Significance
In plants the transition from vegetative growth to flowering is induced by environmental cues. The amplitude of these responses is enhanced by repressors that strongly delay flowering under non-inductive conditions. In Arabidopsis thaliana, the transcription factor SHORT VEGETATIVE PHASE (SVP) has a major role among these repressors. We show that SVP has an unrecognized function in repressing biosynthesis of the plant growth regulator gibberellin (GA) at the shoot apex. Under inductive photoperiods, SVP expression falls, contributing to increased expression of a GA biosynthetic enzyme that accelerates flowering. These results link GA biosynthesis to the established regulatory network controlling flowering and illustrate one of the mechanisms by which the levels of growth regulators are synchronized with the floral transition.
Abstract
In Arabidopsis thaliana environmental and endogenous cues promote flowering by activating expression of a small number of integrator genes. The MADS box transcription factor SHORT VEGETATIVE PHASE (SVP) is a critical inhibitor of flowering that directly represses transcription of these genes. However, we show by genetic analysis that the effect of SVP cannot be fully explained by repressing known floral integrator genes. To identify additional SVP functions, we analyzed genome-wide transcriptome data and show that GIBBERELLIN 20 OXIDASE 2, which encodes an enzyme required for biosynthesis of the growth regulator gibberellin (GA), is upregulated in svp mutants. GA is known to promote flowering, and we find that svp mutants contain elevated levels of GA that correlate with GA-related phenotypes such as early flowering and organ elongation. The ga20ox2 mutation suppresses the elevated GA levels and partially suppresses the growth and early flowering phenotypes of svp mutants. In wild-type plants, SVP expression in the shoot apical meristem falls when plants are exposed to photoperiods that induce flowering, and this correlates with increased expression of GA20ox2. Mutations that impair the photoperiodic flowering pathway prevent this downregulation of SVP and the strong increase in expression of GA20ox2. We conclude that SVP delays flowering by repressing GA biosynthesis as well as integrator gene expression and that, in response to inductive photoperiods, repression of SVP contributes to the rise in GA at the shoot apex, promoting rapid induction of flowering.
In plants, the transition from vegetative growth to flowering is regulated by a complex combination of environmental and internal signals. This developmental transition is controlled by environmental cues, such as seasonal changes in day length (photoperiod) or winter cold (vernalization) as well as ambient conditions including light intensity and spectral quality (1). Furthermore, endogenous signals such as the age of the plant or hormone levels influence flowering time. In Arabidopsis thaliana the genetic architecture of the pathways mediating these effects has been partially elucidated. Defined pathways conferring flowering responses to photoperiod and vernalization have been described (1), whereas the growth regulator gibberellin (GA) and age-related changes in expression of particular microRNAs represent endogenous flowering pathways (2, 3). These diverse pathways converge to regulate the transcription of a small number of integrator genes that promote the floral induction program. Notable among these genes are FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1). FT is transcribed in the leaves and encodes a small protein related to phosphatidylethanolamine-binding proteins that is transported to the shoot apex where it promotes the transcriptional reprogramming of the meristem to initiate flowering (4–10). SOC1 encodes a MADS box transcription factor that is expressed in the shoot meristem during floral induction and is the earliest gene shown to be upregulated by environmental cues such as day length (11–13).
Floral integrator gene expression is repressed by the MADS box transcription factor SHORT VEGETATIVE PHASE (SVP), an inhibitor of flowering. Mutations in SVP cause early flowering under noninductive short days (SD) and under long days (LDs) (14), which correlates with increased levels of the mRNAs of FT, its paralogue TWIN SISTER OF FT (TSF) and SOC1 (15–17). In wild-type plants, the repressive function of SVP is overcome by exposure to LDs, indicating that SVP increases the amplitude of the photoperiodic response by preventing premature flowering under SDs. SVP plays a similar role in response to vernalization where it forms a heterodimer with the MADS box transcription factor FLOWERING LOCUS C (FLC) to strongly repress flowering before exposure to cold (17, 18). Repression of SVP activity also contributes to the early flowering observed under high ambient temperatures (19, 20). Patterns of naturally occurring allelic variation at SVP also suggest that SVP plays a role in adapting flowering time to local conditions (21). Thus, SVP represents a critical node in the control of flowering time of A. thaliana. Genomic studies proposed several hundred SVP direct targets based on ChIP-chip or ChIP-seq analysis (22, 23). This global analysis together with specific ChIP-PCR experiments demonstrated that repression of some flowering genes by SVP, including FT and SOC1, is direct (16, 17). However, further functional analysis of the processes downstream of SVP are required to understand how this transcription factor so effectively represses flowering and thereby increases the amplitude of flowering responses to different environmental cues.
Here we show that an important previously unrecognized function of SVP is to reduce levels of GA by reducing expression of GA20-OXIDASE 2 (GA20ox2), which encodes a rate-limiting enzyme in GA biosynthesis (24–26). We show that svp mutants contain elevated levels of GA and propose that repression of SVP transcription during floral transition leads to an increase in GA20ox2 expression. Our data indicate that the resulting increase in GA levels, for example, during photoperiodic flowering, increases the mRNA levels of genes encoding SQUAMOSA PROMOTER BINDING PROTEIN LIKE (SPL) transcription factors, stably inducing the floral transition. This analysis demonstrates a mechanism for how GA biosynthesis is increased at the shoot apex by environmental cues through the well-established regulatory network that controls flowering (27).
Results
Inhibition of Floral Induction by SVP Cannot Be Fully Explained by Repression of FT, TSF, SOC1, and FUL.
