Significance
Phosphatidyl inositol-3 kinase (PI3K) activity is central to B lymphocyte survival, growth, and differentiation. Recent clinical studies also indicate that inhibiting the activity of the PI3Kδ isoform will be effective in treating human B cell malignancies. Here we focus on the B cell-specific role of the Ser/Thr kinase phosphoinositide-dependent kinase 1 (PDK1), a pivotal downstream effector of PI3K signaling that is required for the activation of most members of the AGC kinase family (e.g., Akt, S6K, RSK, PKC). Using conditional gene-targeting approaches, we show that the B cell receptor and a subset of cytokine receptors require PDK1 for B cell generation in the bone marrow as well as for mature B cell survival and activation.
Abstract
Successful B cell differentiation and prevention of cell transformation depends on balanced and fine-tuned activation of cellular signaling pathways. The phosphatidyl inositol-3 kinase (PI3K) signaling pathway has emerged as a major regulator of B lymphocyte homeostasis and function. Phosphoinositide-dependent protein kinase-1 (PDK1) is the pivotal node in the PI3K pathway, regulating the stability and activity of downstream AGC kinases (including Akt, RSK, S6K, SGK, and PKC). Although the importance of PI3K activity in B cell differentiation is well documented, the role of PDK1 and other downstream effectors is underexplored. Here we used inducible and stage-specific gene targeting approaches to elucidate the role of PDK1 in early and peripheral B cell differentiation. PDK1 ablation enhanced cell cycle entry and apoptosis of IL-7–dependent pro-B cells, blocking Ig synthesis and B cell maturation. PDK1 also was essential for the survival and activation of peripheral B cells via regulation of PKC and Akt-dependent downstream effectors, such as GSK3α/β and Foxo1. We found that PDK1 deletion strongly impaired B cell receptor (BCR) signaling, but IL-4 costimulation was sufficient to restore BCR-induced proliferation. IL-4 also normalized PKCβ activation and hexokinase II expression in BCR-stimulated cells, suggesting that this signaling pathway can act independent of PDK1 to support B cell growth. In summary, our results demonstrate that PDK1 is indispensable for B cell survival, proliferation, and growth regulation.
Activation of the phosphatidyl inositol-3 kinase (PI3K) signaling pathway is critical to early B cell development as well as peripheral B cell survival and activation (1). Although the catalytic p110 subunits of class I PI3K molecules are partially redundant, the combined loss of the p110α and p110δ isoforms results in impaired IL-7R–driven proliferation (2). Conversely, it has been suggested that attenuation of PI3K signaling via IL-7R signaling is required for pre-B cell differentiation into IgM-expressing cells to cease proliferation and promote RAG expression (3).
In peripheral B cells, continued survival requires “tonic” signaling via the B cell receptor (BCR), which can be replaced by constitutive PI3K activity (4). Moreover, generation of the marginal zone (MZ) and B-1 B cell subsets, as well as antigen-driven differentiation into antibody-producing cells, are dependent on PI3K (1). PI3K activity generates PtdIns(3,4,5)P3, which acts as a secondary messenger by binding the pleckstrin homology domains of downstream effector molecules. PtdIns(3,4,5)P3 is also the substrate for the phosphatases PTEN and SHIP, generating PtdIns(4,5)P2 and PtdIns(3,4)P2, respectively. Unrestrained activation of PI3K signaling in B cells lacking PTEN and SHIP results in lethal B cell lymphoma (5).
Phosphoinositide-dependent kinase 1 (PDK1) represents a pivotal downstream effector of PI3K signaling, regulating cellular responses to growth factors, insulin, and numerous other agonists by activating a number of AGC protein kinases. Analysis of Pdk1−/− mouse embryonic stem cells confirmed that the major targets include protein kinase B (PKB)/Akt, p70 and p90 ribosomal S6 kinases (S6K1 and RSK, respectively), serum-and glucocorticoid-induced protein kinase (SGK), and protein kinase C (PKC) isoforms (6). After cell stimulation, phosphorylation of conserved residues in the carboxyl-terminal domain of the AGC kinases creates a docking site for PDK1 (7). Maximum activity and stability of the AGC kinases depend on the subsequent phosphorylation of a key threonine residue in the T or activation loop within the catalytic domain by PDK1 (7). The mechanism of Akt activation is an exception to this general rule. Akt isoforms as well as PDK1 have a pleckstrin homology domain that specifically binds to PtdIns(3,4,5)P3. This interaction induces translocation and colocalization of Akt and PDK1 to the inner membrane, where PDK1 can effectively phosphorylate the T loop of Akt at T308 (8). The Sin1/Rictor-containing mTOR complex (mTORC2) phosphorylates Akt at S473 in the hydrophobic C-terminal motif (9), resulting in dual phosphorylation and full activation. In the present study, we used a genetic approach to explore the functions of PDK1 in B cell development and differentiation.
