Abstract
To investigate the function of prostaglandin H synthase-1 and synthase-2 (PGHS-1 and PGHS-2) in the normal lung and in allergic lung responses, we examined allergen-induced pulmonary inflammation and airway hyperresponsiveness in wild-type mice and in PGHS-1–/– and PGHS-2–/– mice. Among nonimmunized saline-exposed groups, we found no significant differences in lung function or histopathology, although PGE2 was dramatically reduced in bronchoalveolar lavage (BAL) fluid from PGHS-1–/– mice, relative to wild-type or PGHS-2–/– mice. After ovalbumin sensitization and challenge, lung inflammatory indices (BAL cells, proteins, IgE, lung histopathology) were significantly greater in PGHS-1–/– mice compared with PGHS-2–/– mice, and both were far greater than in wild-type mice, as illustrated by the ratio of eosinophils in BAL fluid (8:5:1, respectively). Both allergic PGHS-1–/– and PGHS-2–/– mice exhibited decreased baseline respiratory system compliance, whereas only allergic PGHS-1–/– mice showed increased baseline resistance and responsiveness to methacholine. Ovalbumin exposure caused a modest increase in lung PGHS-2 protein and a corresponding increase in BAL fluid PGE2 in wild-type mice. We conclude that (a) PGHS-1 is the predominant enzyme that biosynthesizes PGE2 in the normal mouse lung; (b) PGHS-1 and PGHS-2 products limit allergic lung inflammation and IgE secretion and promote normal lung function; and (c) airway inflammation can be dissociated from the development of airway hyperresponsiveness in PGHS-2–/– mice.
Introduction
Arachidonic acid, a polyunsaturated fatty acid esterified to cell membrane glycerophospholipids, is released in response to various stimuli and then oxidized by lipoxygenase (LO), cyclooxygenase, or cytochrome P-450 monooxygenase enzymes (1, 2). A wealth of data suggests that the leukotrienes (LTs), products of the arachidonate 5-LO pathway, are proinflammatory mediators that can reproduce many of the pulmonary manifestations of asthma including airway inflammation, bronchoconstriction, increased vascular permeability, and enhanced mucus secretion (1–3). Inhibitors of 5-LO or 5-LO–activating protein and antagonists of the LT receptors possess bronchodilatory/anti-inflammatory effects in the lung and have been used to treat patients with asthma (2, 4, 5). Moreover, recent studies show that 5-LO–deficient mice exhibit reduced allergen-induced airway eosinophilia and hyperresponsiveness compared with their wild-type counterparts (6).
The role of cyclooxygenase metabolites, or prostanoids, in allergic lung disease is less clear. Prostaglandin H synthase (PGHS), the first enzyme in this pathway, converts arachidonic acid to PGG2, then reduces it to PGH2. Other enzymes subsequently convert PGH2 to PGD2, PGE2, PGF2α, prostacyclin (PGI2), or thromboxane A2 (TxA2). Release of PGD2 into the airways is an early event after allergen challenge in sensitized asthmatic patients (7), and both PGD2 and PGF2α cause bronchoconstriction in allergic asthmatic, but not normal, subjects (8, 9). Furthermore, pulmonary expression of PGHS appears to be increased during allergic inflammation in rodents and in human asthmatic subjects (10, 11). Together, these studies have led to the concept that PGHS-derived eicosanoids have detrimental effects in the lung after allergen exposure. However, PGE2 and PGI2 have bronchodilatory effects (9, 12). PGE2 also blocks both the early and late asthmatic responses to allergen challenge in asthmatics (13) and inhibits leukotriene production (14) and IgE synthesis (15). Moreover, some patients with asthma develop airway inflammation and bronchoconstriction after ingestion/inhalation of salicylates or other nonsteroidal anti-inflammatory agents that are known to inhibit PGHS, and these effects are largely prevented by inhalation of PGE2 (16, 17). Thus, PGHS-derived eicosanoids may also have beneficial effects in the lung after allergen exposure.
Two distinct PGHS enzymes have been described in rodents and humans (18). PGHS-1 is constitutively expressed in a number of tissues, including the lung, and is believed to be a “housekeeping” enzyme that produces prostaglandins, which are required for maintenance of normal cell and organ function. In contrast, PGHS-2 is an inducible enzyme that is upregulated by cytokines and phorbol esters, highly expressed in inflamed tissues, and believed to produce prostaglandins involved in inflammatory processes (18). Importantly, the functional significance of these two PGHS enzymes in the lung under normal conditions and their relative importance in the pathogenesis of allergic lung disease remain unknown.
Recently, mice with disrupted Pghs-1 or Pghs-2 genes were generated using gene-targeting strategies (19–21), and the characteristics of the mice have been reviewed (22). Arachidonic acid–induced ear inflammation is reduced in homozygous PGHS-1–deficient (PGHS-1–/–) mice, whereas homozygous PGHS-2–/– mice had normal inflammatory responses to phorbol ester and arachidonic acid treatments (19, 20). To define the roles of these two PGHS enzymes and their bioactive eicosanoid products in normal lung physiology and to investigate their relative roles in the pathogenesis of allergen-induced lung inflammation and airway hyperresponsiveness, we used these mice in an established model of allergic airway disease in which animals are sensitized and challenged with ovalbumin antigen. The objectives of this study were to determine (a) whether there are any differences in airway function and histopathology among control nonallergic wild-type mice, PGHS-1–/– mice, and PGHS-2–/– mice; (b) whether PGHS products contribute to airway inflammatory responses after allergen challenge; (c) whether PGHS products contribute to baseline airway function or responsiveness to methacholine (Mch) in allergic mice; and (d) whether PGHS-1 or PGHS-2 deficiency affect lung lavage eicosanoid and IgE levels in nonallergic or allergic mice.
Methods
Materials.
All chemicals and reagents were purchased from Sigma Chemical Company (St. Louis, Missouri, USA) unless otherwise specified.
Experimental animals.
All animal studies were conducted in accordance with principles and procedures outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at both the National Institute of Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency. Pathogen-free wild-type mice, PGHS-1–/– mice, and PGHS-2–/– mice were obtained from the breeding colony at NIEHS. They were housed under identical conditions and fed NIH 31 rodent chow (Agway, St. Mary, Ohio, USA) ad libitum. All mice were of a hybrid C57BL/6J × 129/Ola genetic background, intercrossed for 15–20 generations. Mice were genotyped using a combination of PCR and Southern blotting using DNA isolated from tail pieces as described (19, 20). Primers used to identify genotypes were as follows: PGHS-1–/– forward: 5′-GCAGCCTCTGTTCCACATACAC-3′; PGHS-1+/+ forward: 5′-AGGAGATGGCTGCTGAGTTGG-3′; both PGHS-1 alleles reverse: 5′-AATCTGACTTTCTGAGTTGCC-3′; PGHS-2–/– forward: 5′-ACGCGTCACCTTAATATGCG-3′; PGHS-2+/+ forward: 5′-ACACACTCTATCACTGGCACC-3′; both PGHS-2 alleles reverse: 5′-ATCCCTTCACTAAATGCCCTC-3′. The PCR amplifications were performed in the presence of 10% dimethylsulfoxide, 2 mM MgCl2, and AmpliTaq DNA polymerase on a Perkin-Elmer 9600 thermal cycler. After an initial 1-minute incubation at 94°C, samples were subjected to 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds, and a final 7-minute incubation at 72°C. The PCR products were electrophoresed on 1.2% agarose gels containing ethidium bromide. The size of the PCR products are 646 bp for PGHS-1–/–, 601 bp for PGHS-1+/+, 905 bp for PGHS-2–/–, and 760 bp for PGHS-2+/+.