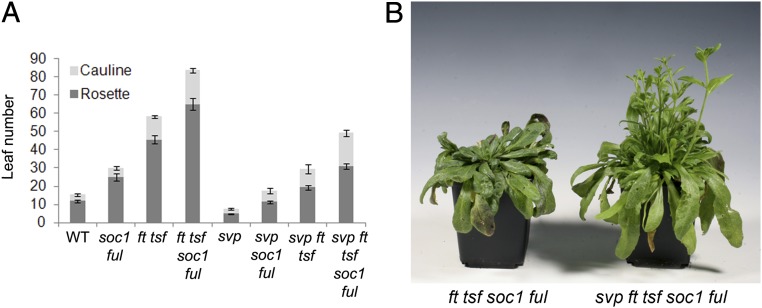
The MADS box transcription factor SVP regulates flowering under SDs and LDs by repressing transcription and reducing steady-state mRNA levels of the floral integrators FT, TSF, and SOC1, which are all required for the photoperiodic flowering response (28). By contrast, the mRNA abundance of FRUITFULL (FUL), which also encodes a MADS box transcription factor that acts in the photoperiod pathway and is partially genetically redundant with SOC1 (9, 29), is affected only by SVP under LDs (Fig. S1 A–D). The relevance of the increase in FT, TSF, SOC1, and FUL mRNA levels for the early flowering phenotype of svp mutants was tested by genetic analysis using the svp-41 null allele (14). The svp-41 ful-2 soc1-2 and svp-41 ft-10 tsf-1 triple mutants flowered significantly later than svp-41 mutants but much earlier than the ful-2 soc1-2 or ft-10 tsf-1 double mutants, respectively (9, 15) (Fig. 1A). Therefore, FUL SOC1 and FT TSF contribute to the early flowering of svp-41 mutants, but these pairs of genes are not responsible for the full early flowering phenotype of svp-41. To test whether this early flowering can be fully explained by all four genes, the quintuple mutant svp-41 ft-10 tsf-1 soc1-2 ful-2 was constructed and its flowering time compared with that of the quadruple mutant ft-10 tsf-1 soc1-2 ful-2. Under inductive LDs the quadruple mutant flowered after forming around 85 leaves, whereas the quintuple mutant flowered after producing around 50 leaves (Fig. 1 A and B). Therefore, the svp-41 mutation causes earlier flowering even in the absence of functional FT TSF SOC1 FUL genes.
Fig. 1.
The svp-41 mutation accelerates flowering in the absence of functional FT TSF SOC1 FUL genes. (A) Leaf number at flowering of plants grown under LD condition. Data are mean ± SD of at least 10 individual plants. (B) Phenotypes of the quadruple ft-10 tsf-1 soc1-2 ful-2 and of the quintuple svp-41 ft-10 tsf-1 soc1-2 mutant plants around 60 d after germination growing under LDs. See also Fig. S1.
SVP Reduces Levels of the GA Growth Regulator by Repressing Transcription of the Gene Encoding the GA-Biosynthetic Enzyme GA20-oxidase 2.
Genome-wide transcriptome analysis was used to identify additional genes regulated by SVP that could contribute to the early flowering of svp-41 ft-10 tsf-1 soc1-2 ful-2 plants. Previously, hybridization of Affymetrix tiling arrays was used to identify genes deregulated in svp-41 mutants compared with wild type (23). Among the genes differentially expressed in svp-41 mutants compared with wild type were several that contribute to the biosynthesis, catabolism, or signaling pathway for the growth regulator GA (Fig. 2A), which promotes flowering of A. thaliana. Expression of genes involved in GA catabolism and signaling was upregulated in svp-41 mutants whereas those contributing to GA biosynthesis were downregulated. A striking exception to this trend was GIBBERELLIN 20-OXIDASE 2 (GA20ox2), which encodes a GA biosynthetic enzyme and showed an increase in mRNA abundance in svp-41 compared with wild type. SVP acts as a transcriptional repressor, and therefore whether it binds directly to the GA20ox2 genomic region in vivo was tested. Mutant svp-41 plants in which the mutation was complemented by a SVP::SVP:GFP were used for ChIP-qPCR. No enrichment of the GA20ox2 locus was detected after ChIP, although positive controls with the known SVP target SEP3 clearly detected binding of SVP:GFP (Fig. S2 A–C). Therefore, SVP reduces the transcription of GA20ox2, but probably does not bind directly to the gene.
Fig. 2.
SVP reduces GA content through the transcriptional repression of GA20ox2. (A) GA-related genes differentially expressed in svp-41 mutant compared with wild-type plants according to the microarray experiments described (23). (B) Phenotype of seedlings of wild-type and svp-41 mutant (Upper) and ga20ox2-1 mutant and svp-41 ga20ox2-1 double mutants (Lower). Bar = 10 mm. (C) Flowering time and (D) chlorophyll content measurement of wild-type, svp-41, and 35S::SVP plants after treatments with GA4 (light bars) or mock (dark bars). All plants in A–D were grown under SDs. n = 10–12. (E) Schematic representation of the non–13-hydroxylated GA-biosynthetic pathway in Arabidopsis (adapted from Yamaguchi, ref. 32) (1)GA2ox7 and -8; (2)GA2ox1, -2, -3, -4, and -6. (F) Concentration of GAs in aerial part of seedlings grown for 2 wk under SDs. The values are the mean ± SEM of three biological replicates (ng/g fresh weight). Letters shared in common between the genotypes indicate no significant difference in GA concentration (pairwise multiple comparison procedures, Student–Newman–Keuls method, P < 0.05). *Two biological replicates. See also Fig. S2 and Table S1.
Increased expression of GA20ox2 mRNA in svp-41 mutants suggested that these plants might contain higher levels of the growth regulator GA than wild-type plants and that this could contribute to the early flowering of svp-41. Consistent with this idea, comparisons of the svp-41 and wild-type plants revealed that the mutants exhibit phenotypes that resemble those of plants over-accumulating GA. For example, in addition to early flowering, svp-41 mutants display a larger rosette radius, lower chlorophyll content, and a longer stem (Fig. 2B and Table S1).
If svp-41 plants are altered in their GA content, then their responses to exogenously applied GA might differ from those of wild-type plants. Treatment of SD-grown wild-type plants with GA4 accelerated flowering and reduced chlorophyll content; by contrast, no significant changes in these phenotypes were observed after application of GA4 to svp-41 mutants (Fig. 2 C and D and Fig. S2D). The insensitivity of svp-41 to exogenous application of GA4 is consistent with svp-41 mutants containing high endogenous levels of the hormone that saturate downstream responses. By contrast, flowering time and chlorophyll content of 35S::SVP plants were hypersensitive to GA4 treatment (Fig. 2 C and D), suggesting that phenotypes associated with high expression of SVP are at least partially due to unusually low levels of GA.
Further support for svp-41 containing increased levels of GA was obtained by direct quantification of GA and by analysis of expression of GA20ox1 (GA5), which is regulated by GA via negative-transcriptional feedback control (30, 31). The microarray data showed that levels of GA20ox1 mRNA were significantly lower in svp-41 mutants than in wild-type plants, consistent with the mutant containing elevated levels of GA (Fig. 2A). To explore this idea further, we quantified the concentration of GA forms belonging to the non–13-hydroxylated pathway that contributes mainly to the biosynthesis of GA4 (Fig. 2E) (32). The levels of the final GA products of this pathway (GA9, GA51, and GA4) were significantly increased in svp-41 and reduced in 35S::SVP compared with wild type (Fig. 2F).
