reperfusion attenuates BLTR-driven PMN infiltration. In addition, because ATL inhibited PMN infiltration, which is approximately the reciprocal level (40–60% change) observed in the 5-LO–/– and BLTR TG mice (Figure 5, a and b; Figure 6b), it emphasizes the importance of the LXA4-LTB4 regulatory system in these genetically defined mice.
Figure 6.
ATL inhibits PMN infiltration into lungs after hindlimb ischemia-reperfusion. (a) BALB/c mice were subjected to ischemia-reperfusion (∼0.8 ng; filled bar) or ischemia alone (∼0.5 ng; hatched bar). After removal of hindlimb tourniquet, left lungs were collected with or without reperfusion. LXA4 present within each lung tissue was quantitated by ELISA (Neogen Corp., Lexington, Kentucky, USA) or LC/MS/MS. (b) hBLTR TG mice were injected with vehicle (hatched bar) or the ATL analogue (10 μg; filled bar) [inset: ATL analogue template R2 = (para-fluoro)-phenoxy]. Leukocyte MPO values were obtained from left lung of each mouse after ischemia-reperfusion and were expressed as percent inhibition of lung PMN infiltration. Values from mice that received ATL analogue (∼7 × 105 PMN) versus vehicle alone (∼12 × 105 PMN) were significantly different (*P = 0.04; n = 3).
Using LC/MS/MS–based analysis, we found that in hBLTR TG mice, 5S-HETE (Figure 7a) (a product of 5-LO; Figure 7b, inset) was selectively and dramatically elevated in peritoneal inflammatory exudates compared with nTG mice (Figure 7b). In contrast, significant differences were not observed in the amount of other mono-HETEs from the major lipoxygenases, including 15S-HETE, 12S-HETE (Figure 7b), or LTB4 (data not shown). It is noteworthy that 5S-HETE is also a PMN chemoattractant, albeit less potent than LTB4 (21). Expression of 5-LO transcript in peritoneal leukocytes was approximately 3.5-fold higher in hBLTR TG mice than in nTG mice. Relative intensities of 5-LO messages were approximately 0.26 and approximately 0.91 for nTG and hBLTR TG mice, respectively (normalized by GAPDH message levels; Figure 7c). The observed increase in 5S-HETE, rather than LTB4, in hBLTR TG mice may reflect enhanced LTB4 utilization and clearance (i.e., further metabolism) in this model. We noted no apparent increases in cycloxygenase-2 message levels or striking elevations in cycloxygenase products (e.g., prostaglandin E2) (data not shown). Thus, the overexpression of BLTR specifically enhances a proinflammatory network by acting at the level of 5-LO gene transcription that is evident in vivo (Figure 7c), suggesting that this positive feedback within the 5-LO pathway could be operative in a wide range of disease states.
Discussion
To our knowledge, our findings provide the first demonstration that overexpression of BLTR profoundly amplifies PMN recruitment, function, and 5-LO signaling in murine models of acute skin inflammation, peritonitis, and reperfusion-initiated second-organ injury. In addition, this is the first direct evidence, to our knowledge, for an in vivo role of BLTR expression in regulating PMN activation and upregulation of the 5-LO pathway. Together, these results emphasize the impact of receptor expression as an important regulatory step in host responses, as well as in ischemia-reperfusion, and are consistent with the regulation of ALXR (22) and BLTR (9) expression by cytokines that are linked to the control of human immune functions. Moreover, these results open new avenues for the utility of counter-regulatory signals, namely ALXR agonists and metabolically stable ATL mimetics, to selectively regulate inflammatory diseases and reperfusion-associated injury events that are characterized and exacerbated by excessive PMN infiltration and activation.