Experimental design.
Male and female mice, 5–9 months old (median 6.7 months), weighing 20–35 g were used. Within each experimental group, the sex ratio was approximately equal, and the ages of the groups did not differ significantly. This age range was used because of the time necessary to breed sufficient numbers of mice and to genotype the animals. On day 1, wild-type, PGHS-1–/–, and PGHS-2–/– mice (n = 13, 13, 11, respectively) were sensitized intraperitoneally with 20 μg of ovalbumin (Grade V; Sigma Chemical Co.) in 0.2 mL aluminum hydroxide adjuvant (Alhydrogel; Accurate Chemical & Scientific Corp., Westbury, New York, USA). Control wild-type, PGHS-1–/–, and PGHS-2–/– mice (n = 8, 8, 7, respectively) received Alhydrogel only. On 6 consecutive days (days 15–20), sensitized mice were challenged for 30 minutes per day with 1% ovalbumin in saline aerosol. Control mice were exposed to saline aerosol only. One day after the last aerosol exposure (day 21), airway function was measured, and the mice were sacrificed to assess inflammatory indices as detailed below.
Airway reactivity measurements.
Mice were anesthetized with urethane (1.5 g/kg) and tracheotomized using a custom-built cannula (0.89-mm inner diameter, 1.27-mm outer diameter) with ports for inspiration, expiration, and monitoring of airflow and pressure, similar to a design described previously (23). Animals were ventilated with a constant flow of air regulated by a mass-flow controller (FC-260; Tylan Manufacturing, Carson, California, USA). Respiratory solenoids (NVZ 110-6GZ-M5; SMC Pneumatics, Indianapolis, Indiana, USA) controlled inspiration (45% of breathing cycle) and allowed passive expiration. Breathing frequency (50 × kg–0.25/min–1) and tidal volume (7.5 mL/kg) were determined by animal body weight. Skeletal muscle movement was blocked by administration of succinylcholine chloride (15 mg/kg, intraperitoneally). Body temperature was maintained at 37°C using an isothermal heating pad, and heart rate and waveform were monitored (SRA-200; Micro-Med, Louisville, Kentucky, USA). A single-point pressure transducer connected to 1 port of the tracheal cannula and a pneumotachograph (SCXL004DN; SenSym, Milpitas, California, USA) connected to 2 ports closer to the point of insertion in the trachea provided pressure and flow signals, respectively. A custom software program (Mouse Reactivity System; U.S. Environmental Protection Agency, Research Triangle Park, North Carolina, USA) was used to control ventilation and calculate respiratory indices. Flow was integrated to obtain volume and maximum airway opening pressure, total respiratory system compliance (CT), resistance (RT), and resistance at 70% tidal volume were calculated for each breath cycle. Compliance and resistance were calculated using the constant-flow inflation method single-compartment model (24). All values were averaged over 6-second intervals and stored in data files. After establishing a stable baseline, mice were challenged by intravenous infusion of doubled doses of Mch in PBS (25, 50, 100, 200 μg/kg; 0.1 μg/μL; 1-second delivery) every 2 minutes using a syringe pump (model 200; KD Scientific, Boston, Massachusetts, USA) for precise timing and delivery of the cholinergic agonist. To standardize lung volumes before the first dose and 1 minute after each dose, the expiratory port was occluded until airway pressure reached ∼30 cm H2O. Resistance of the tracheal cannula (0.25 cm H2O/mL per second; ∼25% of baseline RT) was subtracted from measured values of RT, and responses to each dose were calculated as (peak RT – baseline RT) or (baseline CT – lowest CT) using the baseline value immediately before each dose.
Bronchoalveolar lavage and analysis of bronchoalveolar lavage fluid cells, proteins, and arachidonic acid metabolites.
After measurement of airway function, lungs were lavaged with five 1-mL aliquots of HBSS. Approximately 90% of the total injected volume was consistently recovered. The bronchoalveolar lavage (BAL) fluid was placed on ice and centrifuged at 360 g for 12 minutes at 4°C. Aliquots of BAL fluid supernatants were kept at 4°C for biochemical analysis (see below) or stored at –80°C for IgE or eicosanoid analyses. Cells were resuspended in 1.0 mL of HBSS and counted (Coulter Electronics Ltd., Hialeah, Florida, USA). Slides of BAL fluid cells were prepared (Cytospin 3; Shandon, Pittsburgh, Pennsylvania, USA), stained with Leuko-Stat (Fisher Scientific Co., Pittsburgh, Pennsylvania, USA), and at least 500 cells per sample were differentiated using conventional morphological criteria for macrophages/monocytes, lymphocytes, neutrophils, and eosinophils. Slides were number coded and cells differentiated in a blinded fashion. Assays for total protein, microalbumin, lactate dehydrogenase (LDH) and N-acetyl-β-D-glucosamidase (NAG) were modified for use on the Cobas Fara II centrifugal spectrophotometer (Hoffman LaRoche Inc., Nutley, New Jersey, USA) as described previously (25). PGE2 and LTB4 levels were determined by RIA using kits supplied by Amersham Life Sciences Inc. (Arlington Heights, Illinois, USA) according to the manufacturer’s instructions. LTC4/LTD4/LTE4 levels were determined by enzyme immunoassay (Amersham Life Sciences). For the LTB4 and cysteinyl LT analyses, BAL samples were purified using Amprep C2 ethyl columns (Amersham Life Sciences), according to the manufacturer’s instructions. After BAL, the left lung was frozen in liquid nitrogen, and the remaining lung was fixed as described below.
Protein immunoblotting.
Microsomal fractions were prepared from frozen mouse lungs by differential centrifugation at 4°C as described (26), resuspended in 50 mM Tris-Cl (pH 7.4), 1 mM dithiothreitol, 1 mM EDTA, and 20% (vol/vol) glycerol, and stored at –80°C. Goat anti-mouse PGHS-1 (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) and rabbit anti-mouse PGHS-2 (Cayman Chemical Co., Ann Arbor, Michigan, USA) were specific for their respective PGHS isoforms and used according to the manufacturers’ instructions. Recombinant PGHS-1 and PGHS-2 protein standards were prepared as described by Chulada et al. (27). For immunoblotting, proteins were resolved by electrophoresis in 10% SDS (wt/vol) polyacrylamide gels (80 × 80 × 1 mm) (Novex, San Diego, California, USA) and transferred electrophoretically to nitrocellulose membranes. Membranes were immunoblotted using the primary antibodies, goat anti-rabbit or rabbit anti-goat IgG conjugated to horseradish peroxidase (Bio-Rad Laboratories, Richmond, California, USA), and the ECL Western Blotting Detection System (Amersham International, Buckinghamshire, United Kingdom). Autoradiographs were scanned using a ChemiImager 4000 Low Light Imaging System (Alpha Innotech Corp., San Leandro, California, USA).
Isolation of total RNA and Northern analysis.