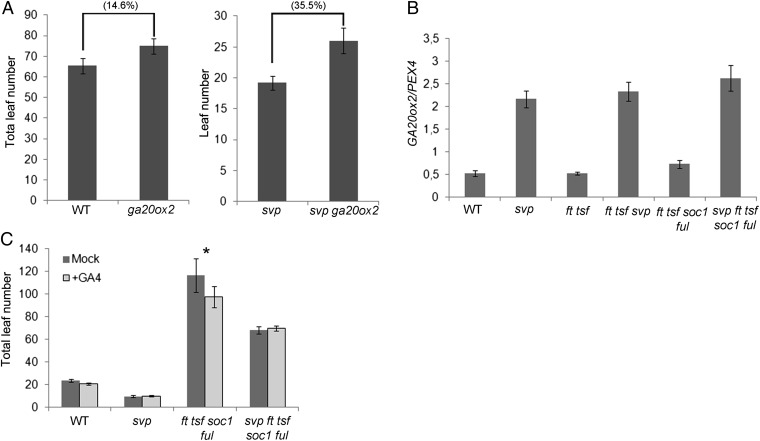
Whether increased expression of GA20ox2 contributes to the over-accumulation of GA and the early flowering phenotype of the svp-41 mutant was then tested. As shown in Fig. 3A and Fig. S2E, the loss-of-function ga20ox2-1 mutant flowered slightly later than wild type (14.6 and 1.9% more leaves under SDs and LDs, respectively); however, when this mutation was introduced into the svp-41 mutant, it strongly delayed flowering (35.5 and 32.5% more leaves under SDs and LDs, respectively). Moreover, the GA over-accumulation phenotypes observed in svp-41, including the leaf radius and chlorophyll content, were largely suppressed in the svp-41 ga20ox2-1 double mutant (Fig. 2B and Table S1). In addition, GA quantification analyses demonstrated that GA20ox2 was the main contributor to the GA9, GA51, and GA4 over-accumulation in the svp-41 mutant because the levels of these forms were strongly reduced in the svp-41 ga20ox2-1 double mutant (Fig. 2E). Therefore, repression of GA20ox2 is an important aspect of the role of SVP in modulating GA biosynthesis and the phenotypes controlled by this pathway, including flowering time.
Fig. 3.
SVP regulates flowering time through transcriptional regulation of GA20ox2. (A) Flowering time of wild-type plants compared with ga20ox2-1 (Left) and svp-41 compared with svp-41 ga20ox2-1 plants (Right) grown under SDs. The numbers in parentheses indicate the differences in flowering time expressed as a percentage. The ANOVA analysis showed that this difference is statistically significant (Holm–Sidak test, P = 0.003). (B) GA20ox2 mRNA levels in 2-wk-old seedlings of ft-10 tsf-1 and soc1-2 ful-2 in the presence or absence of SVP. Wild-type and svp-41 plants were used as controls. Samples were collected 8 h after dawn under SDs. (C) Effect of GA4 treatment on flowering phenotype of svp-41, ft-10 tsf-1 soc1-2 ful-2, and svp-41 ft-10 tsf-1 soc1-2 ful-2 mutants growing under LDs. Treatment was carried out with at least 10 individual plants, and wild type was used as control. The asterisk indicates that there is a statistically significant difference between the treated and untreated ft-10 tsf-1 soc1-2 ful-2 plants (P = 0.007).
The increase in GA20ox2 mRNA was also detected in the svp-41 soc1-2 ful-2 ft-10 tsf-1 quintuple mutant compared with the soc1-2 ful-2 ft-10 tsf-1 quadruple, consistent with it contributing to the earlier flowering phenotype of the quintuple (Fig. 3B). Whether GA20ox2 activity is responsible for all of the residual flowering in the quintuple mutant requires construction of the hextuple mutant svp-41 soc1-2 ful-2 ft-10 tsf-1 ga20ox2, and so far we have not been able to test the flowering time of this genotype. Nevertheless, support for the role of GAs in promoting flowering independently of FT, TSF, SOC1, and FUL was obtained by applying GA4 to the quadruple and quintuple mutants. Strikingly, GA4 treatment accelerated flowering of the quadruple mutant (Fig. 3C), but had no effect on flowering time of the quintuple mutant (Fig. 3C). Taken together, these results suggest that GAs promote flowering by acting either downstream or in parallel to the photoperiodic pathway containing FT, TSF, SOC1, and FUL and that this process is regulated by the floral repressor SVP.
SVP Regulates Flowering and the Expression of GA20ox2 in the SAM.
SVP represses FT and TSF in the leaves and SOC1 and FUL in the SAM. In the absence of FT TSF photoperiodic signals produced in the leaves, the svp-41 mutation still accelerates flowering, and this is associated with an increase of GA20ox2 mRNA. Therefore, SVP might act downstream of FT and TSF to repress GA20ox2 in the SAM. We tested this possibility by quantifying the expression of GA20ox2 mRNA in different plant organs. As shown in Fig. 4A, GA20ox2 mRNA is more abundant in apices than in leaves of wild-type and svp-41 seedlings.
Fig. 4.
SVP controls floral transition and GA20ox2 transcription in the SAM. (A) Levels of GA20ox2 mRNA in apices and leaves of wild-type and svp-41 plants. (B) Effect of the misexpression of SVP in the SAM on flowering time under LDs (Upper) and SDs (Lower). CL: cauline leaves; RL: rosette leaves. (C) Levels of GA20ox2 mRNA in apices of transgenic plants misexpressing SVP compared with WT and svp-41 mutant grown for 2 wk under SDs.
The effect of misexpression of SVP in the SAM on GA20ox2 expression was also tested. A pKNAT1::SVP transgene that drives SVP expression in the shoot meristem was introduced into the svp-41 mutant. The transgenic plants showed a significant delay of flowering under LDs and SDs compared with the svp-41 mutant, indicating that SVP expression in the SAM is sufficient to repress flowering (Fig. 4B and Fig. S3 C and D). In addition, the GA20ox2 mRNA level was lower in apices of these transgenic plants than in apices of svp-41 mutants, confirming that SVP represses the transcription of GA20ox2 in the SAM (Fig. 4C) and that this is associated with delayed flowering. Thus, in wild-type plants SVP represses GA20ox2 expression at the shoot apex. However, when SVP is expressed specifically in leaves by using the phloem-specific promoter pSUC2, it only delays flowering under LDs probably by repressing the transcription of FT and TSF (Fig. S4 A–C).
