Abstract
The pathogenesis of septic shock occurring after Pseudomonas aeruginosa pneumonia was studied in a rabbit model. The airspace instillation of the cytotoxic P. aeruginosa strain PA103 into the rabbit caused a consistent alveolar epithelial injury, progressive bacteremia, and septic shock. The lung instillation of a noncytotoxic, isogenic mutant strain (PA103ΔUT), which is defective for production of type III secreted toxins, did not cause either systemic inflammatory response or septic shock, despite a potent inflammatory response in the lung. The intravenous injection of PA103 did not cause shock or an increase in TNF-α, despite the fact that the animals were bacteremic. The systemic administration of either anti–TNF-α serum or recombinant human IL-10 improved both septic shock and bacteremia in the animals that were instilled with PA103. Radiolabeled TNF-α instilled in the lung significantly leaked into the circulation only in the presence of alveolar epithelial injury. We conclude that injury to the alveolar epithelium allows the release of proinflammatory mediators into the circulation that are primarily responsible for septic shock. Our results demonstrate the importance of compartmentalization of inflammatory mediators in the lung, and the crucial role of bacterial cytotoxins in causing alveolar epithelial damage in the pathogenesis of acute septic shock in P. aeruginosa pneumonia.
Introduction
Nosocomial pneumonia is a leading cause of mortality among critically ill patients (1–3). The most common gram-negative organism associated with nosocomial pneumonia is Pseudomonas aeruginosa (4–6). Patients with P. aeruginosa pneumonia are also more likely to develop multiple organ failure and to die than patients with other types of pneumonia(7, 8). One explanation for the poor prognosis associated with P. aeruginosa pneumonia is that some P. aeruginosa strains cause acute lung epithelial injury and disseminate into the circulation (4, 7, 8).
A tight alveolar epithelium is the barrier that prevents the dissemination of inhaled organisms into the systemic circulation. We have determined the mechanisms for dissemination of P. aeruginosa from the lung into the circulation. First, cytotoxic P. aeruginosa causes necrosis of epithelial cells (9, 10). Instilled P. aeruginosa bacteria disseminate across the injured epithelium (11–14), and production of type III secreted toxins by P. aeruginosa leads to acute epithelial injury and bacterial dissemination (14, 15).
The role of circulating proinflammatory cytokines in the pathogenesis of septic shock has been well described (16, 17). In animal experiments, the sepsis syndrome is frequently mimicked by intravenous administration of bacteria or LPS. However, patients with sepsis are frequently not bacteremic, yet have severe hemodynamic instability (18). We therefore hypothesized that septic shock associated with P. aeruginosa pneumonia was secondary to the leak of mediators generated in the lungs crossing the injured epithelial barrier into the circulation. To test this hypothesis, we used a noncytotoxic isogenic mutant, PA103ΔUT. This strain was genetically engineered to be defective in the production of the known type III secreted toxins, and is thus unable to cause alveolar epithelial injury. We also compared the ability of PA103 to produce septic shock when administered by airspace instillation or intravenous infusion. We used anti-cytokine therapies to test whether cytokine-dependent mechanisms were involved in the development of septic physiology in P. aeruginosa pneumonia. Finally, using radiolabeled TNF-α instilled in the lung, we tested whether the leakage of TNF-α from the lung into the circulation depended on the presence of alveolar epithelial injury.
Methods
Culture conditions.
Parental P. aeruginosa PA103 and its isogenic mutant PA103ΔexoUexoT:Tc (PA103ΔUT) were used in this study (19, 20). PA103ΔUT was engineered by allelic replacement techniques to contain an insertion within exoT and a deletion of exoU (21). The bacterial suspension was prepared as described previously (10). The number of bacteria in the solution was confirmed by serial dilution followed by culture on sheep-blood agar plates.
In-vitro cytotoxicity assay.
A human bronchial epithelial cell line immortalized by adenovirus-12-SV40 hybrid virus (BEAS-2B; American Type Culture Collection, Rockville, Maryland, USA) was used for the cytotoxicity assay, as reported previously (22). Either PA103 or PA103ΔUT was mixed with RPMI-1640 medium buffered with 25 mM HEPES without serum or antibiotics, and applied to the BEAS-2B cells in 1 of 2 bacterial concentrations (108 or 107 CFU/mL). Bacterial-induced cytotoxicity after 4 hours was quantified by counting the number of live and dead cells per field, using trypan blue dye to stain the cells. Two hundred cells were counted, and viability was calculated as the percentage of live cells.
