Significance
The ω-3 long-chain polyunsaturated fatty acids are a class of dietary lipids that are highly enriched in the central nervous system and the retina. We demonstrate that dietary enrichment with ω-3s suppresses choroidal neovascularization in a mouse model of age-related macular degeneration (AMD), a leading cause of blindness. The ω-3s have anti-inflammatory properties and compete with ω-6s for downstream lipid metabolite synthesis at the cytochrome P450 (CYP) level. Specifically, 17,18- epoxyeicosatetraenoic acid and 19,20- epoxydocosapentaenoic acid derived from the CYP pathway were identified by liquid chromatography-tandem MS and found to confer protection. Systemic immune-cell recruitment and adhesion-molecule regulation were dampened significantly in mice receiving ω-3s. These findings provide a unique mechanism whereby specific CYP-derived lipid metabolites regulate angiogenesis in a mouse model of AMD.
Keywords: choroidal neovascularization, immune cell recruitment, PPARγ, adhesion molecules, epoxy-metabolites
Abstract
Ocular neovascularization, including age-related macular degeneration (AMD), is a primary cause of blindness in individuals of industrialized countries. With a projected increase in the prevalence of these blinding neovascular diseases, there is an urgent need for new pharmacological interventions for their treatment or prevention. Increasing evidence has implicated eicosanoid-like metabolites of long-chain polyunsaturated fatty acids (LCPUFAs) in the regulation of neovascular disease. In particular, metabolites generated by the cytochrome P450 (CYP)–epoxygenase pathway have been shown to be potent modulators of angiogenesis, making this pathway a reasonable previously unidentified target for intervention in neovascular ocular disease. Here we show that dietary supplementation with ω-3 LCPUFAs promotes regression of choroidal neovessels in a well-characterized mouse model of neovascular AMD. Leukocyte recruitment and adhesion molecule expression in choroidal neovascular lesions were down-regulated in mice fed ω-3 LCPUFAs. The serum of these mice showed increased levels of anti-inflammatory eicosanoids derived from eicosapentaenoic acid and docosahexaenoic acid. 17,18-epoxyeicosatetraenoic acid and 19,20-epoxydocosapentaenoic acid, the major CYP-generated metabolites of these primary ω-3 LCPUFAs, were identified as key lipid mediators of disease resolution. We conclude that CYP-derived bioactive lipid metabolites from ω-3 LCPUFAs are potent inhibitors of intraocular neovascular disease and show promising therapeutic potential for resolution of neovascular AMD.
Angiogenesis plays a central role in many diseases, including age-related macular degeneration (AMD), a leading cause of blindness. Advanced AMD exists in two forms, “atrophic” and “neovascular,” which are defined by the absence or presence of choroidal neovascularization (CNV), respectively (1). Neovascular AMD is characterized by the formation of abnormal blood vessels that grow from the choroidal vasculature, through breaks in Bruch’s membrane, toward the outer retina (1). These vessels generally are immature in nature and leak fluid below or within the retina (2). Although growth factors are thought to play an important role in the late stage of neovascular AMD progression, they likely do not contribute to the underlying cause of the disease. The current standard of care for individuals with neovascular AMD is based on the targeting of VEGF, which promotes both angiogenesis and vascular permeability (3). However, although VEGF-targeted therapy attenuates angiogenesis and vascular permeability, it does not lead to complete vascular regression or disease resolution (3).
The ω-3 and ω-6 long-chain polyunsaturated fatty acids (LCPUFAs) are two classes of dietary lipids that are essential fatty acids and have opposing physiological effects. The ω-6 LCPUFA, arachidonic acid (AA), and its cytochrome P450 (CYP)-generated metabolites (epoxyeicosatrienoic acids, EETs) recently have attracted much attention as a result of increasing evidence that they play a role in cancer as well as in cardiovascular disease (4–9). EETs are part of the VEGF-activated signaling cascade leading to angiogenesis (10) and promote tumor growth and metastasis (11). The major dietary ω-3 LCPUFAs are docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are highly enriched in the central nervous system including the retina (12). The ω-3 LCPUFAs have antithrombotic, antiangiogenic, and anti-inflammatory properties, and they compete with ω-6 LCPUFAs as substrates for synthesis of downstream metabolites by CYP enzymes, cyclooxygenases (COX), and lipoxygenases (LOX) (6, 13–15). Moreover, dietary enrichment with ω-3 LCPUFAs has been shown to protect against pathological angiogenesis-associated cancer and retinopathy (2, 16–19). Of the three main pathways (COX, LOX, and CYP) involved in eicosanoid biosynthesis, the lipid mediators derived from the CYP branch are the most susceptible to changes in dietary fatty acid composition (20–23). The ω-3 double bond that distinguishes DHA and EPA from their ω-6 counterparts provides a preferred epoxidation site for specific CYP family members (20, 22). In fact, most CYP isoforms can metabolize EPA and DHA with significantly higher catalytic efficiency than AA, making them uniquely susceptible to variations in the availability of these lipids (19–22). CYP epoxygenases target the ω-3 double bond, resulting in an accumulation of 17,18-epoxyeicosatetraenoic acid (17,18-EEQ) derived from EPA and 19,20-epoxydocosapentaenoic acid (19,20-EDP) from DHA (20, 22). Very recently, it was recognized that19,20-EDP inhibits angiogenesis, tumor growth, and metastasis (24). Thus, it appears that the CYP–epoxygenase pathway has the capacity to produce proangiogenic metabolites from ω-6 LCPUFAs (10, 11) and antiangiogenic metabolites from ω-3 LCPUFAs (24). This unique feature of the CYP enzymes may provide a previously unidentified mechanistic link between the ω-6/ω-3 ratio of dietary LCPUFAs and pathological angiogenesis; however, their roles in ocular angiogenesis have been largely unexplored to date.
We now show that dietary enrichment with ω-3 LCPUFAs suppresses CNV, vascular leakage, and immune cell recruitment to the lesion site in a mouse model of laser-induced CNV. We characterized the CYP-dependent pathway by which dietary ω-3 LCPUFAs promote resolution of choroidal neovessels in this model and identified CYP-generated metabolites 17,18-EEQ and 19,20-EDP as mediators of disease resolution. Furthermore, we show that expression of adhesion molecules at the CNV site was down-regulated in association with inhibition of leukocyte recruitment in mice receiving ω-3 LCPUFAs.