Results
PDK1 Is Required for Early B Cell Development.
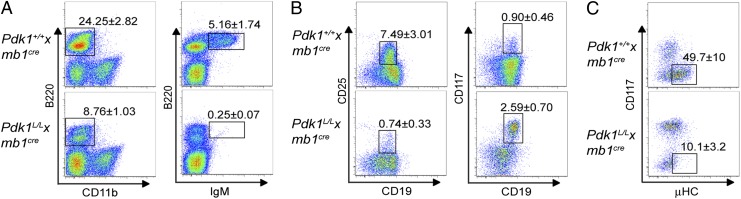
To examine the in vivo role of PDK1 in B cell development, we crossed mice bearing a conditional loxP-flanked Pdk1 allele (Pdk1L) (10) with mb1Cre mice in which the cre recombinase gene has been inserted into the mb1 locus (11). Multicolor flow cytometry analysis of bone marrow (BM) cells from Pdk1L/L × mb1Cre mice revealed a threefold reduction in the frequency of B220+ B cells, encompassing an almost complete loss of mature recirculating (B220hiIgMlo) and immature (B220loIgMhi) B cells (Fig. 1A and Fig. S1B). Analysis of the B cell progenitor compartment revealed a 10-fold reduction in the frequency of resting pre-B cells (B220+CD19+CD25+), whereas the proportion of pro-B cells (B220+CD19+CD117+) may be increased slightly in Pdk1L/L × mb1Cre mice (Fig. 1B and Fig. S1B). Therefore, ablation of Pdk1 prevents the generation of surface IgM+ B cells.
Fig. 1.
PDK1 is required for early B cell development. (A) Frequencies of total B cells (B220+CD11b−) and IgM-expressing B cells (B220+IgM+). (B) B220+ B cells were gated as depicted in A, and the proportions of pre-B cells (CD19+CD25+) (Left) and pro-B cells (CD19+CD117+) (Right) within this gate are shown. (C) Intracellular staining of μHC in B220+surface IgM−CD19+CD25− cells, represented as percentages. The values represent mean ± SD of at least three independent experiments.
To determine the precise stage of B cell development that is affected by Pdk1 deletion, we analyzed the subpopulations within the earliest B cell progenitors according to the Hardy classification scheme (12). Pdk1L/L × mb1Cre and Pdk1+/+ × mb1Cre mice had similar percentages and numbers of fraction A (Fr. A) pre–pro-B cells and Fr. B early pro-B cells in the BM (Fig. S1). Pdk1L/L × mb1Cre mice also showed a normal frequency of Fr. C cells; however, these mice had significantly lower proportions and numbers of Fr. C′ cells, including large cycling pre-B cells expressing the pre-BCR (Fig. S1). To determine whether Pdk1-deficient Fr. C pro-B cells are capable of expressing a productive μ heavy chain (μHC) component of the pre-BCR, we stained BM cells for intracellular μHC. Staining revealed that the frequency of pro-B cells (B220+sIgM−CD19+CD25−) expressing μHC was much lower in Pdk1L/L × mb1Cre mice than in Pdk1+/+ × mb1Cre mice (Fig. 1C). Taken together, these results suggest that PDK1 regulates survival and/or proliferation of B cell progenitors, including the generation and expansion of large-cycling pre-B cells expressing the pre-BCR.
PDK1 Regulates IL-7R–Dependent Proliferation and Survival.