Animal investigation protocol.
The protocol for all animal experiments was approved by the Animal Research Committee of the University of California–San Francisco. Pathogen–free male New Zealand white rabbits (range of body weight 3.6–4.4 kg; Western Oregon Rabbit, Philomath, Oregon, USA) were used for all animal experiments.
Animal preparation and general protocol.
Anesthesia was induced with intravenous sodium pentobarbital (25 mg/kg) and maintained with halothane. A tracheotomy was done, and an endotracheal tube (4.0-mm inner diameter) was inserted. Mechanical ventilation was maintained with a constant-volume pump (Harvard Apparatus Inc., Holliston, Massachusetts, USA), with an inspired oxygen fraction of 1.0 at a tidal volume of 20 mL/kg body weight; positive end-expiratory pressure of 3 cm H2O was applied. The respiratory rate was adjusted to maintain an arterial CO2 level between 35 and 45 mmHg. A polyethylene tube (0.86-mm inner diameter) was inserted through the endotracheal tube into the right lower lung, for subsequent instillation of a bacterial suspension or a vehicle solution. The right carotid artery was catheterized for measurement of blood pressure and sampling of arterial blood. A thermodilution catheter was inserted through the right femoral vein into the pulmonary artery. Then the rabbits were placed in the right lateral decubitus position and were observed for a minimum of 30 minutes before taking baseline measurements.
In all experiments, pressures were monitored regularly as reported (14). All animals received an intravenous infusion of lactated Ringer’s solution (L/R; 4 mL/kg per hour) throughout the experimental period. Blood (700 μL) was sampled every 15 minutes for quantitative cultures of bacteria, for quantitation of the efflux of the airspace-protein tracer into the circulation, and for measurements of cytokines.
Experimental groups.
Table 1 summarizes the experimental groups and treatments. Twenty-eight rabbits were used in experiments comparing hemodynamics and quantities of plasma mediators. Four rabbits (control) received an airspace instillate that did not contain bacteria. Five rabbits (PA103) received an airspace instillate that contained PA103; 4 rabbits (ΔUT) received an airspace instillate that contained PA103ΔUT. All 3 of these groups received continuous infusions of L/R (3 mL/kg) that did not contain bacteria.
Table 1.
Classification of experimental groups
Instillation of P. aeruginosa and airspace-protein tracer.
The instillate (3 mL/kg) contained 125I-labeled human albumin (0.5 μCi; Merck Frosst Canada, Kirkland, Quebec, Canada) as the airspace-protein tracer, and 5% BSA in L/R. In designated experiments, bacteria (108 CFU/mL of PA103 or PA103ΔUT) were also added to the instillates. The instillates were administered using a published method, and calculations were done as described previously (12–14).
Pretreatment with anti–TNF-α serum or recombinant human IL-10.
Five additional rabbits (PA103 with anti–TNF-α) were pretreated with an intravenous injection of 1 mL goat anti-rabbit TNF-α serum (a generous gift from J.C. Mathison and V. Kravchenco, Scripps Research Institute, San Diego, California, USA) 30 minutes before administration of the airspace instillate containing PA103. The anti–TNF-α serum, which was made from blood collected in sterile pyrogen-free and endotoxin-free devices, had more than 106 neutralizing units per milliliter. Because the injection of nonimmune serum as a control for the administration of the anti–TNF-α serum had no effects on either physiological parameters or cytokine release in our preliminary experiments performed in the rabbits instilled with PA103, we compared the anti–TNF-α serum group with the PA103 group that had not received non-immune serum pretreatment. Another 5 rabbits (PA103 with rhIL-10) were pretreated with an intravenous injection of 50 μg/kg of recombinant human IL-10 (rhIL-10; Schering-Plough Corp., Kenilworth, New Jersey, USA) 30 minutes before the administration of an airspace instillate containing PA103. This dose was found to be effective in blocking the biological effects of TNF-α in a previous investigation using rhIL-10 (23).
Intravenous injection of P. aeruginosa.
Five other rabbits (intravenous PA103) received L/R (3 mL/kg body weight) containing PA103 (108 CFU/mL) in an intravenous infusion continuously for 8 hours (i.e., 6.25 × 105 CFU/kg per minute). These rabbits received the same total number of bacteria as the rabbits undergoing the airspace-instillation treatment already described. The rabbits that were given the intravenous infusion of bacteria had their airspaces instilled with vehicle alone (no bacteria).