Results
Dietary Intake of ω-3 LCPUFAs Promotes CNV Resolution.
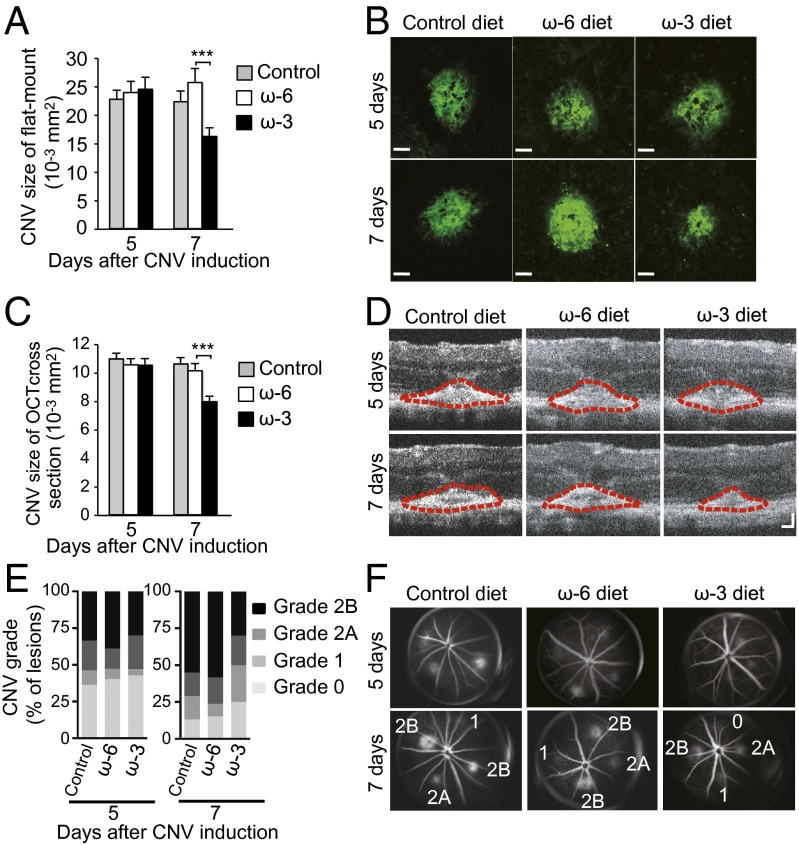
To evaluate the effect of LCPUFAs on CNV development, we fed mice one of three diets beginning 2 wk before CNV induction by laser photocoagulation. The experimental diets are enriched with either ω-3 or ω-6 LCPUFAs; the control diet is devoid of the primary ω-3 or ω-6 LCPUFAs. Examination of CNV lesions in choroidal flat-mount preparations revealed that lesion size at 5 d after photocoagulation [previously shown to be the time of peak size and severity (25)] did not differ significantly between mice fed ω-3 or ω-6 LCPUFAs, but lesions at 7 d were significantly smaller in animals fed ω-3 LCPUFAs (Fig. 1 A and B), indicating that ω-3 LCPUFAs promote disease resolution in the laser-induced AMD model. Lesion size at both 5 d and 7 d after CNV induction did not differ between mice fed the control or the ω-6 LCPUFA diet.
Fig. 1.
Dietary intake of ω-3 LCPUFAs attenuates CNV. (A) Lesion size at 5 and 7 d after CNV induction, as assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4, for mice fed a diet enriched in ω-6 LCPUFAs (n = 61 and 69 lesions, respectively), ω-3 (n = 63 and 65 lesions, respectively) LCPUFAs, or a control diet (n = 73 and 72 lesions, respectively) beginning 2 wk before laser photocoagulation. Data are presented as means ± SEM. ***P < 0.001. (B) Representative staining of CNV lesions quantified in A. (Scale bars: 50 μm.) (C) Cross-sectional area of lesions quantified by SD-OCT at 5 and 7 d after CNV induction in mice fed an ω-6 LCPUFA diet (n = 60 and 53 lesions, respectively), an ω-3 LCPUFA diet (n = 64 and 55 lesions, respectively), or a control diet (n = 69 and 63 lesions, respectively). Data are presented as means ± SEM. ***P < 0.001. (D) Representative SD-OCT images of CNV lesions (demarcated by red dashed lines) quantified in C. (Scale bars: 50 μm.) (E) Quantification of lesion grade at 5 and 7 d after CNV induction in mice fed an ω-6 LCPUFA diet (n = 72 lesions, respectively), an ω-3 LCPUFA diet (n = 96 and 80 lesions, respectively), or a control diet (n = 80 and 76 lesions, respectively). (F) Representative lesions that were graded on an ordinal scale based on the spatial and temporal evolution of fluorescein leakage. Representative fluorescein angiographic images of lesions quantified in E.
Spectral domain-optical coherence tomography (SD-OCT) allows detailed and noninvasive evaluation of the retinal architecture in vivo, and its findings have been found to reflect changes in retinal morphology during AMD progression (26). Therefore we applied SD-OCT to determine the cross-sectional area of CNV lesions in mice fed the control, ω-3 LCPUFA, or ω-6 LCPUFA diets. Although lesion size did not differ among mice of the three groups at 5 d after CNV induction, lesions at 7 d were significantly smaller in mice fed ω-3 LCPUFAs than in those fed the ω-6 LCPUFA diet (Fig. 1 C and D), further supporting a role for ω-3 LCPUFAs in the resolution of CNV lesions. Similarly, lesion size in mice fed the control diet was similar to that in animals on a ω-6 LCPUFA diet at both 5 d and 7 d after CNV induction, indicating that the protection observed is conferred by the ω-3 LCPUFAs.