During Photoperiodic Induction of Flowering, FT Signaling Mediates the Downregulation of SVP and the Induction of GA Biosynthesis.
SVP mRNA levels are reduced in the shoot apical meristem during floral induction (15) and are absent in the inflorescence meristem (33). Our data show that this correlates with increased GA20ox2 mRNA abundance and higher GA levels. To test the dynamics of SVP downregulation, we studied the temporal and spatial expression patterns of SVP mRNA at the SAM of wild-type plants grown in SDs and then transferred to inductive LDs. The SVP mRNA was strongly detected at the meristem of wild-type plants under SDs in agreement with the function of SVP as a repressor of flowering (Fig. 5A). However, after transferring plants to LDs, SVP mRNA decreased from the center of the meristem, and it was detectable only in floral primordia at 5 and 7 LDs, representing a later function of SVP in floral development (34, 35). Thus, during photoperiodic induction LD signals repress activity of the floral repressor SVP in the shoot apical meristem. To test whether this reduction is associated with changes in the levels of GA20ox2 mRNA, quantitative RT-PCR (qRT-PCR) was performed with cDNA extracted from apices of wild-type plants transferred from SDs to LDs. The levels of GA20ox2 mRNA significantly increased at the apex of these plants after exposure to 3, 5, and 7 LDs, consistent with the idea that reduced SVP mRNA level is associated with increased expression of GA20ox2 at the apex (Fig. 5B).
Fig. 5.
Photoperiodic regulation of GA biosynthesis and transcriptional activation of SPLs. (A) Spatial pattern of SVP mRNA detected by in situ hybridization during a time course of ft-10 tsf-1 soc1-2 ful-2 and svp-41 ft-10 tsf-1 soc1-2 ful-2 mutant plants grown for 3 wk in SDs (0 LD) and then transferred to LDs (3, 5, and 7 LDs). A specific probe was used to detect mRNA of SVP at the shoot apex. (B) Temporal expression pattern of GA20ox2 mRNA in apices of wild-type, ft-10 tsf-1, and soc1-2 ful-2 mutant plants grown for 3 wk in SDs (0 LD) and then shifted to LDs (3, 5, and 7 LDs). All samples were harvested 8 h after dawn. (Scale bar: 50 µm.) (C) Histochemical localization of GUS activity at SAM of pGA20ox2::GA20ox2:GUS seedlings harvested at the beginning (8 LDs), during (11 LDs), and after (14 LDs) the transition to flowering. (Scale bar: 100 µm.) (D) Pattern of expression of SPL4 in ft-10 tsf-1 soc1-2 ful-2 and svp-41 ft-10 tsf-1 soc1-2 ful-2 mutant plants grown for 15 (Upper) and 30 LDs (Lower). (Scale bars: 50 µm.) (E) Quantification of the mRNA levels of SPL5 and SPL3 in wild-type, svp-41, ga20ox2-1, and svp-41 ga20ox2-1 seedlings grown for 2 wk under SDs.
To characterize the GA20ox2 spatial expression pattern at the SAM of wild-type plants, GUS staining was performed in pGA20ox2::GA20ox2:GUS plants (36) growing under LDs, and tissue was harvested prior (8 LDs), during (11 LDs), and after (14 LDs) the transition to flowering (Fig. 5C). GUS signal was weakly detected in the center of the SAM of pGA20ox2::GA20ox2:GUS plants 8 LDs after germination (Fig. 5C). However, at 11 LDs, GA20ox2:GUS expression was strongly increased (Fig. 5C) at the base of the SAM in the rib meristem region. After the floral transition, 14 LDs after germination, GUS expression was maintained mainly in the elongating region of the rib meristem (Fig. 5C). Therefore, GA20ox2 expression occurs in a specific area of the SAM and correlates with the switch from vegetative growth to flowering. Furthermore, SVP and GA20ox2 have reverse temporal expression patterns at the SAM during flowering in LDs (Fig. 5 A and B). To assess whether mutation in SVP alters the spatial expression pattern of GA20ox2, the pGA20ox2::GA20ox2:GUS construct was introduced into the svp-41 mutant by crossing. Similar to pGA20ox2::GA20ox2:GUS plants, svp-41 pGA20ox2::GA20ox2:GUS plants showed GUS activity in the rib meristem during the transition to flowering at 12 LDs (Fig. S3A). These experiments suggest that mutation in SVP does not greatly change the spatial pattern of expression of GA20ox2, but it does increase GA20ox2 mRNA levels in the apical region based on the previously described qRT-PCR experiments showing higher levels of GA20ox2 mRNA in several genetic backgrounds containing the svp-41 mutation (Fig. 3B).
In A. thaliana, the photoperiodic response is mediated by increased expression of FT and TSF in the leaf followed by upregulation of SOC1 and FUL in the meristem (28). During floral induction, SOC1 binds directly to the promoters of several floral integrator genes, including SVP (37). Therefore, whether the module SVP/GA20ox2 is controlled by the photoperiod pathway was tested by studying the temporal and spatial expression patterns of SVP in meristems of ft-10 tsf-1 soc1-2 ful-2 mutant plants shifted from SDs to LDs. In contrast to wild-type plants, SVP mRNA was still strongly detectable at the center of the meristem of ft-10 tsf-1 soc1-2 ful-2 plants even after 7 d exposure to LDs, demonstrating that the FT TSF SOC1 FUL pathway is required to repress expression of SVP during LD induction (Fig. 5A). Furthermore, SVP transcript persisted at the meristem of the double mutants soc1-2 ful-2 and ft-10 tsf-1 for at least 7 d after their transfer from SDs to LDs (Fig. S5). In agreement with these results, the levels of GA20ox2 mRNA were significantly reduced in the apex of ft-10 tsf-1 soc1-2 ful-2 plants compared with wild type (Fig. 5B). Continued expression of SVP in the apices of ft-10 tsf-1 likely contributes to the reduction of GA20ox2 mRNA because in apices of svp-41 ft-10 tsf-1 plants GA20ox2 mRNA levels were increased (Fig. S3B). Interestingly, a slight increase of GA20ox2 mRNA was still detected in apices of ft-10 tsf-1 soc1-2 ful-2 plants exposed to LDs (Fig. 5B), indicating that GA20ox2 might also be activated by photoperiod independently of FT, TSF, SOC1, and FUL.
GA20ox2 Is Responsible for the SVP-Mediated Activation of SPL Transcription Factors During Floral Induction.