125I–TNF-α efflux from the airspaces.
Seven additional rabbits were used to determine the movement of radiolabeled TNF-α from the airspaces to the circulation. One microcurie of 125I–TNF-α (1,000 Ci/mmol; Amersham International, Little Chalfont, United Kingdom) was added to the airspace instillate along with PA103 (n = 3), PA103ΔUT (n = 3), or the vehicle solution (n = 1). After the administration of the airspace instillate, blood was collected every 15 minutes for 8 hours, and the efflux of 125I–TNF-α into the circulation was calculated in the same way as the quantitation of airspace-protein tracer efflux was done (see above). One additional rabbit was injected intravenously with both 125I–TNF-α and 131I-albumin, to compare the clearance rates of those 2 compounds from the circulation. Blood samples were collected repeatedly to measure the elimination half-life of these injected radiolabeled proteins from the circulation.
Bronchoalveolar lavage.
Another 8 rabbits underwent the same protocol as the above animals, with the addition of bronchoalveolar lavage (BAL). Their right lungs (containing the instillates) were removed and BAL was performed by instilling ice-cold PBS (50 mL) containing 0.1% EDTA. The BAL fluid (BALF) was centrifuged at 3,400 g at 4°C for 20 minutes, and the supernatant was filtered using a 0.2-μm filter.
Cultures.
To assess bacteremia, arterial blood (100 μL) was obtained every 15 minutes and cultured on tryptic soy agar with 5% sheep-blood plates. To assess the quantity of bacteria remaining in the instilled lungs, right lungs were harvested and homogenized. Bacterial colonies were counted after incubation for 12 hours at 37°C. The quantity of bacteria was determined by multiplying counted colonies by the dilution ratio.
TNF-α and chemokine assays.
A biological TNF-α assay was performed using mouse sarcoma cells (WEHI-13VAR; American Type Culture Collection) as reported (24). TNF-α activity of each sample was calculated by comparing absorbance to a standard curve plotted from dilutions of rabbit TNF-α (PharMingen, San Diego, California, USA) between 1.0 pg/mL and 1.0 ng/mL. The lower limit of detection for this assay was 1.0 pg/mL. ELISA assays were performed using goat polyclonal IgG raised against either recombinant rabbit (rRab) IL-8, rRab growth-regulated oncogene (GRO), or rRab monocyte chemotactic protein-1 (MCP-1), as we have done before (25, 26). The lower limits of detection for the ELISA assays for IL-8, GRO, and MCP-1 were all 100 pg/mL.
Statistical analysis.
All values obtained incrementally during the 7-hour experimental period were analyzed using 2-way ANOVA with repeated measures followed by a Student’s t test. Comparisons at 7 hours are presented. Other data were analyzed with an unpaired Student’s t test. Data are presented as mean ± SEM. A value of P < 0.05 was accepted as statistically significant.
Results
In vitro cytotoxicity of PA103 and its isogenic mutant.
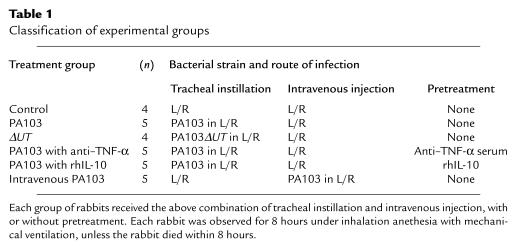
The in vitro cytotoxicity of PA103 and PA103ΔUT was documented using BEAS-2B cells (Figure 1). PA103ΔUT, an isogenic mutant in which the exoT and exoU genes have been inactivated, is noncytotoxic. Therefore, PA103ΔUT could be used to create airspace inflammation without causing alveolar epithelial injury.
Figure 1.
Cytotoxicity of PA103 and its isogenic mutant, PA103ΔUT. BEAS-2B cells (2 × 104 cells per well) were cultured with (a) 108 CFU/mL PA103 or PA103ΔUT, or (b) 107 CFU/mL PA103 or PA103ΔUT, for 4 hours. Triplicate measurements; mean ± SEM of percent change in cell viability. *P < 0.05 vs. group incubated with PA103.
Comparison of airspace instillation of PA103, PA103ΔUT, and vehicle.