Vascular leakage in eyes with neovascular AMD may lead to detachment of the retinal pigment epithelium (RPE) as well as subretinal or intraretinal edema that can give rise to disturbance or loss of vision. We assessed the effect of dietary intake of ω-3 LCPUFAs on vascular leakage in CNV lesions by fluorescein angiography. The extent of leakage from the pathological choroidal vessels at both 5 and 7 d after CNV induction was less pronounced in mice fed ω-3 LCPUFAs than in those fed the ω-6 LCPUFA diet (Fig. 1 E and F). The incidence of clinically significant (grade 2B) CNV lesions at 7 d after photocoagulation was only 30% in mice receiving ω-3 LCPUFAs versus 58% for those fed the ω-6 LCPUFA diet. No change in vascular leakage was observed in mice on the control diet and their ω-6 LCPUFA–fed counterparts.
To gain mechanistic insight into the effect of dietary ω-3 LCPUFAs on CNV regression, we analyzed the lipid profiles of both the retina and serum in mice on either diet at 7 d after CNV induction. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed that the concentrations of the principal ω-3 LCPUFAs (EPA and DHA) and of total ω-3 LCPUFAs as well as the DHA/ω-6 docosapentaenoic acid (DPA) ratio were increased significantly and the ω-6/ω-3 LCPUFA ratio was decreased significantly in serum of mice fed the ω-3 LCPUFA diet as compared with those fed the ω-6 LCPUFA diet (Table S1). The EPA level and the DHA/ω-6 DPA ratio also were increased significantly, and the amounts of AA and ω-6 DPA were reduced, in the retina of mice fed the ω-3 LCPUFA diet. These results suggested that dietary intake of primary ω-3 LCPUFAs alone is able to alter the serum lipid profile substantially, but such effects may be buffered in tissues enriched in these fatty acids such as the retina.
Identification of CYP Metabolites Affected by Dietary Intake of ω-3 or ω-6 LCPUFAs.
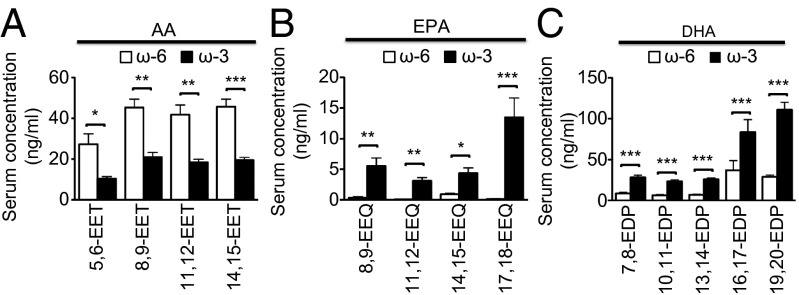
We used LC-MS/MS to measure the amounts of endogenous CYP-generated metabolites in the serum and retina 7 d after CNV induction in mice fed either ω-3 or ω-6 LCPUFAs. The serum levels of AA-derived EETs (5,6-, 8,9-, 11,12-, and 14,15-EET) were reduced significantly (Fig. 2A), whereas those of EPA-derived EEQs (8,9-, 11,12-, 14,15-, and 17,18-EEQ) and DHA-derived EDPs (7,8-, 10,11-, 13,14-, 16,17-, and 19,20-EDP) were increased significantly (Fig. 2 B and C, respectively) in mice fed ω-3 LCPUFA. In contrast to serum, the retinal levels of AA- and DHA-derived epoxyeicosanoid epoxides did not differ between mice fed ω-3 or ω-6 LCPUFAs; only the levels of EPA-derived EEQs were increased significantly in mice fed the ω-3 LCPUFA diet (Fig. S1 A–C). These results suggest that, in mice on a short-term dietary regimen, circulating CYP metabolites might contribute to CNV resolution to a greater extent than CYP metabolites in the retina. A large family of CYP isoforms is known to modulate eicosanoid metabolism. We assessed several of these isoforms for their presence in the retina in mice with CNV. We were able to detect Cyp2c44, Cyp4a12a, Cyp4a12b, and Cyp2j6 but not Cypc29 or Cyp2j5 mRNA at 7 d after CNV induction in mice fed either ω-3 or ω-6 LCPUFAs (Fig. S1 D–I). Expression of Cyp2c44 was increased significantly, whereas that of Cyp2j6 was reduced significantly in mice fed ω-3 LCPUFA. In contrast, expression of Cyp4a12a and Cyp4a12b did not differ between mice fed ω-3 or ω-6 LCPUFAs.
Fig. 2.
Effects of dietary intake of ω-3 and ω-6 LCPUFAs on the serum profile of CYP monoepoxides. CYP bioactive lipid metabolite profiles derived from AA (A), EPA (B), and DHA (C) were determined for serum isolated at 7 d after CNV induction in mice fed a diet enriched in ω-6 or ω-3 LCPUFAs (n = 8). Data are presented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
CYP-Generated Metabolites of ω-3 LCPUFAs Reduce the Size of CNV Lesions.
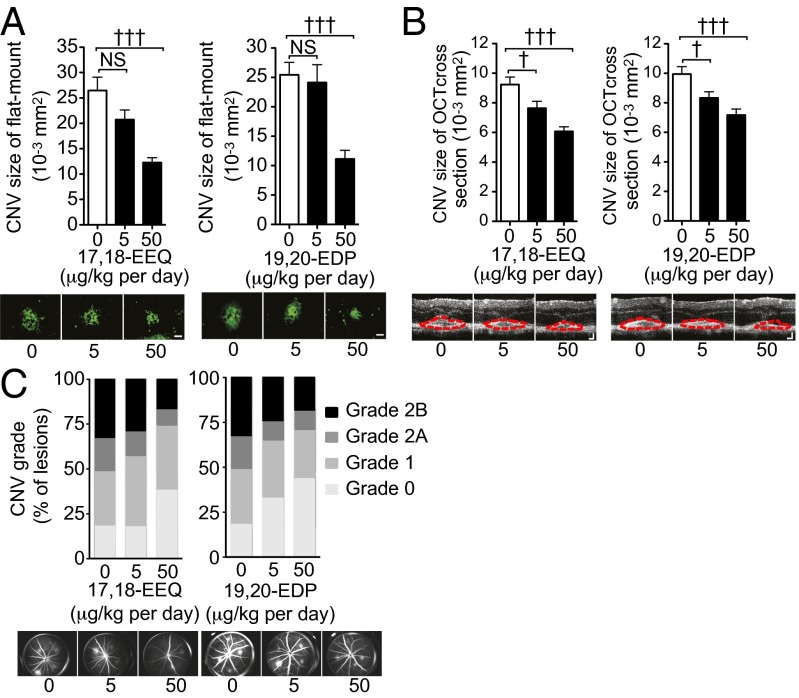
To assess the role of CYP-generated metabolites of ω-3 LCPUFAs in CNV resolution, we examined the effects of 17,18-EEQ and 19,20-EDP in the laser-induced AMD model. We administered exogenous 17,18-EEQ or 19,20-EDP at doses corresponding to the serum levels achieved in mice fed the ω-3 LCPUFA diet. The i.p. injection of 17,18-EEQ conferred significant and dose-dependent protection from laser-induced CNV, as assessed both in choroidal flat-mount preparations (Fig. 3A, Left and Fig. S2A) and by SD-OCT (Fig. 3B, Left and Fig. S2B). In addition, 17,18-EEQ significantly reduced the extent of vascular leakage apparent after CNV induction (Fig. 3C, Left and Fig. S2C). The i.p. administration of 19,20-EDP conferred similar protection against both CNV development and vascular leakage (Fig. 3 A–C, Right and Fig. S2 D–F). These data suggested that CYP-generated metabolites derived from ω-3 LCPUFAs promote laser-induced CNV disease resolution.