Total RNA was prepared from frozen lungs using TRIreagent (Molecular Research Center Inc., Cincinnati, Ohio, USA) according to the manufacturer’s instructions. Northern blots were performed using either a 1.7-kb mouse PGHS-1 cDNA probe (Oxford Biomedical, Oxford, Michigan, USA) or a 2.1-kb mouse PGHS-2 cDNA probe (20) as described previously (19, 20).
Lung histopathology.
Lungs were perfusion fixed at 20 cm H20 in 10% neutral buffered formalin, processed routinely, and embedded in paraffin. Serial sections (5–6 μm) were stained with either hematoxylin and eosin, alcian blue/periodic acid-Schiff, or mucicarmine. A semiquantitative histopathologic scoring system was developed based upon the presence and abundance of the following: (a) lymphoid aggregates (0, absent; 1, identifiable at low magnification around fewer than 50% of vessels and bronchi; 2, identifiable around more than 50% of vessels and bronchi; 3, identifiable around more than 50% of vessels and bronchi with acute inflammation and extension to involve small vessels); (b) large, eosinophilic alveolar macrophages (0, absent; 1, present in fewer than 25% of alveolar spaces; 2, present in 25–75% of alveolar spaces; 3, present in more than 75% of alveoli, often in tightly packed clusters); (c) multinucleated giant cells (0, absent; 1, occasionally identified while examining slides at medium magnification [×10 objective] and not generally visible at low power [×2 or ×4 objective]; 2, present and numerous, easily identified at lower magnification); (d) large eosinophilic intracytoplasmic inclusions (crystals) in macrophages (0, absent; 1, identified in a few cells upon examination at high-power magnification [×20 objective]; 2, present and numerous, sometimes large and extracellular; 3, extremely numerous with many lying free within alveolar spaces); (e) alveolar consolidation with mononuclear and polymorphonuclear leukocytes or mixed inflammation (0, absent; 1, consolidation in less than 25% of alveolar spaces; 2, consolidation in more than 25% of alveoli); (f) eosinophils (0, absent; 0.5, a few identified within inflammatory exudates at high magnification; 1, present in multiple fields but never more than 10% of cells identified); and (g) goblet cells (0, absent; 1, present in fewer than 50% of the airways; 2, abundant in the majority of airways). A total inflammatory score (range 0–16), taken as the sum of the individual scores, was determined by an investigator blinded to genotype and treatment group assignment. Intraobserver variability was determined by reexamination of 20 randomly selected slides for all 7 parameters at a later time. The same value was assigned 80% of the time. In the remaining 20%, the second score differed from the first by 1 grade. The total inflammatory score was highly correlated with other measures of inflammation including total BAL fluid inflammatory cells (r2 = 0.723, P < 0.00001).
Measurement of total IgE.
Total IgE in BAL fluid supernatant was measured by sandwich ELISA, using rat anti-mouse IgE (clone R35-72), purified mouse IgEκ standards (clone IgE-3), biotinylated rat anti-mouse IgE (clone R35-118) and other reagents from PharMingen (San Diego, California, USA) according to the manufacturer’s instructions. Plates were developed using streptavidin-alkaline phosphatase (Kirkegaard and Perry Laboratories Inc., Gaithersburg, Maryland, USA) and p-nitrophenylphosphate disodium, and read at 405 nm on a Thermomax plate reader (Molecular Devices, Menlo Park, California, USA). Concentrations of IgE were calculated from a quadratic equation fit of the standard curve. Values that were too low to be interpolated from the standard curve were assigned the lowest detectable standard value of the assay (6.25 ng/mL).
Statistical analysis.
All values are expressed as mean ± SE. Data were analyzed by ANOVA using SYSTAT software (SYSTAT Inc., Evanston, Illinois, USA). When F values indicated that a significant difference was present, Fisher’s LSD test for multiple comparisons was used. Values were considered significantly different if P was less than 0.05.
Results
BAL fluid inflammatory cells are increased in PGHS-deficient mice after allergen exposure.
There were no significant differences in the numbers or types of BAL fluid cells between nonimmunized wild-type, PGHS-1–/–, and PGHS-2–/– mice (Table 1). After allergen exposure, the total cell number in immunized wild-type mice increased 11-fold, and the majority of these cells were eosinophils (66%) (Table 1). Compared with allergic wild-type mice, the total number of cells in allergic PGHS-1–/– mice was 6-fold greater and the number of eosinophils was 8-fold greater (P < 0.001). Numbers of lymphocytes and neutrophils, but not macrophages, were also significantly increased in allergic PGHS-1–/– vs. allergic wild-type mice. Interestingly, allergic PGHS-1–/– BAL fluid macrophages displayed morphologic characteristics consistent with cellular activation (including increased size, vacuolated cytoplasm, and evidence for phagocytosis of amorphous debris and cells), whereas allergic wild-type BAL fluid macrophages did not (Figure 1). BAL fluid from allergic PGHS-2–/– mice contained 4-fold more total cells than allergic wild-type mice (P < 0.001) but significantly fewer than allergic PGHS-1–/– mice (P < 0.005) (Table 1). As before, the increased number of BAL fluid cells in the PGHS-2–/– animals was largely due to increased numbers of eosinophils (86% of total cells, P < 0.001 vs. wild-type mice). As with allergic PGHS-1–/– macrophages, allergic PGHS-2–/– macrophages appeared activated (Figure 1). These data show that after immunization and allergen exposure (a) PGHS-deficient mice have increased airway inflammatory cell influx (primarily eosinophils and lymphocytes) compared with wild-type mice; (b) the magnitude of inflammatory cell influx is greater in PGHS-1–/– vs. PGHS-2–/– mice; and (c) alveolar macrophages from PGHS-deficient mice display a morphology that is consistent with cellular activation.
Table 1.
Numbers (106) and percentages of inflammatory cells in BAL fluid from nonimmunized and allergic wild-type, PGHS-1–/–, and PGHS-2–/– miceA
Figure 1.
Stained cytospins of BAL fluid cells showing alveolar macrophage morphology in wild-type and PGHS-deficient mice. Wild-type, PGHS-2–/–, and PGHS-2–/– mice were administered adjuvant only and exposed to saline aerosol or sensitized with ovalbumin in adjuvant and challenged with ovalbumin aerosol. BAL cells were collected 1 day after final challenge. (a) Nonimmunized wild-type mice. (b) Allergic wild-type mice. (c) Allergic PGHS-1–/– mice. (d) Allergic PGHS-2–/– mice. ×100.
PGHS-deficient mice have histopathologic evidence for increased lung inflammation after allergen exposure.