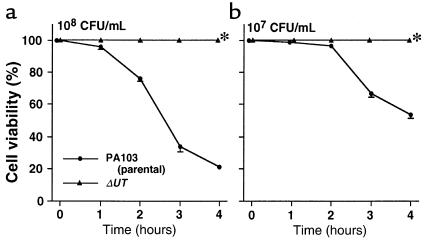
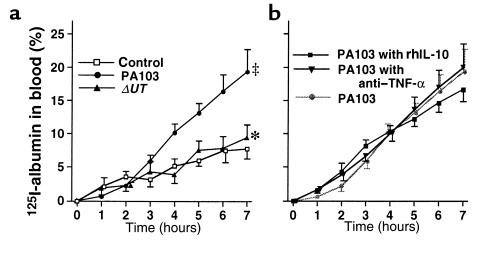
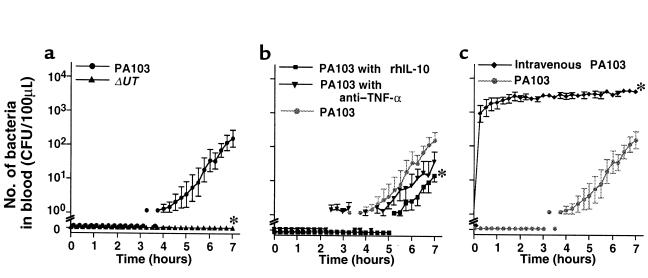
One of the 5 rabbits instilled with PA103 died after 7 hours; therefore, we analyzed the results from all rabbits at 7 hours after treatment. In the control rabbits, which did not receive airspace bacteria, mean arterial pressure (MAP), cardiac output, and base excess remained at baseline values for 7 hours (Figure 2a). Approximately 8% of the airspace-protein tracer crossed the alveolar epithelium into the circulation over the 7-hour interval (Figure 3a). Bacterial cultures were negative in the blood of these animals, and plasma TNF-α did not exceed the baseline value for 7 hours.
Figure 2.
MAP, cardiac output, and base excess for 7 hours. (a) Airspace instillation of PA103, PA103ΔUT, or L/R without bacteria (control group). (b) Pretreatment with intravenously administered anti–TNF-α serum or rhIL-10 followed by airspace instillation of PA103. (c) Intravenous infusion of PA103 and airspace instillation of vehicle. Mean ± SEM. Cardiac output is expressed as percent change from initial measurements. ‡P < 0.05 vs. control group. *P < 0.05 vs. group instilled with PA103.
Figure 3.
Airspace-protein tracer in blood. The quantity of 125I-albumin that left the lungs and entered the circulation was calculated and shown as a percentage of the initial dose. (a) Airspace instillation of PA103, PA103ΔUT, or L/R without bacteria (control group). (b) Pretreatment with intravenously administered anti–TNF-α serum or rhIL-10 followed by airspace instillation of PA103. Mean ± SEM. ‡P < 0.05 vs. control group. *P < 0.05 vs. group instilled with PA103.
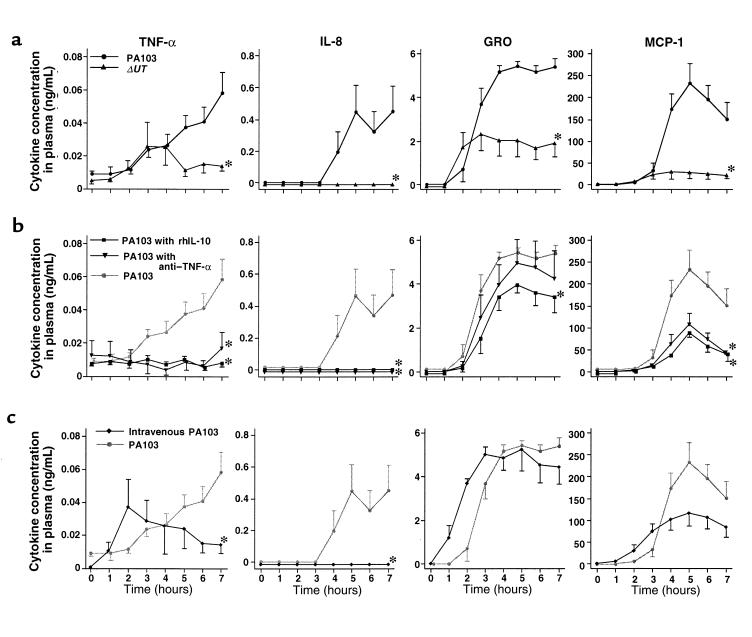
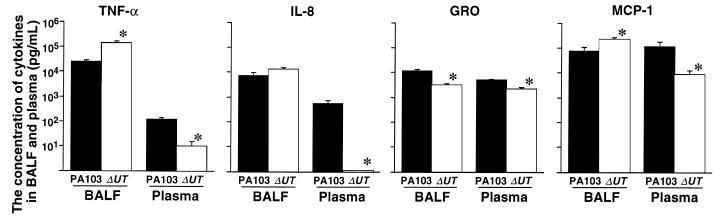
In the rabbits instilled with PA103, measurements of MAP, cardiac output, and base excess measurements decreased; all these animals were in septic shock (defined as a 20% decrease in cardiac output from baseline) after 7 hours (Figure 2a). Twenty percent of the airspace-protein tracer had left the lungs and entered the circulation after 7 hours (Figure 3a). Bacteremia increased exponentially after 4 hours and reached 100 CFU/100 μL after 7 hours (Figure 4a). Plasma TNF-α and chemokine levels increased significantly over the duration of the experiment (Figure 5a). TNF-α levels in the BALF were more than 100 times higher than the TNF-α levels in the plasma (Figure 6).
Figure 4.
Quantity of bacteria in blood. (a) Airspace instillation of PA103 or PA103ΔUT. (b) Pretreatment with intravenously administered anti–TNF-α serum or rhIL-10 followed by airspace instillation of PA103. (c) Intravenous PA103 and airspace instillation of vehicle. Mean ± SEM. *P < 0.05 vs. group instilled with PA103.