Fig. 3.
Metabolites generated by CYP from ω-3 LCPUFAs suppress laser-induced CNV. (A) (Upper) Lesion size as determined by staining of choroidal flat-mount preparations with fluorescent isolectin B4 at 7 d after CNV induction for mice fed a normal diet and injected i.p. with vehicle, 17,18-EEQ at 5 or 50 μg−1⋅kg−1⋅d, or 19,20-EDP at 5 or 50 μg−1⋅kg−1⋅d beginning immediately after photocoagulation. Data are presented as means ± SEM (n = 25–30 lesions). †††P < 0.001 versus the corresponding value for mice injected with vehicle; NS, not significant. (Lower) Representative staining of CNV lesions. (Scale bars: 50 μm.) (B) (Upper) Cross-sectional area of lesions quantified by SD-OCT at 7 d after CNV induction in mice fed a normal diet and injected with vehicle, 17,18-EEQ, or 19,20-EDP. Data are presented as means ± SEM (n = 50–58 lesions). †P < 0.05, †††P < 0.001 versus the corresponding value for mice injected with vehicle. (Lower) Representative SD-OCT images of CNV lesions. (Scale bars: 50 μm.) (C) (Upper) Fluorescein angiography of lesions (n = 64–76) at 7 d after CNV induction in mice fed a normal diet and injected with vehicle, 17,18-EEQ, or 19,20-EDP. (Lower) Representative fluorescein angiographic images.
Peroxisome Proliferator-Activated Receptor-Gamma Expression and Activity Are Increased in ω-3 LCPUFA-Fed Mice.
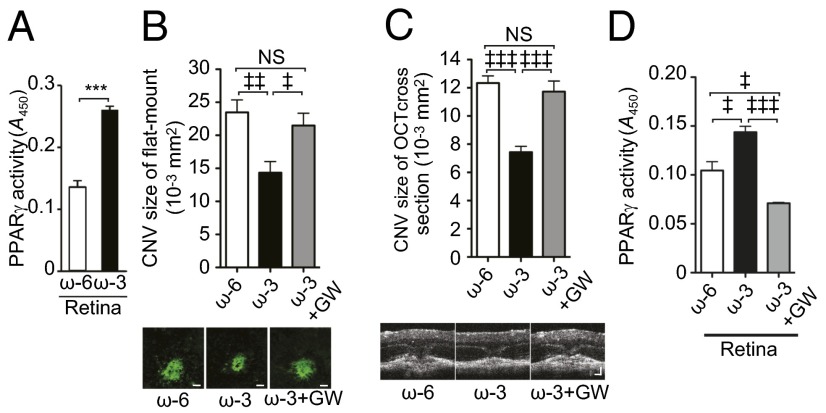
To decipher further the mechanism by which dietary ω-3 LCPUFAs promote CNV resolution, we investigated the possible role of peroxisome proliferator-activated receptor-gamma (PPARγ) (27). The ω-3 LCPUFAs previously were shown to attenuate retinal neovascularization via a mechanism involving the activation of PPARγ, which serves as an endogenous ω-3 LCPUFA receptor (18). Real-time PCR analysis showed that the amount of Pparγ mRNA in the choroid at 7 d after CNV induction was increased significantly in mice fed ω-3 LCPUFAs compared with those fed an ω-6 LCPUFA diet (Fig. S3A), whereas immunoblot analysis revealed that the abundance of Pparγ protein was increased significantly in both the retina and choroid of the animals fed ω-3 LCPUFAs (Fig. S3 B and C). The transactivation activity of Pparγ in retinal nuclear extracts also was significantly higher for mice fed ω-3 LCPUFAs (Fig. 4A). These findings suggest that dietary intake of ω-3 LCPUFAs promotes Pparγ signaling and that such signaling might contribute to the resolution of laser-induced CNV. To assess the role of Pparγ signaling in CNV regression in mice fed the ω-3 LCPUFA diet, we examined the effect of the potent PPARγ inhibitor GW9662 (18). Daily i.p. injections of this agent beginning immediately after CNV induction resulted in significant attenuation of the protective effect of ω-3 LCPUFAs on CNV size (Fig. 4 B and C). We confirmed that GW9662 also significantly reduced the activity of Pparγ in the retina (Fig. 4D). These results suggest that Pparγ plays a key role in CNV regression induced by dietary ω-3 LCPUFAs.
Fig. 4.