Depletion of GA and reduction of GA signaling in the shoot apical meristem was previously shown to reduce expression of genes encoding SPL transcription factors during floral induction under LDs (38, 39). In addition, the levels of SPL3, -4, and -5 transcripts are regulated by FT, by TSF, and by the downstream acting genes SOC1 and FUL (3, 9). We used the svp-41 mutation to distinguish the roles of the FT, TSF, SOC1, and FUL pathway and GA biosynthesis in the transcriptional activation of SPL3, SPL4, and SPL5. The spatial and temporal expression pattern of SPL4 was compared by in situ hybridization in shoot apical meristems of svp-41 ft-10 tsf-1 soc1-2 ful-2 and ft-10 tsf-1 soc1-2 ful-2 plants grown under LDs. SPL4 mRNA was strongly detected in the meristem of 30-d-old svp-41 ft-10 tsf-1 soc1-2 ful-2 plants grown continuously under LDs that were undergoing the transition to flowering whereas the meristem of ft-10 tsf-1 soc1-2 ful-2 showed no SPL4 mRNA at the same time (Fig. 5D and Fig. S6B). A similar experiment was carried out in these genotypes transferred from SDs to LDs. No SPL4 expression was detected in either genotype under SDs, but in svp-41 ft-10 tsf-1 soc1-2 ful-2 plants SPL4 mRNA was detected at the base and on the flanks of the shoot apical meristem after exposure to 7 LDs (Fig. S6A) in a similar pattern to 25-d-old svp-41 ft-10 tsf-1 soc1-2 ful-2 grown continuously in LDs (Fig. S6B). By contrast, in the meristem of ft-10 tsf-1 soc1-2 ful-2, no SPL4 mRNA was detectable after similar treatments (Fig. S6 A and B). Thus, the presence of the svp-41 mutation accelerates expression of SPL4 in the absence of FT, TSF, SOC1, and FUL, which could be due to the increased GA levels present in the svp-41 mutant. To test this further, the transcript levels of SPL3 and SPL5 were quantified in apices of svp-41 ga20ox2-1 double mutants and compared with svp-41, ga20ox2-1, and wild type. The transcript levels of SPL3 and SPL5 were higher in svp-41 apices compared with wild-type and ga20ox2-1 (Fig. 5E). By contrast, in apices of svp-41 ga20ox2-1, the abundance of SPL3 and SPL5 mRNA was reduced compared with svp-41 and similar to wild type and ga20ox2-1. Therefore, the increased levels of SPL3 and SPL5 mRNAs in svp-41 mutants are dependent on GA20ox2 activity.
Discussion
In A. thaliana, several genetic pathways determine the timing of floral induction (1). These genetically separable pathways mediate responses to seasonal cues such as day length and winter temperatures as well as to endogenous signals including the growth regulator GA. However, whether and how the environmentally regulated pathways controlling floral transition are linked to those regulating GA metabolism is not clear. Here we show that SVP, a MADS box transcription factor with a central role in flowering-time control in response to day length, vernalization, and ambient temperature represses GA biosynthesis. Mutations in SVP are associated with higher levels of GA4, the main bioactive GA in Arabidopsis, which was previously shown to promote flowering (40). SVP expression reduces transcription of GA20ox2, which encodes a rate-limiting enzyme in synthesis of GA4 (24, 25, 41). In wild-type plants, GA20ox2 expression rises in the meristem in response to LDs that induce flowering, and we show that this is mediated by FT TSF. We propose that, in the early stages of the floral transition in response to LDs, FT TSF mediates the repression of SVP and that this contributes to an increase in GA20ox2 expression and GA biosynthesis in the shoot meristem. Such mechanisms might be broadly conserved in other plant species, as overexpression of an FT gene in wheat was recently shown to increase GA levels (42).
Regulation of GA Biosynthesis by Day Length.
GA contributes to flowering under inductive LDs and noninductive SDs. Under SDs, flowering is delayed and correlates with a gradual increase in bioactive GA at the shoot apex (40). Furthermore, mutations that impair GA biosynthesis prevent flowering under SDs (43). Such observations led to the idea that GA is essential for flowering under SDs, whereas under LDs the requirement for GA is reduced because the photoperiodic flowering pathway acting through CONSTANS (CO) and FT TSF accelerates flowering (43, 44). Nevertheless, genetic analysis also argues for a role for GA in floral induction under LDs. Mutations or transgenic approaches that inactivate the GA receptors, impair GA signaling, or strongly reduce GA biosynthesis delay flowering under LDs (38, 39, 45, 46). GA biosynthesis is also increased by exposure to LDs in rosette species such as A. thaliana or spinach, which is associated with increased expression of GA20ox isoforms and is linked to shoot elongation as well as earlier flowering (47, 48). Similarly, the GIBBERELLIN 3-OXIDASE 1 (GA3OX1) and GA3ox2 genes of A. thaliana are coregulated with FT by the TEMPRANILLO transcription factors (49). Here, we provide a mechanism by which increased GA levels at the shoot apex are coordinated with floral transition under LDs. Our data demonstrate that under LDs the GA and photoperiodic pathways do not simply act in parallel and converge on integrator genes such as SOC1, but that GA biosynthesis is regulated by the photoperiodic pathway at least partially through downregulation of SVP and thus increased expression of GA biosynthetic genes.
We monitored the expression pattern of pGA20ox2::GA20ox2:GUS (36) in the meristem and found that under LDs GA20ox2 expression rises in the region of the rib meristem during floral induction. Attempts to support this pattern using in situ hybridization failed, presumably due to the low level of expression of this gene. The expression domains of SVP and GA20ox2 may overlap during the vegetative phase when the SVP expression domain encompasses a large part of the SAM (Fig. 5A). However, detailed analysis of how much their expression overlaps will require visualizing the patterns of expression of both genes in the same apices during the floral transition, for example, by using fluorescent marker proteins.