Acknowledgments
N. Chiang is the 1998 recipient of the McDuffie Postdoctoral Fellowship Award from the Arthritis Foundation, and K. Gronert is a recipient of the Arthritis Foundation Postdoctoral Fellowship. We thank Tomoko Takano for expertise in validating the hindlimb ischemia-reperfusion; Mohamed Hachicha (Pharmacopeia Inc., Princeton, New Jersey, USA) for calcium mobilization experiments; and Nicos Petasis for synthetic 15-epi-LXA4 analogue. We also acknowledge helpful discussion with M. Amin Arnaout (Renal Unit, Massachusetts General Hospital, Boston, Massachusetts, USA), and Mary Halm Small for assistance in manuscript preparation. This work was supported in part by grants GM-38765 and DK-50305 (to C.N. Serhan) from the National Institutes of Health.
Footnotes
Nan Chiang and Karsten Gronert contributed equally to this work.
References
- 1.Weissmann G, Smolen JE, Korchak HM. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980;303:27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- 2.Sammuelsson B. Leukotrienes: mediators of inflammation and immediate hypersensitivity. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 3.Owman C, et al. The leukotriene B4 receptor functions as a novel type of coreceptor mediating entry of primary HIV-1 isolates into CD4-positive cells. Proc Natl Acad Sci USA. 1998;95:9530–9534. doi: 10.1073/pnas.95.16.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus AJ. Aspirin as prophylaxis against colorectal cancer. N Engl J Med. 1995;333:656–658. doi: 10.1056/NEJM199509073331011. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL) Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 6.Chiang N, Takano T, Clish CB, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: development of a specific 15-epi-LXA4 ELISA. J Pharmacol Exp Ther. 1998;287:779–790. [PubMed] [Google Scholar]
- 7.Takano T, Clish CB, Gronert K, Petasis NA, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1997;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein–coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 9.Huang W-W, et al. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med. 1998;188:1063–1074. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin V, et al. Leukotriene binding, signaling, and analysis of HIV coreceptor function in mouse and human leukotriene B4 receptor-transfected cells. J Biol Chem. 1999;174:8597–8603. doi: 10.1074/jbc.274.13.8597. [DOI] [PubMed] [Google Scholar]
- 11.Toh H, Ichikawa A, Narumiya S. Molecular evolution of receptors for eicosanoids. FEBS Lett. 1995;361:17–21. doi: 10.1016/0014-5793(95)00129-w. [DOI] [PubMed] [Google Scholar]
- 12.Funk, C.D. 1999. Lipid-mediator–deficient mice in models of inflammation. In Molecular and cellular basis of inflammation. C.N. Serhan and P.A. Ward, editors. Humana Press. Totowa, NJ. 109–125.
- 13.Fiore S, Romano M, Reardon EM, Serhan CN. Induction of functional lipoxin A4 receptors in HL-60 cells. Blood. 1993;81:3395–3403. [PubMed] [Google Scholar]
- 14.Dziennis S, et al. The CD11b promoter directs high-level expression of reporter genes in macrophages in transgenic mice. Blood. 1995;85:319–329. [PubMed] [Google Scholar]
- 15.Ng CF, Sun FF, Taylor BM, Wolin MS, Wong PY. Functional properties of guinea pig eosinophil leukotriene B4 receptors. J Immunol. 1991;147:3096–3103. [PubMed] [Google Scholar]
- 16.Gronert K, Colgan SP, Serhan CN. Characterization of human neutrophil and endothelial cell ligand-operated extracellular acidification rate by microphysiometry: impact of reoxygenation. J Pharmacol Exp Ther. 1998;285:252–261. [PubMed] [Google Scholar]
- 17.Goldman G, et al. Mast cells and leukotrienes mediate neutrophil sequestration and lung edema after remote ischemia in rodents. Surgery. 1992;112:578–586. [PubMed] [Google Scholar]
- 18.Chen X-S, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 19.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Seekamp A, Ward PA. Ischemia-reperfusion injury. Agents Actions Suppl. 1993;41:137–152. [PubMed] [Google Scholar]
- 21.Capodici C, Pillinger MH, Han G, Philips MR, Weissmann G. Integrin-dependent homotypic adhesion of neutrophils. Arachidonic acid activates Raf-1/Mek/Erk via a 5-lipoxygenase-dependent pathway. J Clin Invest. 1998;102:165–175. doi: 10.1172/JCI592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon γ and inhibits tumor necrosis factor α–induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]