Dietary ω-3 LCPUFAs up-regulate PPARγ signaling and attenuate laser-induced CNV in a PPARγ-dependent manner. (A) PPARγ activity as determined with an ELISA in nuclear extracts of the retina at 7 d after CNV induction in mice fed ω-6 or ω-3 LCPUFAs. Data are expressed as means ± SEM of absorbance at 450 nm (A450) (n = 6). ***P < 0.001. (B) (Upper) Lesion size as determined in choroidal flat-mount preparations at 7 d after CNV induction for mice fed ω-3 LCPUFAs, mice fed ω-6 LCPUFAs, or mice fed ω-3 LCPUFAs injected i.p. with the PPARγ inhibitor GW9662 (GW, 1 mg/kg) daily beginning immediately after photocoagulation. Data are presented as means ± SEM (n = 50–73 lesions). ‡P < 0.05, ‡‡P < 0.01. (Lower) Representative staining of CNV. (Scale bar: 50 μm.) (C) (Upper) Cross-sectional area of lesions as determined by SD-OCT for mice treated as in B. Data are presented as means ± SEM (n = 36–69 lesions). ‡‡‡P < 0.001. (Lower) Representative images. (Scale bars: 50 μm.) (D) PPARγ activity in nuclear extracts of the retina for mice treated as in B. Data are presented as means ± SEM (n = 6). ‡P < 0.05, ‡‡‡P < 0.001.
Dietary ω-3 LCPUFAs Suppress Intercellular Adhesion Module-1 and E-Selectin Expression in CNV Lesions.
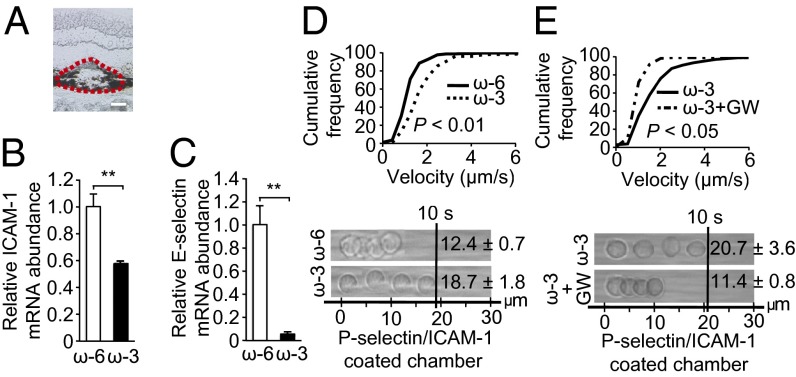
Dietary ω-3 LCPUFAs previously were found to down-regulate the expression of adhesion molecules in retinal neovessels of mice in a Pparγ-dependent manner (18). Therefore we next examined the possible effects of the ω-3-LCPUFA diet on adhesion molecule expression in the laser-induced CNV model. Mice fed ω-3 LCPUFAs had significantly reduced levels of intracellular adhesion molecule 1 (Icam-1) and E-selectin mRNAs in the retina or choroid at 7 d after CNV induction compared with mice on an ω-6 LCPUFA diet (Fig. S4 A and B). In contrast, there was no difference between the two groups of animals in the amounts of Vcam-1 or P-selectin mRNAs in the retina or choroid (Fig. S4 C and D). Furthermore, the levels of Icam-1 and E-selectin proteins in the choroid between 2 and 7 d after CNV induction were reduced significantly in mice fed ω-3 LCPUFAs (Fig. S4 E and F). Laser-capture microdissection of CNV lesions confirmed that the abundance of Icam-1 and E-selectin mRNAs was reduced in lesions of mice fed the ω-3 LCPUFA diet (Fig. 5 A–C). These data indicated that dietary ω-3 LCPUFAs regulate adhesion molecule expression in CNV lesions and therefore might affect recruitment of systemic leukocytes to these lesions.
Fig. 5.
Dietary ω-3 LCPUFA intervention suppresses adhesion molecule expression in CNV lesions and increases leukocyte rolling velocity. (A) Representative isolectin B4 staining of a CNV lesion in a chorioretinal section. The red dashed line indicates the border of the sample isolated by laser-capture microdissection. (Scale bar: 50 μm.) (B and C) Real-time PCR analysis of Icam-1 (B) and E-selectin (C) mRNAs in laser-captured CNV lesions at 7 d after CNV induction in mice fed ω-6 or ω-3 LCPUFAs. Data are presented as means ± SEM (n = 3). **P < 0.01. (D and E) (Upper) Cumulative frequency of leukocyte rolling velocity in a chamber coated with both P-selectin and Icam-1 at 3 d after CNV induction in mice fed ω-6 (n = 6) or ω-3 (n = 6) LCPUFAs. (E) Mice on ω-3 feed were injected i.p. with the PPARγ inhibitor GW9662 (GW, 1 mg/kg) (n = 4) or vehicle (n = 3) daily beginning immediately after CNV induction. (Lower) Representative overlay images of individual leukocytes tracked over 10 s.
Functional Down-Regulation of the ICAM-1 Ligand on Leukocytes by Dietary ω-3 LCPUFAs.
Leukocyte recruitment to CNV lesions is thought to increase disease severity (28). To assess the impact of ω-3 LCPUFAs on systemic leukocyte recruitment to the CNV site, we measured the rolling velocity of peripheral blood leukocytes (PBLs) at 3 d after CNV induction (the peak of immune cell infiltration in this model) (29) by using an autoperfused microflow chamber coated with P-selectin or the combination of P-selectin and Icam-1 (Movie S1). The rolling velocity of PBLs on the combination of P-selectin and Icam-1 was significantly greater for mice fed ω-3 LCPUFAs (1.87 ± 0.18 µm/s) than for those fed the ω-6 LCPUFA diet (1.24 ± 0.07 µm/s) (Fig. 5D). The increased rolling velocity of ω-3 LCPUFAs was attenuated by the PPARγ inhibitor GW9662 (Fig. 5E). PBLs from mice fed ω-3 or ω-6 LCPUFAs rolled at similar velocities on immobilized P-selectin (Fig. S5A). Hemodynamic conditions within the chamber and the number of interacting leukocytes did not differ between groups (Table S2 and Fig. S5 B and C). These data thus suggested functional down-regulation of the Icam-1 ligand on the surface of leukocytes in mice fed the ω-3 LCPUFA diet.