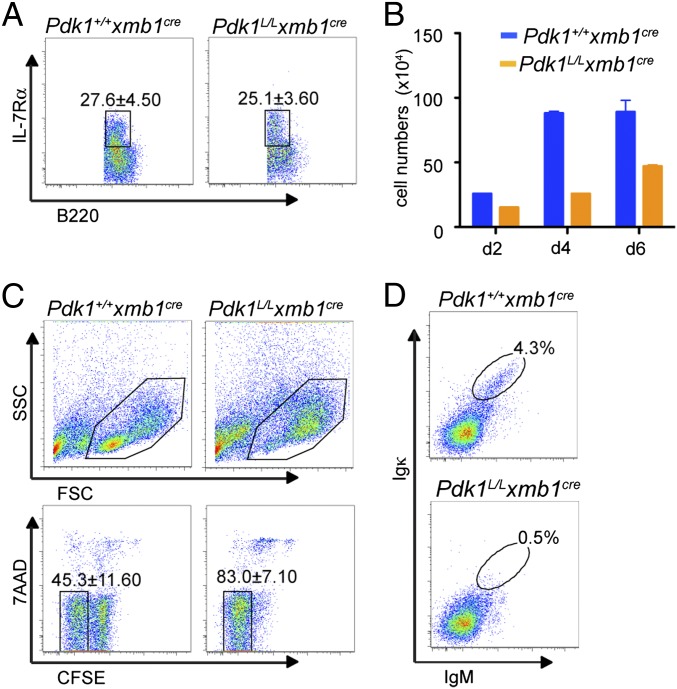
We examined whether, in addition to impaired pre-BCR assembly and/or signaling, alterations in IL-7R signaling also could contribute to the blockage of B cell development observed in Pdk1L/L × mb1Cre mice. The Pdk1L/L × mb1Cre and control mice had similar frequencies of B220+IL-7Rα+ BM cells (Fig. 2A); however, fewer viable Pdk1L/L × mb1Cre BM B cells were recovered after 2, 4, or 6 d of culture with IL-7 compared with Pdk1+/+ × mb1Cre cells (Fig. 2B), suggesting a critical role for PDK1 in IL-7–mediated proliferation and/or survival. To distinguish between these two possibilities, we loaded equal numbers of purified B220+ BM cells with carboxyfluorescein succinimidyl ester (CFSE) before stimulation with IL-7. Analysis of the CFSE dilution profile (as a measure of cell division) vs. 7AAD exclusion (as a measure of viability) showed that Pdk1L/L × mb1Cre cells responded to IL-7 stimulation and actually divided more rapidly than control cells early in culture, indicating that the diminished numbers of Pdk1-deficient cells in IL-7 cultures was caused by defects in cell survival, not be defects in cell proliferation (Fig. 2C). On removal of IL-7, cultured B cell progenitors continued to undergo Ig gene rearrangement to become surface Ig+; however, in the absence of PDK1, formation of IgM+Igκ+ B cells was blocked (Fig. 2D). Taken together, these results suggest that PDK1 regulates IL-7–mediated cell survival and cell cycle progression, which is critical for Ig gene rearrangement and pre-B cell maturation.
Fig. 2.
PDK1 regulates IL-7R–dependent proliferation and survival. (A) Frequency of IL-7Rα+ cells among B220+ cells in the BM. (B) Bar graph showing the average number of B220+ cells in IL-7 cultures at the indicated time points. (C) Light-scatter density plots (Upper) and proportion of live cells (7AAD−B220+CFSElow) that underwent division (Lower) after 2 d of culture in IL-7. (D) IgM and Igκ expression in cells that were cultured for 3 d with IL-7 and for 2 additional days without IL-7. Data in A and C represent mean ± SD of three independent experiments. Data in B and D are representative of three different experiments.