Lungs from nonimmunized wild-type, PGHS-1–/–, and PGHS-2–/– mice were histologically normal (Figure 2, a and e, shown only for wild-type mice). After allergen exposure, immunized wild-type mice exhibited mild inflammation confined mostly to perivascular/peribronchial regions, characterized by the presence of lymphoid aggregates, focal areas of consolidation with mononuclear and polymorphonuclear leukocytes, occasional large eosinophilic alveolar macrophages, and rare multinucleated giant cells (Figure 2, b and f). In contrast, lungs from allergic PGHS-1–/– mice had severe inflammation that not only involved perivascular/peribronchial regions but also extended into the airspace (Figure 2, c and g). Lymphoid aggregates were identifiable around most blood vessels and bronchi and often involved the smallest vessels. Airway epithelium was thickened, alveoli were packed with clusters of large eosinophilic macrophages and eosinophils, multinucleated giant cells were numerous (Figure 2i), and eosinophilic crystals were present within the cytoplasm of macrophages and sometimes in the extracellular space (Figure 2j). Ultrastructurally, the crystals were electron dense and were usually surrounded by a single membrane. Their morphology was distinctly different from the typical hexagonal appearance of Charcot-Leyden crystals. There was no evidence for airway remodeling or changes in smooth muscle mass in allergic wild-type or PGHS-deficient mice, probably because of the relatively short time course of allergen exposure. Lungs from allergic PGHS-2–/– mice exhibited inflammation that was less intense than allergic PGHS-1–/– mice, but more severe than allergic wild-type mice (Figure 2, d and h). Compared with allergic PGHS-1–/– mice, the inflammation observed in allergic PGHS-2–/– mice was more focal and confined mainly to interstitial regions surrounding vessels and bronchi. In addition, allergic PGHS-2–/– mice showed loss of fine alveolar septae in comparison with allergic wild-type mice (Figure 2f), and remaining septae were thickened. These alterations in parenchymal alveolar structure were comparable to those observed in allergic PGHS-1–/– mice. Airway mucus production, as determined by staining with alcian blue/periodic acid-Schiff and mucicarmine stains, was more pronounced in allergic PGHS-1–/– (Figure 2k) and PGHS-2–/– mice than in allergic wild-type or nonimmunized mice of any genotype (Figure 2l).
Figure 2.
(a–l) Lung histopathology showing increased inflammation in PGHS-deficient mice compared with wild-type mice. (a, e, and l) Nonimmunized wild-type mice. (b and f) Allergic wild-type mice. (c, g, i, j, and k) Allergic PGHS-1–/– mice. Arrow in i indicates multinucleated giant cell. Arrow in j indicates eosinophilic crystals within macrophage. (d and h) Allergic PGHS-2–/– mice. All sections were stained with hematoxylin and eosin except k and l, which were stained with alcian blue/periodic acid-Schiff. ×10 (a–d), ×40 (e–h, k, and l), and ×80 (i and j).
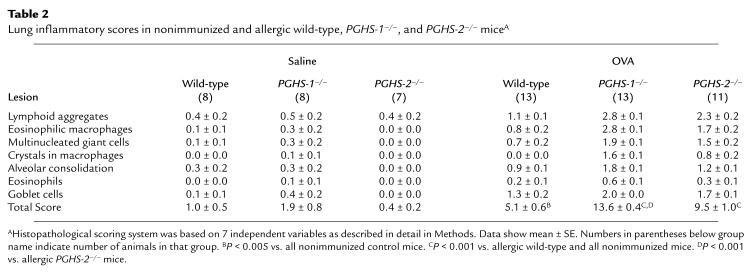
A semiquantitative, histopathologic lung scoring system was used to evaluate the degree of inflammation in the different experimental groups. Nonimmunized wild-type mice had a total lung inflammatory score of 1.0 ± 0.5 units, which was not significantly different from that of nonimmunized PGHS-1–/– and PGHS-2–/– mice (Table 2). After allergen challenge, immunized wild-type mice had a total lung inflammatory score of 5.1 ± 0.6 units, which was significantly greater than nonimmunized mice of any genotype (P < 0.005). In contrast, allergic PGHS-1–/– and PGHS-2–/– mice exhibited significantly more lung inflammation (total inflammatory scores 13.6 ± 0.4 and 9.5 ± 1.0 units, respectively; P < 0.001 vs. wild-type mice). The degree of lung inflammation in allergic PGHS-1–/– mice was significantly greater than that in allergic PGHS-2–/– mice (P < 0.001). Furthermore, the scores for each of the individual histologic variables except goblet cell metaplasia was significantly higher in allergic PGHS-1–/– mice than in allergic PGHS-2–/– mice (P < 0.05; Table 2). Taken together, these data confirm that allergic PGHS-deficient mice have significantly increased lung inflammation compared with allergic wild-type mice and that PGHS-1–/– mice exhibit the most profound histopathologic alterations.
Table 2.
Lung inflammatory scores in nonimmunized and allergic wild-type, PGHS-1–/–, and PGHS-2–/– miceA
PGHS-deficient mice exhibit increased alveolar epithelial permeability, lysosomal enzyme release, and cytotoxicity after allergen exposure.
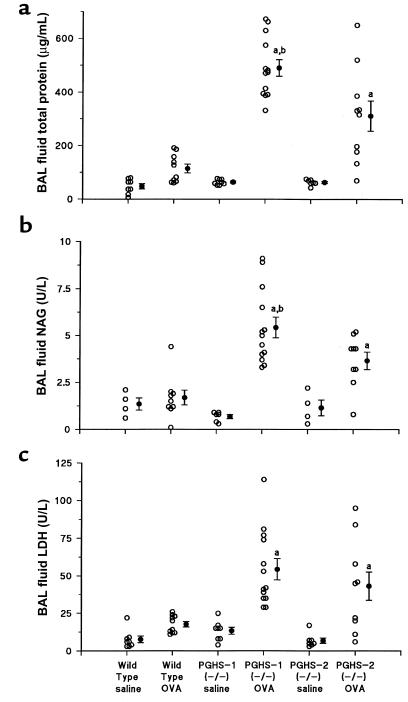
To determine if the increased lung inflammation present in PGHS-1–/– and PGHS-2–/– mice after allergen exposure was accompanied by changes in alveolar epithelial permeability, BAL fluid total protein and microalbumin were measured (Figure 3). Protein levels in nonimmunized wild-type, PGHS-1–/–, and PGHS-2–/– mice were not significantly different (48 ± 10, 64 ± 4, and 63 ± 4 mg/mL, respectively). BAL fluid protein levels in allergic PGHS-1–/– mice were 4-fold higher than in allergic wild-type mice (491 ± 31 vs. 114 ± 16 mg/mL; P < 0.001) suggesting that increased epithelial permeability accompanied the inflammatory process. Allergic PGHS-2–/– mice also had higher BAL fluid protein (311 ± 56 mg/mL; P < 0.001 vs. immunized wild-type mice), although these levels were significantly less than in PGHS-1–/– mice (P < 0.001). Analysis of BAL fluid microalbumin gave similar results (data not shown).
Figure 3.
BAL fluid proteins are increased in allergic PGHS-deficient mice compared with wild-type mice. Wild-type, PGHS-1–/–, and PGHS-2–/– mice were divided into treatment groups and BAL fluid total protein, NAG, and LDH were determined as described in Methods. Each open circle represents results from 1 animal. Filled circles and error bars show mean ± SE. (a) BAL fluid total protein. (b) BAL fluid NAG. (c) BAL fluid LDH. aP < 0.01 vs. all nonimmunized (saline) and wild-type ovalbumin (OVA) groups. bP < 0.01 vs. PGHS-2–/– OVA.
To explore whether the allergen-induced inflammation in the PGHS-1–/– and PGHS-2–/– mice was accompanied by increased lysosomal enzyme release, we measured BAL fluid NAG, an established marker of alveolar macrophage activation (28). Compared with allergic wild-type mice, allergic PGHS-1–/– mice exhibited a 3-fold increase in BAL fluid NAG (P < 0.001 vs. allergic wild-type mice), and allergic PGHS-2–/– mice had a 2-fold increase in BAL fluid NAG (P < 0.005 vs. allergic wild-type mice; P < 0.01 vs. allergic PGHS-1–/– mice) (Figure 3b). BAL LDH was measured as an index of cellular toxicity. There were no significant differences in this variable between allergic wild-type mice and nonallergic mice of any genotype (Figure 3c). In contrast, allergic PGHS-1–/– and PGHS-2–/– mice had 2- to 3-fold higher BAL fluid LDH levels than did allergic wild-type mice (P < 0.005). These results suggest that the increased inflammation in PGHS-deficient mice is associated with increased alveolar epithelial permeability, release of lysosomal enzymes from activated macrophages, and cellular toxicity.