To investigate further the increase in the Icam-1–dependent rolling velocity of PBLs in mice fed ω-3 LCPUFAs, we measured the expression of the Icam-1 ligand CD11b/CD18 on circulating leukocytes. Flow cytometry revealed that the surface expression levels of both CD11b and CD18 on PBLs were significantly lower for mice fed ω-3 LCPUFAs than for those fed the ω-6 LCPUFA diet (Fig. S5 D and E), in accordance with the notion that the increased rolling velocity of leukocytes from mice fed ω-3 is attributable, at least in part, to down-regulation of CD11b and CD18.
Invasion of Leukocytes Is Suppressed in the Choroid and Retina of CNV Lesions in Mice Fed ω-3 LCPUFA.
To investigate whether down-regulation of leukocyte adhesion molecules during ω-3 LCPUFA intervention affects leukocyte infiltration in the eye, we quantified the number of leukocytes in the retina and choroid after CNV induction in Cx3cr1 GFP/+ mice, which express GFP under the control of the Cx3cr1 promoter (30). Cx3cr1 GFP/+ mice fed an ω-3 LCPUFA diet manifested significantly fewer leukocytes in the retina (Fig. 6 A and B) and choroid (Fig. 6 C and D) than did Cx3cr1 GFP/+ mice fed the ω-6 LCPUFA diet. These data suggest that ω-3 LCPUFAs suppress the recruitment of immune cells to the retina and choroid of CNV lesions.
Fig. 6.
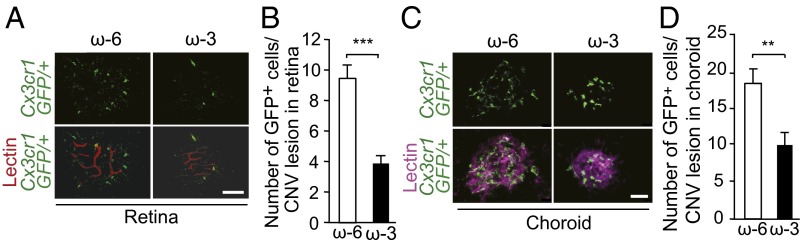
Dietary ω-3 LCPUFA intervention suppresses leukocyte invasion in CNV lesions. (A) Leukocyte invasion in CNV lesions at the back surface of the retina was determined in retinal flat-mount preparations at 7 d after CNV induction in Cx3cr1 GFP/+ mice fed ω-6 or ω-3. (Scale bar: 20 μm.) (B) Quantitation of leukocyte (GFP+ cell) infiltration in the retina in A. Data are presented as means ± SEM for 15 and 13 lesions in ω-6– or ω-3–fed mice, respectively. ***P < 0.001. (C) Leukocyte invasion in CNV lesions in flat-mount preparations of the choroid examined as in A. (Scale bar: 20 μm.) (D) Quantitation of leukocyte infiltration in the choroid in C. Data are presented as means ± SEM for 15 and 13 lesions of mice fed ω-6 or ω-3 LCPUFAs, respectively. **P < 0.01.
Dietary ω-3 LCPUFAs Suppress VEGF Expression in the Retina and Choroid.
Infiltrated macrophages are a potential source of proangiogenic molecules such as VEGF (28). To investigate the possible role of VEGF in CNV resolution, we examined VEGF expression. The amount of Vegfa mRNA in the retina or choroid did not differ between mice fed ω-3 or ω-6 LCPUFA diets (Fig. S6A). In contrast, the abundance of Vegf protein in both the retina and choroid at 7 d after CNV induction was significantly lower in mice fed ω-3 than in mice fed ω-6 LCPUFA diets (Fig. S6 B and C). This pattern of Vegf expression thus correlates with CNV size and vascular leakage (Fig. 1), suggesting that Vegf produced by infiltrated leukocytes might contribute to CNV size in the mouse model of AMD.
Discussion
We have shown that the primary ω-3 LCPUFAs, EPA and DHA, promote vascular regression in a manner dependent in part on the CYP epoxygenase pathway. The ω-3 LCPUFAs (and their CYP-generated metabolites) reduced disease severity by suppressing conditions favorable to both angiogenesis and leukocyte recruitment (Fig. S7). Mice fed ω-3 LCPUFAs showed generally increased circulating levels of EPA- and DHA-derived CYP eicosanoids (EEQs and EDPs), whereas the levels of AA-derived proangiogenic EETs were reduced significantly. We identified two specific bioactive products (17,18-EEQ and 19,20-EDP) as being important for disease resolution in a mouse model of neovascular AMD. We also show that mice fed an ω-3 LCPUFA diet manifest greater Pparγ expression and activity in the retina or choroid than seen in their counterparts fed ω-6 LCPUFA and that the effect of ω-3 LCPUFAs on the size of CNV lesions is blocked by a specific inhibitor of Pparγ. The recruitment of circulating immune cells to CNV lesions was dampened significantly in mice receiving ω-3 LCPUFAs. In these mice, expression of the key endothelial adhesion molecules Icam-1 and E-selectin was down-regulated significantly in the choroidal vasculature, and the ICAM-1 ligand CD11b-CD18 was suppressed on circulating PBLs.
We found that administration of the CYP-derived ω-3 LCPUFA metabolites 17,18-EEQ or 19,20-EDP reduced CNV lesion size and vascular leakage, indicating that enrichment of these metabolites in serum by dietary supplementation can reduce choroidal neovessel growth and pathology significantly. Up-regulation of these CYP-generated metabolites in serum was observed previously in rats and man after dietary EPA/DHA supplementation (21, 23). EETs, EDPs, and EEQs are synthesized by the same CYP enzymes in both hepatic and extrahepatic tissues and act as second messengers with potent biological activities (7–9). Although we identified several CYP family members localized to the retina, further investigation is needed to determine how these enzymes are modulated both locally in the retina and systemically. This information should give us greater insight into how these CYP-derived lipid metabolites are regulated in response to disease.
Three distinct enzyme classes are responsible for the metabolism of LCPUFAs: CYP, COX, and LOX. EPA and DHA compete with AA for binding to and metabolism by these enzymes, with such competition affecting the production, bioactivity, and bioavailability of epoxides, prostanoids, and leukotrienes, respectively. Chronic aspirin administration recently was shown to be associated with an increased risk of neovascular AMD (31). Given the ability of aspirin to affect the formation of both ω-3 and ω-6 LCPUFA metabolites, such long-term aspirin use may influence leukocyte recruitment and angiogenesis.