Figure 5.
Concentrations of plasma cytokines. (a) Airspace instillation of PA103 or PA103ΔUT. (b) Pretreatment with intravenously administered anti–TNF-α serum or rhIL-10 followed by airspace instillation of PA103. (c) Intravenous PA103 and airspace instillation of vehicle. Mean ± SEM. *P < 0.05 vs. group instilled with PA103.
Figure 6.
Concentrations of cytokines in BALF and plasma. Comparison of instillation with PA103 and instillation with PA103ΔUT. Mean ± SEM. *P < 0.05 vs. group instilled with PA103.
In the rabbits instilled with PA103ΔUT, MAP, cardiac output, and base excess remained at baseline levels for the entire 7-hour interval (Figure 2a). Efflux of the airspace-protein tracer into the circulation was approximately 9%, which was similar to that seen in the control animals, and significantly lower than the efflux seen after the airspace instillation of PA103 (Figure 3a). There was no bacteremia in the rabbits instilled with PA103ΔUT (Figure 4a). The plasma levels of TNF-α, IL-8, GRO, and MCP-1 were all significantly lower than those detected in the animals instilled with PA103 (Figure 5a). TNF-α levels in BALF were significantly higher in the animals instilled with PA103ΔUT than in PA103-instilled animals. When TNF-α levels in plasma and BALF were compared in PA103ΔUT-instilled animals, there was a 10,000-fold higher level in BALF than in plasma (Figure 6). The TNF-α levels in the BALF confirms that both bacterial strains caused generation of high levels of TNF-α in the airspaces of instilled lungs; however, it appears that the TNF-α remained confined in the lungs of the animals instilled with PA103ΔUT.
Efflux of airspace-instilled 125I–TNF-α from the lungs into the circulation, and clearance of intravenous 125I–TNF-α from the circulation.
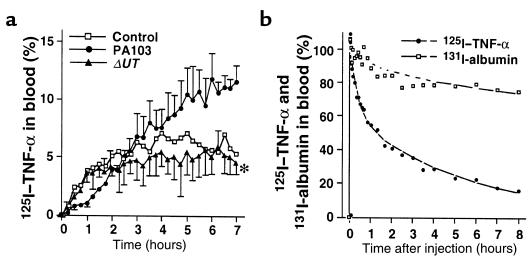
To compare the efflux of TNF-α from lungs instilled with PA103, PA103ΔUT, or vehicle (control group), we added 125I–TNF-α to the instillates. The efflux of 125I–TNF-α into the circulation of the rabbits instilled with PA103 was greater than the efflux in the control rabbits or in the rabbits instilled with PA103ΔUT (Figure 7a). In other rabbits, 125I–TNF-α and 131I-albumin were injected intravenously to determine the clearance of TNF-α and albumin from the circulation. 125I–TNF-α clears from the circulation significantly faster than does 131I-albumin (Figure 7b). The half-life for elimination of 125I–TNF-α was only 4 hours, whereas the half-life for elimination of 131I-albumin was 31 hours. The faster clearance of 125I–TNF-α from the circulation contributed to the differences between the measured plasma levels of 125I–TNF-α (Figure 7a) and 125I-albumin (Figure 3a).