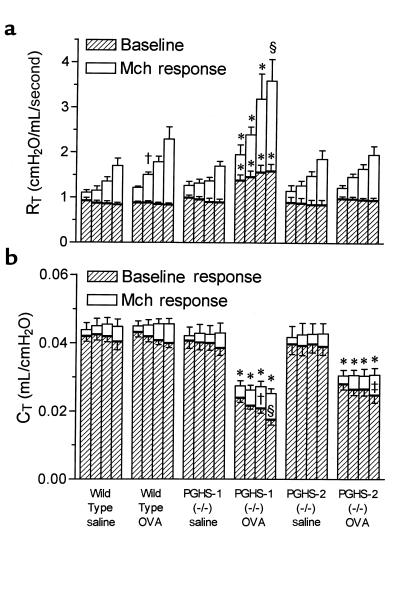
Both allergic PGHS-1–/– and PGHS-2–/– mice exhibit decreased baseline respiratory system compliance, whereas only allergic PGHS-1–/– mice exhibit increased respiratory system resistance and reactivity to Mch.
Baseline total respiratory system resistance (RT) was not significantly different among nonimmunized wild-type, PGHS-1–/–, and PGHS-2–/– mice (Figure 4a). In contrast, allergic PGHS-1–/– mice exhibited significantly increased baseline RT, ranging from 47% greater before the first dose (25 μg/kg) of Mch to 80% greater before the last dose (200 μg/kg), in comparison with all other groups. In addition, Mch induced significantly greater increases in RT in allergic PGHS-1–/– mice compared with all other groups, except at 200 μg/kg Mch, where the increase was not significantly different from that in allergic wild-type mice. Allergic wild-type mice were hyperresponsive compared with nonimmunized wild-type mice after dosing with 50 μg/kg Mch. Relative to baseline resistance [(Mch response – baseline)/baseline], allergic wild-type and PGHS-1–/– mice were equally hyperresponsive compared with their respective nonallergic controls at the first 3 doses of Mch (data not shown). Interestingly, there were no differences between nonallergic and allergic PGHS-2–/– mice with respect to baseline RT or Mch-induced increases in RT.
Figure 4.
Airway responsiveness to methacholine in PGHS-deficient and wild-type mice. Wild-type, PGHS-1–/–, and PGHS-2–/– mice were divided into treatment groups as described in Figure 1. Airway responses to intravenous Mch were measured 1 day after final aerosol exposure, as described in Methods. Bars represent mean ± SE of 7–13 mice per group. (a) RT before dosing with Mch and at peak of response to 25, 50, 100, and 200 μg/kg Mch (left to right for each group). For each dose, significant differences among groups were determined for associated baselines (shown above hatched bars) and increases in RT due to Mch (shown above open bars). *P < 0.05 vs. all other groups. †P < 0.05 vs. wild-type saline. §P < 0.05 vs. all groups except wild-type OVA. (b) Total respiratory system-compliance (CT), measured simultaneously with RT, before dosing with Mch and at nadir of response to Mch. For each dose, significant differences among groups were determined for associated baselines (hatched bars + open bars; shown above open bars) and decreases in CT due to Mch (shown within open bars). *P < 0.05 vs. wild-type saline, wild-type OVA, PGHS-1–/– saline, and PGHS-2–/– saline. †P < 0.05 vs. PGHS-1–/– saline and PGHS-2–/– OVA. §P < 0.05 vs. wild-type OVA and PGHS-1–/– saline. ‡P < 0.05 vs. PGHS-2–/– saline.
In contrast with RT, baseline respiratory system compliance (CT) was significantly reduced in both allergic PGHS-1–/– and PGHS-2–/– groups compared with allergic wild-type mice and all nonimmunized groups (Figure 4b). There was no significant difference in baseline CT between allergic PGHS-1–/– and PGHS-2–/– mice, and the reduction in compliance averaged 35% for both groups in comparison with the other 4 groups. Mch infusion decreased CT to a significantly greater degree in allergic PGHS-1–/– and PGHS-2–/– mice compared with nonimmunized PGHS-1–/– and PGHS-2–/– mice, respectively, at higher doses of Mch. At these doses, the Mch-induced decreases in CT in allergic PGHS-1–/– mice were greater than in allergic wild-type or allergic PGHS-2–/– mice, although the overall change was not great.
PGHS-1 is the major enzyme that biosynthesizes PGE2 in mouse airways under basal conditions, whereas lung PGHS-2 protein is upregulated after allergen exposure.
Protein immunoblotting with antibodies specific for PGHS-1 and PGHS-2 revealed that nonimmunized wild-type mice constitutively expressed both PGHS-1 and PGHS-2 proteins in the lung, whereas PGHS-1–/– mice expressed only PGHS-2 protein and PGHS-2–/– mice expressed only PGHS-1 protein (Figure 5). Furthermore, the levels of expression of PGHS-1 and PGHS-2 in PGHS-2–/– and PGHS-1–/– lungs, respectively, were comparable to those in wild-type lungs, indicating that PGHS-1 and PGHS-2 proteins are not coordinately regulated in vivo.
Figure 5.
PGHS-1 and PGHS-2 expression in nonimmunized and allergic wild-type, PGHS-1–/–, and PGHS-2–/– mice. Wild-type, PGHS-1–/–, and PGHS-2–/– mice were divided into treatment groups, and the abundance of PGHS-1 and PGHS-2 protein in the lungs was determined by Western blotting using immunospecific antibodies as described in Methods. Results are representative of experiments with 18 different animals (3 mice in each of the 6 groups). Lane 1, PGHS-1–/– mice, allergic; lane 2, PGHS-2–/– mice, allergic; lane 3, wild type mice, allergic; lane 4, PGHS-1–/– mice, nonimmunized; lane 5, PGHS-2–/– mice, nonimmunized; lane 6, wild-type mice, nonimmunized.
To determine which of the two PGHS isoforms was primarily responsible for PGE2 biosynthesis in mouse airways under basal conditions, PGE2 levels were measured in BAL fluid from nonimmunized wild-type, PGHS-1–/–, and PGHS-2–/– mice. BAL fluid obtained from nonimmunized wild-type and PGHS-2–/– mice contained equivalent amounts of PGE2 (206 ± 42 and 201 ± 38 pg/mL, respectively) (Figure 6a). In contrast, BAL fluid obtained from nonimmunized PGHS-1–/– mice contained significantly less PGE2 (56 ± 7 pg/mL; P < 0.05 vs. wild-type and PGHS-2–/– mice) indicating that PGHS-1 is the major enzyme responsible for basal production of PGE2 in mouse airways. After allergen exposure, PGE2 levels in immunized wild-type mice (383 ± 55 pg/mL) were significantly increased relative to all nonallergic groups and allergic PGHS-1–/– mice (P < 0.005), but not relative to allergic PGHS-2–/– mice. Accompanying this increase in BAL fluid PGE2 was a corresponding increase in lung PGHS-2, but not PGHS-1 immunoreactive protein (Figure 5). Lung PGHS-2 protein was also increased, albeit to a lesser extent, in allergic PGHS-1–/– mice compared with nonimmunized PGHS-1–/– mice, but BAL fluid PGE2 levels were not significantly different (Figures 5 and 6a). The increase in PGHS-2 protein was not accompanied by a corresponding increase in PGHS-2 mRNA (data not shown), suggesting that these allergen-induced changes likely occur at the posttranscriptional level. These data show that PGHS-1 is the predominant enzyme that biosynthesizes PGE2 in mouse lung under basal conditions and that lung PGHS-2 protein is upregulated after allergen exposure.