The bioactive ω-3 LCPUFA–derived mediators neuroprotectin D, resolvin D1, and resolvin E1 suppress angiogenesis (16). Neuroprotectin D, a potent anti-inflammatory molecule, was found to reduce the extent of clinically relevant vascular leakage in a mouse model of CNV (17). Resolvin D1 and E1 dampen leukocyte responses and facilitate the resolution of inflammation (13), and they also have been shown to attenuate angiogenesis associated with oxygen-induced retinopathy (16). We have now identified additional ω-3 LCPUFA derivatives, 17,18-EEQ and 19,20-EDP, that reduce the size of CNV lesions and suppress vascular leakage in a mouse model of CNV. CYP-generated metabolites of ω-3 LCPUFAs thus not only promote CNV resolution but also facilitate vascular repair and regression.
Immune cell recruitment plays a key role in innate host responses including local inflammation (32), neovascularization (33), and wound healing (34) as well as in various diseases including inflammatory neurodegeneration (35), atherosclerosis (36), and cancer (13). Our data now suggest that dietary intake of ω-3 LCPUFAs results in functional down-regulation of both Icam-1 on endothelial cells and the ICAM-1 ligand on the surface of leukocytes and also suppresses macrophage invasion into CNV lesions.
The results of three randomized, controlled, prospective cohort trials (37–39), four case-control studies (40–43), and two cross-sectional studies (44, 45) showed that high dietary ω-3 fatty acid intakes were associated with a 38% reduction in the risk of late AMD [odds ratio (OR): 0.62; 95% confidence interval (CI): 0.48–0.82]. Eating fish at least twice a week was associated with a reduced risk of both early AMD (OR: 0.76; 95% CI: 0.64–0.90) and late AMD (OR: 0.67; 95% CI: 0.53–0.85) (46). In contrast, two recent randomized, prospective, placebo-controlled, clinical trials tested the efficacy of ω-3 supplementation on late AMD development and found no beneficial effect (47, 48). In the National Institutes of Health-initiated Age-Related Eye Disease Study 2 (AREDS2) subjects were given supplements containing lutein plus zeaxanthin, ω-3 LCPUFAs [DHA (350 mg) + EPA (650 mg) from ethyl esters], or both on top of the original AREDS formulation. It was found that ω-3 supplementation did not reduce the 5-y risk of progression from early to late AMD (progression of either geographic atrophy or neovascular AMD was assessed) (47). The Nutritional AMD Treatment 2 (NAT2) study found no effect with 3-y ω-3 supplementation [DHA (840 mg) and EPA (270 mg) from fish oil] on neovascular AMD development in the unaffected eye from the patients with CNV in the contralateral eye (48).
We were able to impose strict dietary controls on our subject animals so that they had access only to either ω-3 (DHA and EPA) or ω-6 LCPUFAs during the experimental window (before disease onset and through the experimental end point). This dietary restriction to only ω-3 or ω-6 lipids was not possible in the supplemented AREDS2 and NAT2 study (47, 48). Collectively these results indicate that dietary sources alone might not be sufficient to alter an individual’s systemic lipid profile. Indeed, identification and subsequent administration of potent bioactive lipid metabolites may be critical to therapeutic treatment for a large majority of the population. Last, the AREDS2 and NAT2 studies comprised a population that generally consisted of highly educated and well-nourished individuals with an overall incidence of late AMD lower than expected from observational studies, and this study population already may have had adequate ω-3 levels. The elevated ω-3 serum levels of the placebo groups in both trials suggest this possibility and also might suggest that trial effects (e.g., self supplementation) might have reduced the power of the statistics in both randomized trials.
A recent study showed that patients who were able to achieve a high EPA+DHA content in their red blood cell membranes (RBCM) had a significant (48%) reduction in the odds of developing neovascular AMD (43). Interestingly, this effect also was observed in a subset of individuals in the NAT2 study, which found that patients with high EPA+DHA content in their RBCM were significantly protected from progression of neovascular disease (48). The authors correctly conclude that RBCM lipid content is a more valid reflection of long-term incorporation of these lipids into tissues, because these lipids likely are introduced during hematopoiesis, whereas serum lipid profiles largely reflect recent, short-term dietary changes (43). Unfortunately only serum profiles were assessed in AREDS2, so tissue incorporation could not be assessed (47). These results suggest that incorporation of these lipids is vital to overall bioavailability and that therapeutic treatment with the downstream bioactive lipid metabolites could be of significant importance in individuals who are unable to achieve an elevated level of ω-3s. However, given the high variability among individuals with different tissue-specific levels of EPA/DHA, dietary intake of ω-3 LCPUFAs alone may not be sufficient to achieve protection against neovascular AMD. Therefore, identifying the bioactive lipid metabolites with antiangiogenic and anti-inflammatory functions, as defined in this study, is of significant interest for dietary nonresponders who may not be able to alter their tissue lipid metabolite profiles despite supplementation. In our animal models, we can regulate the diets tightly and therefore are able to draw strong conclusions on the actions of individual eicosanoids.
Moreover, dietary incorporation of these lipids into tissues seems to occur largely in an age-dependent manner. This dependence is illustrated by our inability to shift the retinal tissue profile in the adult mice in our current study, in contrast with our earlier work, in which these lipids were incorporated readily into the retinas of neonatal mice (16). Similarly, age-dependent alterations have been identified in a number of CYP isoforms (49) and could reflect how downstream lipid metabolite formation might change drastically with age, thereby providing an important clue into the pathogenesis of AMD development.
In conclusion, we have found that CYP-generated metabolites of dietary ω-3 LCPUFAs are responsible, at least in part, for attenuation of pathological angiogenesis in vivo. Importantly, this beneficial effect can be achieved with physiologically relevant increases in the dietary content of ω-3 LCPUFAs. Although our studies were performed with a mouse model of AMD, our findings also may be relevant to other diseases in which angiogenesis and inflammation play key roles, including atherosclerosis, arthritis, and cancer.