Figure 7.
125I–TNF-α tracer studies. (a) 125I–TNF-α was instilled with PA103, PA103ΔUT, or vehicle. The quantity of 125I–TNF-α in the circulation is shown as a percentage of the initial dose instilled into the airspaces. Mean ± SEM. *P < 0.05 vs. group instilled with airspace PA103. (b) Plasma concentration curves of intravenously injected 125I–TNF-α and 131I-albumin. Data are percentages of the peak values at 1 minute after the injection.
Systemic blockade of TNF-α.
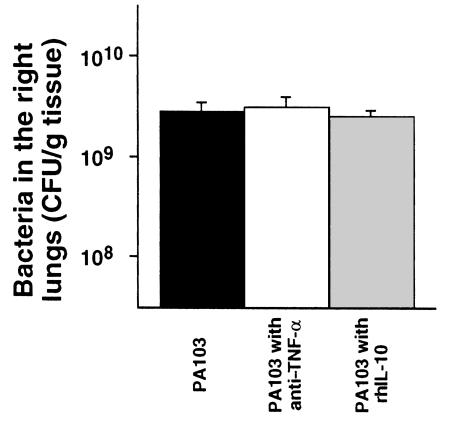
To test the hypothesis that the efflux of mediators across the injured epithelium was the cause of the septic shock, animals were pretreated intravenously with anti–TNF-α serum or rhIL-10. Pretreated animals had significantly improved MAP, cardiac outputs, and base excesses compared with those measurements in nontreated animals instilled with PA103 (Figure 2b). However, the efflux of the airspace-protein tracer into the circulation was similar in all 3 groups (Figure 3b), indicating a similar degree of alveolar epithelial injury among all 3. As shown in Figure 8, the number of bacteria in the instilled lungs at the end of the experimental period was not affected by the pretreatments. However, bacteremia was significantly improved by pretreatment with rhIL-10 before the instillation of PA103 (Figure 4b).
Figure 8.
Number of bacteria in the right lung at the end of experiments (7 hours). The quantity of bacteria in the lungs was determined by multiplying colonies by the dilution ratio. Mean ± SEM.
TNF-α activity did not exceed baseline levels during the 7-hour period in the animals that were both pretreated with anti–TNF-α or rhIL-10 and infected with PA103 (Figure 5b). Therefore, both pretreatments significantly decreased TNF-α activity for the duration of the experiment. IL-8 levels similarly did not exceed baseline in the pretreated animals (Figure 5b). MCP-1 levels were also significantly lower in animals pretreated with anti–TNF-α or rhIL-10 (Figure 5b). However, GRO levels were significantly lowered only by pretreatment with rhIL-10.
Intravenous infusion of PA103.
To determine the effects of PA103 in the circulation in the absence of alveolar epithelial lung injury, intravenous infusions of PA103 were given to animals that had vehicle instilled into their airspaces. Animals receiving infusions of PA103 did not develop septic shock; their MAP, cardiac output, and base excess remained at baseline levels for 7 hours (Figure 2c). All these rabbits survived the duration of the experiments despite constant high-level bacteremia (Figure 4c). Plasma TNF-α levels increased moderately for the first 2 hours and then declined in the animals infused with PA103. After 7 hours, the TNF-α plasma levels in these animals were significantly lower than the TNF-α levels in the animals receiving airspace instillation of PA103 (Figure 5c). The infusion of PA103 did not lead to detectable levels of IL-8 in the plasma. Plasma GRO and MCP-1 levels rose earlier in the animals receiving intravenous PA103 than in animals instilled with PA103; however there were no significant differences in these values after 7 hours (Figure 5c).