Figure 6.
BAL fluid eicosanoid levels in PGHS-deficient and wild-type mice. BAL fluid PGE2 (a) and LTB4 (b) were determined by RIA in wild-type, PGHS-1–/–, and PGHS-2–/– mice (groups described in Figure 1) as described in Methods. Each open circle represents results from 1 animal. Filled circles and error bars show mean ± SE. aP < 0.05 vs. wild-type groups and PGHS-2–/– saline groups. bP < 0.005 vs. saline-exposed wild-type and OVA-exposed PGHS-1–/– groups. cP < 0.005 vs. all other groups.
Increased LT biosynthesis occurs in allergic PGHS-1–/– but not PGHS-2–/– mice.
To determine if increased metabolism along the 5-LO pathway occurs in allergic PGHS-deficient mice, we measured levels of LTB4 and the cysteinyl leukotrienes in BAL fluid. LTB4 levels were increased in BAL fluid from allergic PGHS-1–/– mice compared with allergic wild-type and PGHS-2–/– mice and nonimmunized mice of any genotype (Figure 6b). Cysteinyl leukotrienes were also increased in BAL fluid from allergic PGHS-1–/– mice compared with allergic PGHS-2–/– and wild-type mice (21.4 ± 2.9, 8.2 ± 3.1, and 7.1 ± 2.9 pg/mL, respectively; P < 0.05). These results suggest that increased metabolism along the 5-LO pathway could contribute, in part, to the observed differences in pathology, inflammation, and lung function in allergen-challenged PGHS-1–/– mice.
Total IgE levels in BAL fluid are increased in both PGHS-1–/– and PGHS-2–/– mice, but not wild-type mice, after allergen challenge.
Total BAL fluid IgE levels were minimal in all nonimmunized groups of mice (Figure 7). There was no significant increase in IgE in allergic wild-type mice in comparison with nonimmunized mice. In contrast, there were significant increases in total IgE in both PGHS-1–/– and PGHS-2–/– allergic groups in comparison with nonimmunized groups and allergic wild-type mice (P < 0.001). The difference between allergic PGHS-1–/– and PGHS-2–/– mice was significant (P < 0.01).
Figure 7.
Total IgE levels in BAL fluid of PGHS-deficient and wild-type mice. Total IgE levels in BAL fluid from nonimmunized and allergic wild-type, PGHS-1–/–, and PGHS-2–/– mice were analyzed as described in Methods. Each open circle represents results from animal. Filled circles and error bars show mean ± SE. aP < 0.001 vs. all saline control groups and wild-type OVA. bP < 0.01 vs. PGHS-2–/– OVA.
Discussion
The purpose of this study was to define the relative functional roles of the two PGHS enzymes and their eicosanoid products in the lung under normal conditions and to investigate their involvement in the pathogenesis of allergen-induced lung inflammation and airway hyperresponsiveness. Mice with disrupted Pghs-1 or Pghs-2 genes were used to circumvent potential problems related to lack of isoform specificity of available PGHS inhibitors and possible effects of these agents on unrelated pathways (18, 29). Under basal conditions, both PGHS-1–/– and PGHS-2–/– mice were indistinguishable from wild-type mice with respect to various lung histologic, functional, and biochemical variables, although PGHS-1–/– mice had markedly reduced BAL fluid PGE2. Thus, the constitutive “housekeeping” PGHS isoform responsible for the majority of PGE2 biosynthesis within the lung was apparently not required to maintain normal lung cell and organ function under ordinary conditions. After allergen exposure, lung inflammation, airway hyperresponsiveness, and indices of lung injury were significantly greater in immunized PGHS-1–/– mice compared with wild-type mice. Thus, PGHS-1 products have mainly protective effects in the allergic mouse lung. Immunized PGHS-2–/– mice also showed greater lung inflammation after allergen exposure, suggesting that PGHS-2–derived eicosanoids are also primarily protective. The increased inflammatory response of allergic PGHS-2–/– mice is compatible with recent data showing an acquired deficiency of PGHS-2 expression in another proinflammatory lung condition, human idiopathic pulmonary fibrosis (30). Interestingly, the increased airway inflammation in allergic PGHS-2–/– mice was not associated with airway hyperresponsiveness to Mch. Although airway inflammation is frequently correlated with airway hyperresponsiveness, and treatment of inflammation often improves this condition, this correlation has not been observed in some studies of animals and humans (4, 31).
We found decreased baseline compliance in both allergic PGHS-1–/– and PGHS-2–/– mice, compared with allergic wild-type mice. In contrast, only allergic PGHS-1–/– mice showed increased baseline respiratory system resistance and hyperresponsiveness to Mch challenge. However, relative to baseline resistance values, wild-type and PGHS-1–/– allergic groups were equally hyperresponsive to Mch, because baseline values were significantly increased only in allergic PGHS-1–/– mice. This result indicates that the sensitivity of the 2 groups to nonspecific contractile agonists was equivalent. Thus, allergic wild-type mice displayed the expected hyperresponsiveness phenotype using this criteria, but relative to the overall greater resistance in allergic PGHS-1–/– mice, these changes were largely insignificant. These differences in responsiveness appear to be consistent with the observed pathology in that extensive airway and interstitial inflammatory infiltrates were observed in allergic PGHS-1–/– mice, whereas only focal interstitial infiltrates were observed in allergic PGHS-2–/– mice. Increased airway resistance and hyperresponsiveness are generally associated with alterations in airway structure and function. Decreased compliance is typically associated with changes in alveolar parenchymal structure, although structure is less important than smooth muscle tone and surfactant function in determining lung compliance. Therefore, after allergen challenge, it appears that both PGHS-1 and PGHS-2 products are involved in maintaining lung parenchymal structure and compliance, whereas only PGHS-1 products limit changes in airway inflammation, resistance, and responsiveness.
The leukotrienes, products of the 5-LO pathway, are generally considered to be proinflammatory mediators that have detrimental effects in the lung and are thought to be critical for the development of airway hyperresponsiveness after allergen exposure (1–6). Indeed, recent studies with 5-LO–deficient mice have shown dramatic reductions in airway responsiveness and degree of inflammation after ovalbumin sensitization and exposure in comparison with wild-type mice (6). In contrast, PGHS-derived eicosanoids have been reported to have both detrimental and beneficial effects in the airway, thus their role in the development of allergic lung disease is more complex. For example, PGD2, PGF2α, and TxA2 cause bronchoconstriction and are thought to be important in the development of airway hyperresponsiveness in animals and humans (8, 9, 32–34). On the other hand, PGE2 is a bronchodilator, blocks the early and late asthmatic responses to allergen challenge, and prevents the bronchoconstriction that accompanies nonsteroidal anti-inflammatory agent ingestion in aspirin-sensitive asthmatics (9, 13, 15–17). Prostaglandins of the E-series and their analogs have also been shown to inhibit recruitment of inflammatory cells into the lung, decrease their survival, and inhibit their activation (14, 35–37). Thus, whereas the mechanisms for these effects are unknown, the data presented here that shows accentuation of allergen-induced lung inflammation in both PGHS-1–/– and PGHS-2–/– mice supports the contention that overall, cyclooxygenase products are critical for maintenance of normal lung function and limitation of pulmonary inflammation after allergen exposure.