Materials and Methods
The study adhered to the Statement for the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology and was approved by the Animal Care Committee of the Massachusetts Eye and Ear Infirmary (www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research/).
Detailed descriptions of the animals, regents, animal studies, analysis of CYP-generated metabolites and fatty acids, RNA isolation, cDNA preparation, real-time PCR, laser-capture microdissection, measurement of PPARγ activity, flow cytometric analysis, ELISA, and immunoblot analysis are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by a Special Scholar Award (to K.M.C.) and an unrestricted grant (to J.W.M.) from Research to Prevent Blindness; the Massachusetts Lions Eye Research Fund and an award from the Japan Eye Bank Association (to R.Y.); and by Grants R01EY022084/S1 (to K.M.C.), T32EY007145 (to H.S.), and P30EY014104 from the National Eye Institute of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.A.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401191111/-/DCSupplemental.
References
- 1.Miller JW. Age-related macular degeneration revisited—piecing the puzzle: The LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013;155(1):1–35, e13. doi: 10.1016/j.ajo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 2.SanGiovanni JP, Agrón E, Clemons TE, Chew EY. Omega-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch Ophthalmol. 2009;127(1):110–112. doi: 10.1001/archophthalmol.2008.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 4.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 5.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814(1):210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30(3-4):525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Wang D, Dubois RN. Epoxyeicosatrienoic acids: A double-edged sword in cardiovascular diseases and cancer. J Clin Invest. 2012;122(1):19–22. doi: 10.1172/JCI61453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92(1):101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webler AC, et al. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295(5):C1292–C1301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panigrahy D, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122(1):178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazan NG. Cell survival matters: Docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morin C, Sirois M, Echavé V, Albadine R, Rousseau E. 17,18-epoxyeicosatetraenoic acid targets PPARγ and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: Role of soluble epoxide hydrolase. Am J Respir Cell Mol Biol. 2010;43(5):564–575. doi: 10.1165/rcmb.2009-0155OC. [DOI] [PubMed] [Google Scholar]
- 15.Kubota T, et al. Eicosapentaenoic acid is converted via ω-3 epoxygenation to the anti-inflammatory metabolite 12-hydroxy-17,18-epoxyeicosatetraenoic acid. FASEB J. 2014;28(2):586–593. doi: 10.1096/fj.13-236224. [DOI] [PubMed] [Google Scholar]
- 16.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheets KG, et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl A, et al. Short communication: PPAR γ mediates a direct antiangiogenic effect of ω 3-PUFAs in proliferative retinopathy. Circ Res. 2010;107(4):495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JX, Liu A. The role of the tissue omega-6/omega-3 fatty acid ratio in regulating tumor angiogenesis. Cancer Metastasis Rev. 2013;32(1-2):201–210. doi: 10.1007/s10555-012-9401-9. [DOI] [PubMed] [Google Scholar]
- 20.Fer M, et al. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471(2):116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62(3):536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 22.Arnold C, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids. J Biol Chem. 2010;285(43):32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer R, et al. Dietary Omega-3 Fatty Acids Modulate the Eicosanoid Profile in Man Primarily via the CYP-epoxygenase Pathway. J Lipid Res. 2014 doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci USA. 2013;110(16):6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giani A, et al. Spectral-domain optical coherence tomography as an indicator of fluorescein angiography leakage from choroidal neovascularization. Invest Ophthalmol Vis Sci. 2011;52(8):5579–5586. doi: 10.1167/iovs.10-6617. [DOI] [PubMed] [Google Scholar]
- 26.Sulzbacher F, et al. Correlation of OCT characteristics and retinal sensitivity in neovascular age-related macular degeneration in the course of monthly ranibizumab treatment. Invest Ophthalmol Vis Sci. 2013;54(2):1310–1315. doi: 10.1167/iovs.12-11046. [DOI] [PubMed] [Google Scholar]
- 27.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 28.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda K, et al. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J. 2008;22(8):2928–2935. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 31.Klein BE, et al. Long-term use of aspirin and age-related macular degeneration. JAMA. 2012;308(23):2469–2478. doi: 10.1001/jama.2012.65406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 33.Ishida S, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9(6):781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 34.Cotran RS. 1982. The endothelium and inflammation: New insights. Current Topics in Inflammation and Infection, eds Majno G, Cotran RS, Kaufman N (Williams & Wilkins, Baltimore), pp 18–37.
- 35.London A, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208(1):23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho E, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001;73(2):209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- 38.Arnarsson A, et al. Risk factors for five-year incident age-related macular degeneration: The Reykjavik Eye Study. Am J Ophthalmol. 2006;142(3):419–428. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Chua B, et al. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol. 2006;124(7):981–986. doi: 10.1001/archopht.124.7.981. [DOI] [PubMed] [Google Scholar]
- 40.Seddon JM, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119(8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 41.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: The US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124(7):995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 42.SanGiovanni JP, et al. Age-Related Eye Disease Study Research Group The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125(5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 43.Merle BM, et al. Nutritional AMD Treatment 2 Study Group Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):2010–2019. doi: 10.1167/iovs.14-13916. [DOI] [PubMed] [Google Scholar]
- 44.Mares-Perlman JA, et al. Dietary fat and age-related maculopathy. Arch Ophthalmol. 1995;113(6):743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- 45.Delcourt C, Carrière I, Cristol JP, Lacroux A, Gerber M. Dietary fat and the risk of age-related maculopathy: The POLANUT study. Eur J Clin Nutr. 2007;61(11):1341–1344. doi: 10.1038/sj.ejcn.1602685. [DOI] [PubMed] [Google Scholar]
- 46.SanGiovanni JP, et al. Age-Related Eye Disease Study Research Group The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126(9):1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chew EY, et al. Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 48.Souied EH, et al. Nutritional AMD Treatment 2 Study Group Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The Nutritional AMD Treatment 2 study. Ophthalmology. 2013;120(8):1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Wauthier V, Verbeeck RK, Calderon PB. The effect of ageing on cytochrome p450 enzymes: Consequences for drug biotransformation in the elderly. Curr Med Chem. 2007;14(7):745–757. doi: 10.2174/092986707780090981. [DOI] [PubMed] [Google Scholar]