Discussion
We previously documented that strains of P. aeruginosa can be classified as cytotoxic or noncytotoxic to the lungs (19). The airspace instillation of a cytotoxic strain, PA103, consistently produces alveolar epithelial lung injury and bacteremia (14, 15, 20). This experimental paradigm is unique in that septic shock and bacteremia are consistently produced, thereby allowing investigation of the mechanisms causing their development, as well as the relationship of these conditions to lung epithelial injury. Our previous studies documented that instillation of noncytotoxic P. aeruginosa strains into the lungs of mice or rats did not produce lung injury or death when compared with the instillation of cytotoxic strains (10, 19). An isogenic mutant bacteria lacking specific cytotoxins (PA103ΔUT) was instilled to create lung inflammation without epithelial injury or bacteremia.
These investigations showed that instillation of PA103ΔUT led to significantly elevated levels of TNF-α in the BALF of rabbit lungs — levels 10,000-fold higher than in the plasma of the same animals. These experiments support our prior observations that lung inflammation can occur without increased epithelial permeability (27, 28). The difference between TNF-α levels in the airspaces and the plasma is consistent with the containment of TNF-α within the lung by an intact epithelial barrier. Further evidence for this comes from the experiments in which radiolabeled TNF-α was instilled along with PA103 or PA103ΔUT. The efflux of labeled TNF-α was significantly increased only when the cytotoxic PA103 was instilled into the airspaces. However, plasma TNF-α levels resulting from PA103 instillation were much higher than those predicted assuming that the only source of circulating TNF-α was the lung. This suggests that leakage of proinflammatory mediators from the lung into the circulation stimulates systemic TNF-α production.
The instillation of PA103ΔUT also led to the generation of levels of IL-8 in the airspaces that were comparable to those generated in response to instilled PA103. However, IL-8 was undetectable in the circulation of the animals instilled with PA103ΔUT. This can be explained by the fact that little lung injury occurred, or by the fact that IL-8 coming from the lung was absorbed onto circulating receptors (29). Plasma IL-8 levels were detectable in the animals instilled with PA103; this was consistent with the efflux of airspace IL-8 across the injured epithelium in amounts that exceeded the binding capacity of circulating receptors.
A previous investigation by Tutor et al. documented leakage of TNF-α from isolated, perfused rat lungs injured with α-naphthylthiourea (30). Clinical investigations measuring proinflammatory mediators in patients with acute lung injury have documented high concentrations of inflammatory mediators in BALF and in pulmonary-edema fluid (31, 32). One investigation that compared mediator levels in a patient’s pulmonary-edema fluid to levels in the same patient’s plasma found increased concentrations of the inflammatory mediators in the lung fluids, and low concentrations of the same mediators in the plasma (33). Plasma cytokine levels vary markedly in the course of experimental and clinical septic shock; therefore the timing of plasma sampling may explain the discrepancy between those results and our own. Our investigation documents that significant bacterial-induced alveolar epithelial injury causes a progressive increase in circulating TNF-α and IL-8 that is most likely due to leakage from the airspaces.
The systemic administration of anti–TNF-α serum or rhIL-10 blocked the increase of proinflammatory mediators in the circulation, and prevented hypotension and decreased cardiac output. Pretreatment with anti–TNF-α serum did not significantly decrease bacteremia; however, shock was prevented. The rhIL-10 pretreatment significantly decreased bacteremia without affecting the degree of alveolar epithelial injury. This suggests that the presence of rhIL-10 led to improved clearance of circulating bacteria. Thus, despite the presence of bacteria in the lung and in the circulation, and the development of lung injury, septic physiology did not develop when the systemic effects of the lung-generated inflammatory mediators had been blocked by anti–TNF-α or by rhIL-10.
Finally, intravenous infusion of PA103 did not cause septic shock. The presence of P. aeruginosa in the circulation was not sufficient for the development of septic physiology, even though the concentration of circulating bacteria was greater than that found circulating in the blood of septic patients (34). Notably, the circulatory levels of some of the proinflammatory mediators were different in the PA103-infused animals compared with the PA103-instilled animals. Plasma TNF-α levels in the animals infused with PA103 transiently increased early and then significantly declined later. This phenomenon is similar to the changes observed in serum TNF-α levels after LPS injections in rats (35) and in primates (36). Plasma IL-8 levels were significantly different between treatments; there was no detectable plasma IL-8 in the animals infused with PA103, whereas there was a large increase in plasma IL-8 in the animals instilled with PA103.