This region of the meristem promotes stem elongation (bolting), and floral promoter genes change in expression in this region in Arabidopsis after exposure to LDs (9, 50). This indicates that GA20ox2 expression in this region might have roles in the onset of bolting and floral development and in synchronizing these events during the onset of reproductive development in Arabidopsis (50). Furthermore, the spatial expression pattern of pGA20ox2::GA20ox2:GUS at the resolution tested was not altered in the svp mutant, suggesting that the early flowering caused by increased GA20ox2 mRNA levels in the svp mutant is due to elevated GA20ox2 activity in the rib meristem region. These results are in agreement with previous observations that GA20 oxidases are involved in stem elongation and that mutations in GA20ox2 delay flowering under LDs (24, 48). The flowering-time defect of the ga20ox2-1 mutant under LDs is enhanced by mutations in two other paralogues (36), suggesting that these also contribute to GA biosynthesis under these conditions. Nevertheless, in our experiments only GA20ox2 was negatively regulated by SVP, suggesting that the boost in GA biosynthesis conferred by svp mutations and associated with downregulation of SVP during floral induction acts predominately through this paralogue. The increase in GA20ox2 expression observed in the rib meristem under LDs indicates that GA biosynthesis increases specifically in the meristem after downregulation of SVP. This result contrasts with the gradual increase in GA levels under SDs, which could not be correlated with elevated expression in GA biosynthetic genes, suggesting that under these conditions GA is synthesized in other tissues and transported to the meristem (40). The GA synthesized via GA20ox2 expression in the rib meristem might move locally into other regions of the shoot meristem because GA influences the expression of genes such as LEAFY and SPL9 in more apical regions of the meristem (38, 51). However, it cannot be excluded that non–cell-autonomous factors acting downstream of GA move from the rib meristem into more apical regions.
SVP Mediates Between the Photoperiodic Pathway and GA Regulation.
A progressive decrease in SVP mRNA in wild-type plants shifted from SDs to LDs is accompanied by a complementary increase in GA20ox2 mRNA. The reduction of SVP mRNA requires the activity of the FT, TSF, SOC1, and FUL genes because SVP mRNA strongly accumulates at the meristem of the quadruple mutant ft-10 tsf-1 soc1-2 ful-2 even after several days under LDs. This effect probably occurs mainly at the meristem because mutations of either FT or CO genes did not result in a significant decrease of SVP mRNA level in entire seedlings at early stages of development, as previously shown (17). Therefore, under LDs FT and TSF and their downstream target genes SOC1 and FUL act to repress SVP, which contributes to increases in GA20ox2 mRNA and GA levels at the SAM. FT and TSF might also act independently of SVP repression to increase GA levels.
SOC1 binds directly within an intron of SVP (37) where it might contribute to the repression of SVP during floral induction. On the other hand, SOC1 expression is upregulated in svp-41 mutants (15), and SVP binds directly to the SOC1 promoter (17, 23), indicating that SVP directly represses SOC1. These data demonstrate reciprocal repression of SVP/SOC1, so that SVP represses expression of SOC1 and vice versa. Consistent with this model, SVP and SOC1 show mutually exclusive temporal expression patterns at the shoot apical meristem with SVP being expressed during the vegetative phase whereas SOC1 is activated during the transition to flowering (15). Thus, one possibility is that in the vegetative shoot apex SVP is activated early during development and acts to repress SOC1, whereas during flowering the strong induction of SOC1 by FT TSF overcomes SVP repression and allows SOC1 to repress SVP (37) (Fig. 6). In SD, GAs gradually induce SOC1 expression, which in turn represses SVP transcription, and this could explain the repressive effect of the GA pathway upstream of SVP observed under these conditions (17) (Fig. 6).
Fig. 6.
Proposed model for the activation of GA biosynthesis in the shoot apical meristem during photoperiodic flowering. In plants exposed to LDs, the transcription of FT and TSF is induced in the leaves. The FT protein moves to the SAM (black dashed line) where FD is expressed. The FT FD module is proposed to activate the transcription of downstream floral promoter genes, such as AP1, SOC1, and FUL. SOC1 (and probably also FUL) directly binds to SVP and contributes to its repression. Downregulation of SVP transcription contributes to increased expression of GA20ox2 and higher GA content at the shoot apex. Higher GA levels increase transcription of the SPL genes and release SPL proteins from DELLA repression during photoperiodic flowering.
Influence of GA on Shoot Apical Meristem Activity.
The influence of GA on meristem activity was demonstrated by the finding that homeobox transcription factors involved in meristem identity and maintenance control GA levels. In the shoot meristem, GA levels are reduced by these factors, preventing differentiation and maintaining meristem activity, whereas on the flanks of the meristem where these transcription factors are not expressed, GA levels rise and contribute to organ differentiation (52, 53). In maize, KNOTTED is expressed in the vegetative meristem and binds directly to a gene encoding GA2ox, an enzyme that reduces bioactive GA levels, to activate its expression (53). Similarly, in A. thaliana the SHOOTMERISTEMLESS homeobox transcription factor reduces expression of GA20ox1 in the shoot meristem (52). This led to models in which homeobox transcription factors repress GA levels in the shoot meristem, preventing differentiation and maintaining meristem activity, whereas, on the flanks of the meristem where these transcription factors are not expressed, GA levels rise and contribute to organ differentiation (52, 53). Our data demonstrate that the MADS domain transcription factor SVP also participates in the control of GA by repressing GA20ox2 mRNA levels in the vegetative meristem. It remains to be tested whether the action of the homeobox transcription factors and SVP are related or whether they independently repress GA biosynthesis, perhaps by repressing different GA20ox paralogues.
During floral induction GA levels rise in the meristem, and our data indicate that this is in part due to repression of SVP transcription. It has been shown that the transcription of genes with defined roles in floral transition responds to increasing GA levels (54, 55). Several genes encoding SPL transcription factors, including SPL3, SPL4, SPL5, and SPL9, are activated in response to GA (38, 39). In agreement with these data, the expression of SPL4 is increased in svp-41 mutants (9) even in the absence of FT and TSF or SOC1 and FUL, supporting the idea that SVP acts downstream of the photoperiod pathway to regulate GA levels and therefore SPL gene transcription. The primary mechanism by which GA acts to regulate transcription is likely to be by promoting DELLA protein degradation and thereby releasing transcription factors to regulate transcription of their target genes (56, 57). SPL transcription factors are also targets of GA regulation at this posttranslational level (58). Thus, SPL transcription factors may be targets for activation by GA at different levels of regulation, and these in turn are direct activators of FUL and LFY (3, 59), perhaps providing one mechanism by which LFY, a floral meristem identity gene, is activated by GA (55).
Materials and Methods
Growth Conditions and Plant Materials.