Disruption of the Pghs-1 and Pghs-2 genes might lead to increased cellular arachidonic acid availability and subsequent shunting of the limited substrate down alternative eicosanoid metabolism pathways. Hence, one hypothesis to explain the increased inflammation observed in the PGHS-deficient mice might involve increased production of proinflammatory mediators such as LTB4 and the cysteinyl leukotrienes (LTC4/LTD4/LTE4). Recent studies with 5-LO–/– mice have produced conflicting results as to whether this shunting phenomenon actually occurs. While Goulet et al. have reported enhanced release of PGE2 and thromboxane B2 in peritoneal macrophages from 5-LO–/– mice in vitro (38), Funk does not find evidence for such shunting (39). We found no significant differences in BAL fluid LTB4 levels between nonimmunized PGHS-deficient and wild-type mice; however, LTB4 and the cysteinyl leukotrienes were significantly increased in BAL fluid from allergic PGHS-1–/– mice compared with allergic wild-type and PGHS-2–/– mice. These results suggest that increased 5-LO metabolism may be one mechanism whereby allergic lung responses are accentuated in the PGHS-1–/– mice.
We found that wild-type mice constitutively express both PGHS-1 and PGHS-2 proteins in the lung. Demoly et al. have recently reported PGHS-1 and PGHS-2 immunoreactivity in bronchial epithelium of normal and asthmatic humans (40). These results are distinctly different than recent in vitro data, which demonstrate that the dominant isoform in cultured human lung epithelial cells is PGHS-2 (41, 42). We also observed that expression of PGHS-1 and PGHS-2 in nonimmunized PGHS-2–/– and PGHS-1–/– mouse lungs, respectively, were comparable to expression in nonimmunized wild-type mouse lungs. Thus, there did not appear to be a compensatory upregulation of the alternate PGHS isoform in either PGHS-1–/– or PGHS-2–/– mice. In contrast, it was recently reported that PGHS-1 and PGHS-2 proteins are coordinately regulated in vitro in immortalized lung cells derived from PGHS-1–/– and PGHS-2–/– mice (43). These in vivo/in vitro differences in expression and regulation of PGHS isoforms indicate caution when interpreting the results of studies with cultured cells.
PGHS-2 protein was constitutively expressed in the lung, but did not appear to contribute significantly to basal PGE2 generation. Indeed, nonimmunized PGHS-2–/– mice had essentially normal BAL fluid PGE2 levels. PGE2 levels in allergic wild-type and PGHS-2–/– mice were not significantly different, suggesting that PGHS-1 is also primarily responsible for PGE2 production after allergen challenge. Multiple factors may influence BAL PGE2 levels after allergen challenge, including changes in PGHS-2 protein expression, changes in PGHS-1 or PGHS-2 activity, and/or alterations in substrate availability. We observed increased lung PGHS-2 protein expression in allergen-exposed wild-type and PGHS-1–/– mice. Interestingly, allergen induced PGHS-2 protein more in wild-type than in PGHS-1–/– mice. This suggests the possibility that PGE2 or other PGHS-1–derived eicosanoids may be necessary for optimal induction of PGHS-2 to occur after allergen challenge. Niki et al. have recently reported increased expression of PGHS-2 in an air pouch model of allergic inflammation (10). Others have reported increased PGHS-2 expression in inflamed tissues and after treatment with proinflammatory cytokines, lipopolysaccharide, and phorbol esters (18, 19, 41–43).
It should be noted that we designed our experimental protocol to examine differences between wild-type and PGHS-deficient mice in the late asthmatic response. Animals were sacrificed 1 day after the last of repeated exposures to ovalbumin, at a time when recruitment and activation of inflammatory cells had occurred and bronchial hyperresponsiveness to Mch was present. We did not examine the early changes that occur after allergen challenge, including mast-cell degranulation and initial bronchoconstriction. However, levels of total BAL fluid IgE in allergic PGHS-1–/– and PGHS-2–/– mice were significantly greater compared with allergic wild-type mice, indicating that the former groups would respond to an allergen challenge with heightened immediate responses. The failure of the wild-type mice to produce increased IgE after allergen challenge may simply reflect a lower level of IgE synthesis compared with PGHS-deficient mice. Another possibility is that the level of inflammation in the lungs of PGHS-deficient mice is sufficient to alter alveolar epithelial permeability and allow significantly increased transudation of serum IgE from the vasculature compared with allergic wild-type mice. Further work will be necessary to determine if differences in airway inflammation and lung function also exist between PGHS-deficient and wild-type mice in the early asthmatic response.
In summary, we observed no differences between control wild-type, PGHS-1–/–, and PGHS-2–/– mice with respect to baseline respiratory system resistance and compliance or lung histopathology, despite the fact that the PGHS-1–/– mice had markedly reduced BAL fluid PGE2. After ovalbumin sensitization and exposure, PGHS-1–/– mice exhibited significantly increased lung inflammation and airway hyperresponsiveness compared with wild-type mice. The allergen-induced lung inflammatory response was also more pronounced in PGHS-2–/– mice compared with wild-type mice, but this was not accompanied by an increase in airway responsiveness. Based upon these findings, we conclude that (a) PGHS-1 is the predominant enzyme that biosynthesizes PGE2 in normal mouse lung; (b) PGHS-1 and PGHS-2 products have mainly protective effects in the lung after allergen exposure; and (c) there is a dissociation between the presence of airway inflammation and the development of airway hyperresponsiveness in PGHS-2–/– mice. Additional studies of PGHS-1–/– and PGHS-2–/– mice will likely provide further insights into the pathogenesis and mechanisms of allergic lung disease and asthma in humans.
Acknowledgments
The authors thank Judy Richards and Paul Evansky for technical assistance, and James Snapper, Thomas Eling, Paul Nettesheim, Linda Birnbaum, Dan Costa, and James Samet for their helpful comments during preparation of this manuscript. Portions of this work were presented at the 1996 International Conference of the American Thoracic Society.