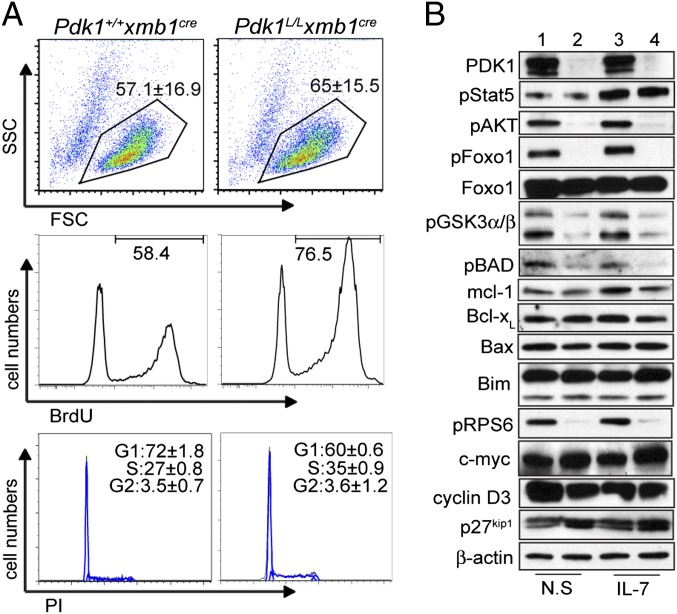
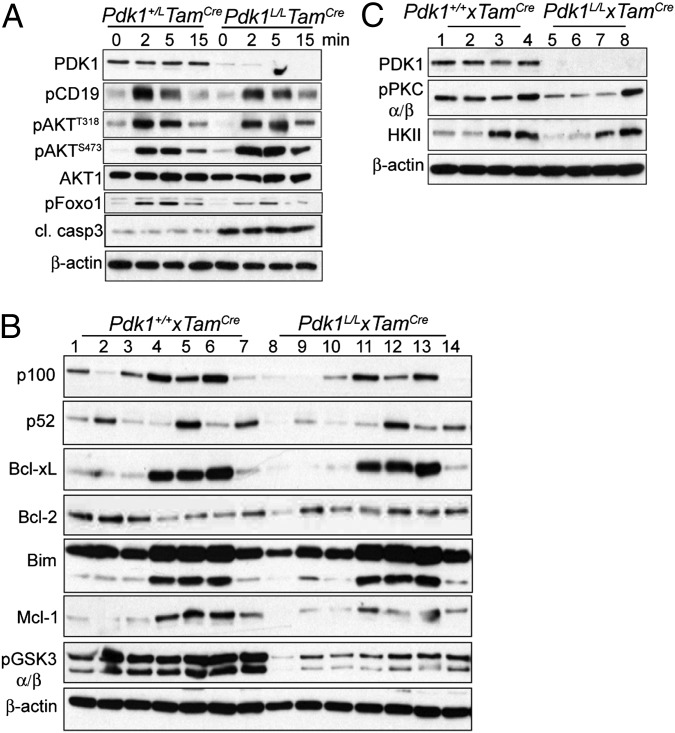
Consistent with the reduction in μHC+ cells, light scatter analysis showed a lack of small pre-B cells in Pdk1L/L × mb1Cre B220+ cell cultures (Fig. 2C). To further investigate the mechanisms involved in the diminished survival of Pdk1-deficient cells, we established a long-term homogeneous pro-B cell culture from lineage-depleted BM cells. After 10 d in culture, cells derived from Pdk1+/+ × mb1Cre and Pdk1L/L × mb1Cre mice were of similar size (Fig. 3A). Cells were counted and further cultured in the presence of BrdU for proliferation analysis. Cultures of Pdk1L/L × mb1Cre mice showed an increased proportion of BrdU+ cells relative to control cultures. Furthermore, cell cycle analysis by propidium iodine (PI) staining revealed a higher proportion of cells in S phase, confirming the enhanced proliferative index of Pdk1-deficient cells (Fig. 3A). Conversely, Pdk1-deficient cultures showed a higher percentage of cells expressing active caspase-3 (Fig. S2), suggesting that these cells were undergoing apoptosis.
Fig. 3.
Analysis of PDK1 effectors mediating early B cell expansion. (A) Proportion of viable proliferating cells in HSC-derived pro-B cell cultures as measured by cell scatter (Top), BrdU incorporation (Middle), or cell cycle analysis with PI (Bottom). Data represent mean ± SD of at least three independent experiments. (B) Immunoblot analyses of HSC-derived pro-B cells from Pdk1+/+ × mb1Cre (lanes 1 and 3) or Pdk1L/L × mb1Cre (lanes 2 and 4) mice after 10 d in culture and on restimulation with IL-7 or not restimulated (N.S.). Blots are representative of three experiments.