For all studies A. thaliana (L.) ecotype Columbia (Col-0) was used as wild type. Plants were grown on soil under controlled conditions of LDs (16 h light/8 h dark) and SDs (8 h light/16 h dark) at 20 °C. The level of photosynthetic active radiation was 150 µmol⋅m−2⋅s−1 under both conditions. The svp-41 mutant and the 35S::SVP transgenic plants were previously described (14); the double ft-10 tsf-1 and triple ft-10 tsf-1 svp-41 mutants were described (15) as was the double mutant soc1-2 ful-2 (9). These plants were crossed to generate the quadruple ft-10 tsf-1 soc1-2 ful-2 and the quintuple ft-10 tsf-1 soc1-2 ful-2 svp-41 mutants. The GA biosynthetic mutants ga20ox2-1 and ga20ox1-3 were reported before (24) as well as the GA20OX2::GA20OX2:GUS lines (36). The SVP::SVP:GFP svp-41 transgenic line used for ChIP experiments (SI Materials and Methods) has been previously described (60).
GA Treatment.
The GA4 stock (Sigma) was prepared in 100% ethanol with final concentration of 1 mM. GA treatments were performed by spraying 10–12 plants with either a GA solution (GA4 10 µM, Silwet 77 0.02%) or a mock solution (ethanol 1%, Silwet 77 0.02%).
Quantification of Gibberellins.
About 100–200 mg (fresh weight) of frozen material were used to extract and purify the GAs, as described (61). Separated GAs were analyzed by electrospray ionization and targeted selected ion monitoring using a Q-Exactive spectrometer (Orbitrap detector; ThermoFisher Scientific). The [17,17-2H]GAs were added to the extracts as internal standards for quantification, and the concentrations of GAs were determined using embedded calibration curves and the Xcalibur program 2.2 SP1 build 48. The full description of these methods can be found as SI Materials and Methods.
Flowering-Time Analysis.
Flowering time was determined by counting the number of cauline and rosette leaves of at least 10 individual plants.
In Situ Hybridization and GUS Staining.
In situ hybridization was performed according to the method already described (38, 62). Probes used were the following: SPL3 (3, 63), SVP (9), and SPL4 (38). GUS staining was performed as described (64).
Plasmid Construction, Plant Transformation, and Transformant Selection.
Full-length SVP cDNAs were amplified by PCR and used to generate an entry clone via BP reaction (Invitrogen). The entry clones were subcloned via the LR reaction into the binary vector pKNAT1::GW or pSUC2::GW (65) to generate pKNAT1::SVP svp-41 and pSUC2::SVP svp-41, respectively. The plasmids were then introduced into Agrobacterium strain GV3101 (pMP90RK) to transform svp-41 mutant plants by floral dip (66).
Determination of Chlorophyll Concentration, Leaf Radius, and Stem Length.
Chlorophyll concentration was estimated by using the SPAD-502 leaf chlorophyll meter (67). Leaf radius and stem length were determined manually by using a ruler.
RNA Extraction and Quantitative Real-Time PCR.
Total RNA was isolated from plant tissues by using RNAeasy extraction kit (Qiagen) and treated with DNA-free DNase (Ambion) to remove residual genomic DNA. One microgram of total RNA was used for reverse transcription (Superscript III, Invitrogen). Transcript levels were quantified by quantitative PCR in a LightCycler 480 instrument (Roche) using the PEX4 gene (AT5G25760) as a standard. The sequences of the primers to quantify de-expression of SVP, SOC1, FUL, and SVP are described in Torti et al. (9) and the ones for SPL3, SPL4, and GA20OX1 are described in Porri et al. (38).
Statistical Analysis.
All of the statistical analyses were performed by using SigmaStat 3.5 software.
Supplementary Material
Acknowledgments
We thank Peter Huijser (Max Planck Institute for Plant Breeding Research) and Peter Hedden (Rothamsted Research Centre) for generously providing materials and Amaury de Montaigu, René Richter, Maarten Koornneef, and Luis Barboza for comments on the manuscript. This work was financially supported by the Deutsche Forschung Gemeinschaft through the European Research Area–Net Plant Genomics Programme and Cluster of Excellence in Plant Science, the European Union via the SYSFLO Training Network (project code 237909), a Marie Curie Intra-European Fellowship for Career Development (Project Intra-European Grant Agreement-2009-251839) (to F.A.), a von Humboldt postdoctoral fellowship (to J.M.), and the Max Planck Society through a core grant (to G.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409567111/-/DCSupplemental.
References
- 1.Andrés F, Coupland G. The genetic basis of flowering responses to seasonal cues. Nat Rev Genet. 2012;13(9):627–639. doi: 10.1038/nrg3291. [DOI] [PubMed] [Google Scholar]
- 2.Mutasa-Göttgens E, Hedden P. Gibberellin as a factor in floral regulatory networks. J Exp Bot. 2009;60(7):1979–1989. doi: 10.1093/jxb/erp040. [DOI] [PubMed] [Google Scholar]
- 3.Wang JW, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138(4):738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286(5446):1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 5.Kardailsky I, et al. Activation tagging of the floral inducer FT. Science. 1999;286(5446):1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 6.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316(5827):1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17(12):1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17(12):1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Torti S, et al. Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell. 2012;24(2):444–462. doi: 10.1105/tpc.111.092791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid M, et al. Dissection of floral induction pathways using global expression analysis. Development. 2003;130(24):6001–6012. doi: 10.1242/dev.00842. [DOI] [PubMed] [Google Scholar]
- 11.Samach A, et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288(5471):1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- 12.Borner R, et al. A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 2000;24(5):591–599. doi: 10.1046/j.1365-313x.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, et al. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14(18):2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann U, et al. Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 2000;21(4):351–360. doi: 10.1046/j.1365-313x.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang S, Torti S, Coupland G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009;60(4):614–625. doi: 10.1111/j.1365-313X.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, et al. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007;21(4):397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, et al. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell. 2008;15(1):110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara S, et al. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell. 2008;20(11):2960–2971. doi: 10.1105/tpc.108.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, et al. Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science. 2013;342(6158):628–632. doi: 10.1126/science.1241097. [DOI] [PubMed] [Google Scholar]
- 20.Posé D, et al. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature. 2013;503(7476):414–417. doi: 10.1038/nature12633. [DOI] [PubMed] [Google Scholar]
- 21.Méndez-Vigo B, Martínez-Zapater JM, Alonso-Blanco C. The flowering repressor SVP underlies a novel Arabidopsis thaliana QTL interacting with the genetic background. PLoS Genet. 2013;9(1):e1003289. doi: 10.1371/journal.pgen.1003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao Z, et al. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J. 2012;70(4):549–561. doi: 10.1111/j.1365-313X.2012.04919.x. [DOI] [PubMed] [Google Scholar]