This report has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- 1.Holtzman MJ. Arachidonic acid metabolism: implications of biological chemistry for lung function and disease. Am Rev Respir Dis. 1991;143:188–203. doi: 10.1164/ajrccm/143.1.188. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. 1997;17:3S–12S. [PubMed] [Google Scholar]
- 3.Adelroth E, Morris MM, Hargreave FE, O’Byrne PM. Airway responsiveness to leukotrienes C4 and D4 and to methacholine in patients with asthma and normal controls. N Engl J Med. 1986;315:480–484. doi: 10.1056/NEJM198608213150803. [DOI] [PubMed] [Google Scholar]
- 4.Henderson WR, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 6.Irvin CG, Tu Y-P, Sheller JR, Funk CD. 5-Lipoxygenase products are necessary for ovalbumin-induced airway responsiveness in mice. Am J Physiol. 1997;272:L1053–L1058. doi: 10.1152/ajplung.1997.272.6.L1053. [DOI] [PubMed] [Google Scholar]
- 7.Murray JJ, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315:800–804. doi: 10.1056/NEJM198609253151304. [DOI] [PubMed] [Google Scholar]
- 8.Hardy CC, Obinson CR, Tattersfield AE, Holgate ST. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. N Engl J Med. 1984;311:209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- 9.Mathe AA, Hedqvist P. Effect of prostaglandins F2 alpha and E2 on airway conductance in healthy subjects and asthmatic patients. Am Rev Respir Dis. 1975;111:313–319. doi: 10.1164/arrd.1975.111.3.313. [DOI] [PubMed] [Google Scholar]
- 10.Niki H, Tominaga Y, Watanabe-Kobayashi M, Mue S, Ohuchi K. Possible participation of cyclooxygenase-2 in the recurrence of allergic inflammation in rats. Eur J Pharmacol. 1997;320:193–200. doi: 10.1016/s0014-2999(96)00898-9. [DOI] [PubMed] [Google Scholar]
- 11.Kuitert LM, Newton R, Barnes NC, Adcock IM, Barnes PJ. Eicosanoid mediator expression in mononuclear and polymorphonuclear cells in normal subjects and patients with atopic asthma and cystic fibrosis. Thorax. 1996;51:1223–1228. doi: 10.1136/thx.51.12.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy CC, Bradding P, Robinson C, Holgate ST. Bronchoconstrictor and antibronchoconstrictor properties of inhaled prostacyclin in asthma. J Appl Physiol. 1988;64:1567–1574. doi: 10.1152/jappl.1988.64.4.1567. [DOI] [PubMed] [Google Scholar]
- 13.Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am Rev Respir Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- 14.Christman BW, Christman JW, Dworski R, Blair IA, Prakash C. Prostaglandin E2 limits arachidonic acid availability and inhibits leukotriene B4 synthesis in rat alveolar macrophages by a nonphospholipase A2 mechanism. J Immunol. 1993;151:2096–2104. [PubMed] [Google Scholar]
- 15.Pene J, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons γ and α and prostaglandin E2. Proc Natl Acad Sci USA. 1988;85:6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sladek K, et al. Eicosanoids in bronchoalveolar lavage fluid of aspirin-intolerant patients with asthma after aspirin challenge. Am J Respir Crit Care Med. 1994;190:940–946. doi: 10.1164/ajrccm.149.4.8143059. [DOI] [PubMed] [Google Scholar]
- 17.Sestini P, et al. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1996;153:572–575. doi: 10.1164/ajrccm.153.2.8564100. [DOI] [PubMed] [Google Scholar]
- 18.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 19.Langenbach R, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 20.Morham SG, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 21.Dinchuk JE, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 22.Langenbach, R., Loftin, C., Lee, C., and Tiano, H. 1999. Cyclooxygenase knockout mice: models for elucidating isoform specific functions. Biochem. Pharmacol. In press. [DOI] [PubMed]
- 23.Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol. 1995;79:560–566. doi: 10.1152/jappl.1995.79.2.560. [DOI] [PubMed] [Google Scholar]
- 24.Bates JH, Rossi A, Milic-Emili J. Analysis of the behavior of the respiratory system with constant inspiratory flow. J Appl Physiol. 1985;58:1840–1848. doi: 10.1152/jappl.1985.58.6.1840. [DOI] [PubMed] [Google Scholar]
- 25.Gavett SH, et al. Metal and sulfate composition of residual oil fly ash determines airway hyperreactivity and lung injury in rats. Environ Res. 1997;72:162–172. doi: 10.1006/enrs.1997.3732. [DOI] [PubMed] [Google Scholar]
- 26.Zeldin DC, et al. The rabbit pulmonary cytochrome P-450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest. 1995;95:2150–2160. doi: 10.1172/JCI117904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chulada PC, et al. Relative activities of retrovirally expressed murine prostaglandin synthase-1 and -2 depend on source of arachidonic acid. Arch Biochem Biophys. 1996;330:301–313. doi: 10.1006/abbi.1996.0257. [DOI] [PubMed] [Google Scholar]
- 28.du Bois RM, Townsend PJ, Cole PJ, Haslam PL, Turner-Warwick M. Bronchoalveolar macrophages in sarcoidosis and cryptogenic fibrosing alveolitis. Clin Allergy. 1981;11:409–419. doi: 10.1111/j.1365-2222.1981.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 29.Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev. 1996;16:181–206. doi: 10.1002/(SICI)1098-1128(199603)16:2<181::AID-MED3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Wilborn J, et al. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renz H, et al. Aerosolized antigen exposure without adjuvant causes increased IgE production and increased airway responsiveness in the mouse. J Allergy Clin Immunol. 1992;89:1127–1138. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- 32.O’Byrne PM, et al. Prostaglandin F2α increases airway responsiveness of pulmonary airways in dogs. Prostaglandins. 1984;28:537–543. doi: 10.1016/0090-6980(84)90242-9. [DOI] [PubMed] [Google Scholar]
- 33.Walters EH, Parrish RW, Bevan C, Smith AP. Induction of bronchial hypersensitivity: evidence for a role of prostaglandins. Thorax. 1981;36:571–574. doi: 10.1136/thx.36.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung KF, et al. Inhibition of antigen-induced airway hyperresponsiveness by a thromboxane synthetase inhibitor (OKY046) in allergic dogs. Am Rev Respir Dis. 1986;134:258–261. doi: 10.1164/arrd.1986.134.2.258. [DOI] [PubMed] [Google Scholar]
- 35.Smith WG, Thompson JM, Kowalski DL, McKearn JP. Inhaled misoprostol blocks guinea pig antigen-induced bronchoconstriction and airway inflammation. Am Rev Respir Dis. 1996;154:295–299. doi: 10.1164/ajrccm.154.2.8756797. [DOI] [PubMed] [Google Scholar]
- 36.Chouaib S, Robb RJ, Welte K, Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. J Clin Invest. 1987;80:333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam R, et al. Selective inhibition of the cutaneous late but not immediate allergic response to antigens by misoprostol, a PGE analog. Am Rev Respir Dis. 1993;148:1066–1070. doi: 10.1164/ajrccm/148.4_Pt_1.1066. [DOI] [PubMed] [Google Scholar]
- 38.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc Natl Acad Sci USA. 1994;91:12852–12856. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funk CD. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta. 1996;1304:65–84. doi: 10.1016/s0005-2760(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 40.Demoly P, et al. Prostaglandin H synthase 1 and 2 immunoreactivities in the bronchial mucosa of asthmatics. Am J Respir Crit Care Med. 1997;155:670–675. doi: 10.1164/ajrccm.155.2.9032211. [DOI] [PubMed] [Google Scholar]
- 41.Walanga RW, et al. Constitutive expression of prostaglandin endoperoxide G/h synthase (PGHS)-2 but not PGHS-1 in human tracheal epithelial cells in vitro. Prostaglandins. 1996;52:341–359. doi: 10.1016/s0090-6980(96)00101-3. [DOI] [PubMed] [Google Scholar]
- 42.Asano K, Lilly CM, Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol. 1996;271:L126–L131. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- 43.Kirtikara K, et al. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J Exp Med. 1998;187:517–523. doi: 10.1084/jem.187.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]