PDK1 is a pivotal effector enzyme in the PI3K pathway that regulates branching of downstream pathways. Western blot analysis demonstrated efficient deletion of PDK1 in cultured Pdk1L/L × mb1Cre pro-B cells (Fig. 3B). In response to IL-7 stimulation, PI3K-independent phosphorylation of STAT5Y694 was normal, as expected. In contrast, loss of PDK1 led to a dramatic reduction in the phosphorylation of AktT308 and downstream mTORC1/S6K target ribosomal protein S6 (RPS6S245) (Fig. 3B), suggestive of cell growth defects. Reduced phosphorylation of the Akt substrates Foxo1S256, GSK3α/βS21/9, and BADS136 was observed as well (Fig. 3B), suggesting that this dysregulation may contribute to impaired cell survival. GSK3α/βS21/9 phosphorylation inhibits kinase activity and has been shown to prevent phosphorylation-dependent degradation of Mcl-1 (13). Correspondingly, IL-7–stimulated Pdk1L/L × mb1Cre pre-B cells exhibited a modest decrease in Mcl-1 expression relative to controls (Fig. 3B). Even though Bim is a target of the FoxO factors, its expression was not altered in Pdk1L/L × mb1Cre pro-B cells expressing hypophosphorylated nuclear Foxo1 (Fig. 3B). In addition, we did not detect significantly altered levels of the antiapoptotic factor Bcl-xL or the proapoptotic factor Bax in cultured Pdk1L/L × mb1Cre pre-B cells (Fig. 3B). Thus, the primary survival defect may result from impaired phosphorylation of BADS136 by Akt, which disrupts binding with Bcl-2 and Bcl-xL and promotes survival. The levels of cyclin D3, c-myc, and p27kip1 were normal in the Pdk1-deficient cells (Fig. 3B), suggesting that alterations in other unidentified cell cycle factors contribute to the augmented proliferation observed in these cells.
PDK1 Is Required for Peripheral B-Cell Homeostasis.
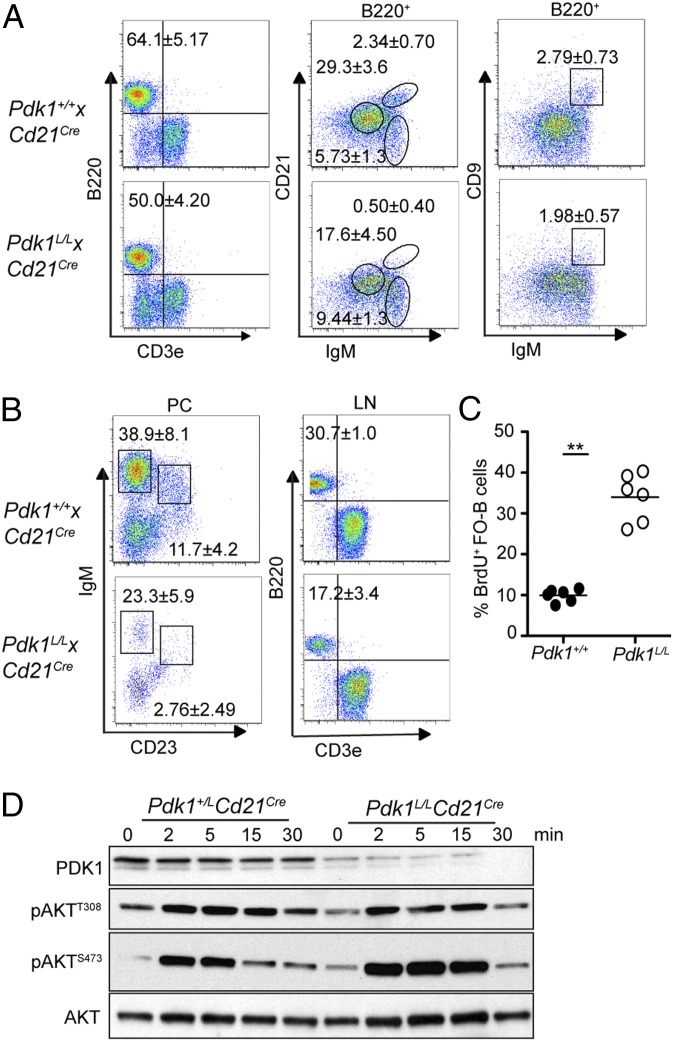
The severity of the early B cell defect observed in Pdk1L/L × mb1Cre mice precluded the analysis of PDK1 function in peripheral B cells. Consequently, we established Pdk1L/L × Cd21Cre transgenic mice. In Cd21Cre transgenic mice, Cre is induced in transitional B cells and sustained in mature B cells (14). On flow cytometry analysis, Pdk1L/L × Cd21Cre mice displayed significantly lower proportions and numbers of B cells compared with age-matched littermate controls (Pdk1+/+× Cd21Cre) (Fig. 4A and Fig. S3). Further analysis revealed significant reductions in the number of follicular B cells (FOB; B220+CD23hiIgMloCD21lo), MZ B cells (MZB; B220+CD23loIgMhiCD21hi), and the combined T2/MZ precursors (MZP; B220+CD23+IgMhiCD21hi). No reduction in the number of transitional 1 (T1) cell subset (B220+CD23−IgMhiCD21lo) was observed, consistent with the induction of CD21Cre expression at this developmental stage (14). In addition, analysis of B cells in the peritoneal cavity revealed a reduction in the proportion of both B-1 (IgMhiCD23−) and B-2 (IgMloCD23+) cell subsets (Fig. 4B). These results indicate that PDK1 is necessary for the formation of both subsets of “innate-like” B cells that produce natural IgM.