- 23.Gregis V, et al. Identification of pathways directly regulated by SHORT VEGETATIVE PHASE during vegetative and reproductive development in Arabidopsis. Genome Biol. 2013;14(6):R56. doi: 10.1186/gb-2013-14-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieu I, et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008;53(3):488–504. doi: 10.1111/j.1365-313X.2007.03356.x. [DOI] [PubMed] [Google Scholar]
- 25.Coles JP, et al. Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J. 1999;17(5):547–556. doi: 10.1046/j.1365-313x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, et al. Overexpression of 20-oxidase confers a gibberellin-overproduction phenotype in Arabidopsis. Plant Physiol. 1998;118(3):773–781. doi: 10.1104/pp.118.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornara F, de Montaigu A, Coupland G. SnapShot: Control of flowering in Arabidopsis. Cell. 2010;141(3) doi: 10.1016/j.cell.2010.04.024. 550, e1–e2. [DOI] [PubMed] [Google Scholar]
- 28.Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- 29.Melzer S, et al. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet. 2008;40(12):1489–1492. doi: 10.1038/ng.253. [DOI] [PubMed] [Google Scholar]
- 30.Phillips AL, et al. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108(3):1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu YL, et al. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: Molecular cloning and functional expression. Proc Natl Acad Sci USA. 1995;92(14):6640–6644. doi: 10.1073/pnas.92.14.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, et al. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development. 2007;134(10):1901–1910. doi: 10.1242/dev.003103. [DOI] [PubMed] [Google Scholar]
- 34.Gregis V, Sessa A, Colombo L, Kater MM. AGAMOUS-LIKE24 and SHORT VEGETATIVE PHASE determine floral meristem identity in Arabidopsis. Plant J. 2008;56(6):891–902. doi: 10.1111/j.1365-313X.2008.03648.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Xi W, Shen L, Tan C, Yu H. Regulation of floral patterning by flowering time genes. Dev Cell. 2009;16(5):711–722. doi: 10.1016/j.devcel.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Plackett AR, et al. Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell. 2012;24(3):941–960. doi: 10.1105/tpc.111.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immink RG, et al. Characterization of SOC1’s central role in flowering by the identification of its upstream and downstream regulators. Plant Physiol. 2012;160(1):433–449. doi: 10.1104/pp.112.202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porri A, Torti S, Romera-Branchat M, Coupland G. Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods. Development. 2012;139(12):2198–2209. doi: 10.1242/dev.077164. [DOI] [PubMed] [Google Scholar]
- 39.Galvão VC, Horrer D, Küttner F, Schmid M. Spatial control of flowering by DELLA proteins in Arabidopsis thaliana. Development. 2012;139(21):4072–4082. doi: 10.1242/dev.080879. [DOI] [PubMed] [Google Scholar]
- 40.Eriksson S, Böhlenius H, Moritz T, Nilsson O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell. 2006;18(9):2172–2181. doi: 10.1105/tpc.106.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedden P, Phillips AL. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000;5(12):523–530. doi: 10.1016/s1360-1385(00)01790-8. [DOI] [PubMed] [Google Scholar]
- 42.Pearce S, Vanzetti LS, Dubcovsky J. Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION1. Plant Physiol. 2013;163(3):1433–1445. doi: 10.1104/pp.113.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992;100(1):403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves PH, Coupland G. Analysis of flowering time control in Arabidopsis by comparison of double and triple mutants. Plant Physiol. 2001;126(3):1085–1091. doi: 10.1104/pp.126.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willige BC, et al. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell. 2007;19(4):1209–1220. doi: 10.1105/tpc.107.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffiths J, et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18(12):3399–3414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DJ, Zeevaart JA. Regulation of gibberellin 20-oxidase1 expression in spinach by photoperiod. Planta. 2007;226(1):35–44. doi: 10.1007/s00425-006-0463-1. [DOI] [PubMed] [Google Scholar]
- 48.Xu YL, Gage DA, Zeevaart JAD. Gibberellins and stem growth in Arabidopsis thaliana. Effects of photoperiod on expression of the GA4 and GA5 loci. Plant Physiol. 1997;114(4):1471–1476. doi: 10.1104/pp.114.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osnato M, Castillejo C, Matías-Hernández L, Pelaz S. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat Commun. 2012;3:808. doi: 10.1038/ncomms1810. [DOI] [PubMed] [Google Scholar]
- 50.Jacqmard A, Gadisseur I, Bernier G. Cell division and morphological changes in the shoot apex of Arabidopsis thaliana during floral transition. Ann Bot (Lond) 2003;91(5):571–576. doi: 10.1093/aob/mcg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blázquez MA, Weigel D. Integration of floral inductive signals in Arabidopsis. Nature. 2000;404(6780):889–892. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- 52.Hay A, et al. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol. 2002;12(18):1557–1565. doi: 10.1016/s0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 53.Bolduc N, Hake S. The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell. 2009;21(6):1647–1658. doi: 10.1105/tpc.109.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon J, et al. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003;35(5):613–623. doi: 10.1046/j.1365-313x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- 55.Blazquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell. 1998;10(5):791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Lucas M, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451(7177):480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 57.Feng S, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451(7177):475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu S, et al. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA promoter binding-like transcription factors. Plant Cell. 2012;24(8):3320–3332. doi: 10.1105/tpc.112.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi A, et al. The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell. 2009;17(2):268–278. doi: 10.1016/j.devcel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gregis V, Sessa A, Dorca-Fornell C, Kater MM. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 2009;60(4):626–637. doi: 10.1111/j.1365-313X.2009.03985.x. [DOI] [PubMed] [Google Scholar]
- 61.Seo M, Jikumaru Y, Kamiya Y. Profiling of hormones and related metabolites in seed dormancy and germination studies. Methods Mol Biol. 2011;773:99–111. doi: 10.1007/978-1-61779-231-1_7. [DOI] [PubMed] [Google Scholar]
- 62.Bradley D, Carpenter R, Sommer H, Hartley N, Coen E. Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell. 1993;72(1):85–95. doi: 10.1016/0092-8674(93)90052-r. [DOI] [PubMed] [Google Scholar]
- 63.Wu G, et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138(4):750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adrian J, et al. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell. 2010;22(5):1425–1440. doi: 10.1105/tpc.110.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.An H, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131(15):3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 66.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 67.Markwell J, Osterman JC, Mitchell JL. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res. 1995;46(3):467–472. doi: 10.1007/BF00032301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.