These results show the crucial importance of a tissue infection, as opposed to bacteremia, in the pathogenesis of septic shock. The results are consistent with the interpretation that mediators leaked from the lung in response to bacterial cytotoxicity and contributed to the development of septic shock. We recently demonstrated that the toxin secretion system of P.aeruginosa could be blocked by antibody therapy (37). Therapeutic strategies that decrease or prevent epithelial permeability in patients with pneumonia or lung injury should limit the development of septic shock.
Acknowledgments
We thank Richard Shanks for his technical assistance. K. Kurahashi especially wishes to thank I. Kudoh and F. Okumura (Department of Anesthesiology, School of Medicine, Yokohama City University, Yokohama, Japan) for providing an opportunity to participate in this project in the USA. This work was supported by grants from the National Institutes of Health (HL-49810 and HL-59239 to J.P. Wiener-Kronish; AI-31665 and AI-01289 to D.W. Frank; HL-30542, GM-37696, and AI-29103 to T.R. Martin).
References
- 1.Fagon JY, et al. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 2.Rouby JJ. Nosocomial infection in the critically ill. The lung as a target organ. Anesthesiology. 1996;84:757–758. doi: 10.1097/00000542-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rouby JJ. Histology and microbiology of ventilator-associated pneumonias. Semin Respir Infect. 1996;11:54–61. [PubMed] [Google Scholar]
- 4.Brewer SC, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 5.Craven DE, Steger K. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect. 1996;11:32–53. [PubMed] [Google Scholar]
- 6.Torres A, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- 7.Parrillo JE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann Intern Med. 1990;113:227–237. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 8.Taylor GD, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Bacteremic nosocomial pneumonia. A 7-year experience in one institution. Chest. 1994;108:786–788. doi: 10.1378/chest.108.3.786. [DOI] [PubMed] [Google Scholar]
- 9.Apodaca G, et al. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawa T, et al. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun. 1998;65:579–586. doi: 10.1128/iai.66.7.3242-3249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiener-Kronish, J.P., Sawa, T., Kurahashi, K., Ohara, M., and Gropper, M.A. 1998. Pulmonary edema associated with bacterial pneumonia. In Pulmonary edema. M. Matthay and D. Ingbar, editors. Marcel Dekker Inc. New York, NY. 247–267.
- 12.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiener-Kronish JP, et al. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]
- 14.Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet JF, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267:L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 15.Sawa T, et al. IL-10 improves lung injury and survival in Pseudomonas pneumonia. J Immunol. 1997;159:2858–2866. [PubMed] [Google Scholar]
- 16.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 17.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 18.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 19.Fleiszig SMJ, et al. Cytotoxic and invasive strains of Pseudomonas aeruginosa are genotypically distinct at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finck-Barbancon V, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 21.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, a novel adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddel RR, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- 23.Hurn RD, et al. Pharmacokinetics and immunomodulatory properties of intravenously administered recombinant human interleukin-10 in healthy volunteers. Blood. 1996;87:699–705. [PubMed] [Google Scholar]
- 24.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 25.Kajikawa O, Goodman RB, Johnson MC, II, Konishi K, Martin TR. Sensitive and specific immunoassays to detect rabbit IL-8 and MCP-1: cytokines that mediate leukocyte recruitment to the lungs. J Immunol Methods. 1996;197:19–29. doi: 10.1016/0022-1759(96)00101-9. [DOI] [PubMed] [Google Scholar]
- 26.Kajikawa O, Johnson MC, II, Goodman RB, Frevert CW, Martin TR. A sensitive immunoassay to detect the α-chemokine GRO in rabbit blood and lung fluids. J Immunol Methods. 1997;205:135–143. doi: 10.1016/s0022-1759(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 27.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darbonne WC, et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tutor JD, et al. Loss of compartmentalization of alveolar tumor necrosis factor after lung injury. Am J Respir Crit Care Med. 1994;149:1107–1111. doi: 10.1164/ajrccm.149.5.8173748. [DOI] [PubMed] [Google Scholar]
- 31.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153:1850–1866. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 32.Goodman RB, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 33.Pugin J, Verghese G, Widmer M-C, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 34.Yagupsky P, Nolte FS. Quantitative aspects of septicemia. Clin Microbiol Rev. 1990;3:269–279. doi: 10.1128/cmr.3.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson S, et al. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989;159:189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- 36.Leturcq DJ, et al. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 1996;98:1533–1538. doi: 10.1172/JCI118945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawa T, et al. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]