Fig. 4.
PDK1 is required for peripheral B cell homeostasis. (A) Percentage of total B cells and B cell subsets [T1 (B220+IgMhi+CD21−), T2/MZP (B220+IgMhi+CD21hi), FOB (B220+IgMlo+CD21lo), and MZB (B220+IgMhi+CD9+)] in spleens. (B) Analysis of peritoneal B1 (IgMhiCD23−) and B2 (IgMloCD23+) cells and lymph node (LN) B cells. (C) Intracellular BrdU staining of splenic FOB cells (B220+CD21loCD23hi) from mice that had been fed with BrdU for 10 d. (D) Immunoblot analyses of splenic FOB cells (CD43−CD9−) stimulated with 10 μg/mL anti-IgM (Fab′)2. Data represent mean ± SD of at least three experiments.
The dramatic reduction in the number of FOB cells in Pdk1L/L × Cd21Cre mice suggests that PDK1 controls the maturation and/or survival of this B cell subset. To investigate the role of PDK1 in the survival of mature recirculating B cells, we administered BrdU in the drinking water of Pdk1+/+ × Cd21Cre and Pdk1L/L × Cd21Cre mice for 10 d to label proliferating B cell precursors in the BM. We used flow cytometry analysis to determine the frequency of BrdU+ cells that differentiated in the BM and emigrated to the spleen during the 10-d period. Compared with WT controls, the Pdk1L/L × Cd21Cre mice showed a 3.5-fold increase in the percentage of BrdU+ cells within the FOB cell pool, indicating a higher rate of peripheral B cell turnover in these mice (Fig. 4C). This result suggests that Pdk1-deficient B cells in the periphery are short-lived and are continually replenished by newly formed B cells from the BM.
To gain insight into PDK1-mediated survival mechanisms in mature recirculating B cells, we examined signal transduction via the PI3K pathway in FOB cells isolated from Pdk1L/L × Cd21Cre and WT mice. Splenic FOB cells (CD43−CD9−) were enriched by depletion of all non-B cells (CD43+) and MZB cells (CD9+) and stimulated with anti-IgM (Fab′)2 before immunoblot analysis of whole-cell lysates. Surprisingly, Pdk1-deficient cells exhibited only a modest reduction in pAktT308, which is the residue directly phosphorylated by PDK1; moreover, pAktS473 was substantially increased in PDK1-deficient cells (Fig. 4D). The modest effect on phosphorylation of AktT308 likely reflects the robust activity of residual PDK1, as has been noted in other systems (15). Moreover, enhanced phosphorylation of AktS473 is known to occur after derepression of a negative feedback mechanism involving the mTORC1 complex (16).
To examine B cell function, we measured basal Ig levels by ELISA. Serum IgM and IgG levels were reduced (Fig. S4A), consistent with impaired B cell survival and strong reductions in the MZ and B-1 B cell compartments. However, serum IgA and IgE was maintained or significantly elevated, respectively (Fig. S4A). Notwithstanding the isotype-specific variations in basal Ig levels, Pdk1L/L × Cd21Cre B cells were unable to mount an efficient antigen-specific antibody response to the T cell-independent polymeric antigen, 2,4,6-trinitrophenyl (TNP)-Ficoll (Fig. S4B). In addition, germinal center B cell differentiation was strongly impaired in the spleen after immunization with sheep red blood cells (SRBCs) (Fig. S4 C and D), and spontaneous germinal center formation in the Peyer’s patches (Fig. S4E). These findings suggest that PDK1 is essential for antigen-driven clonal expansion.
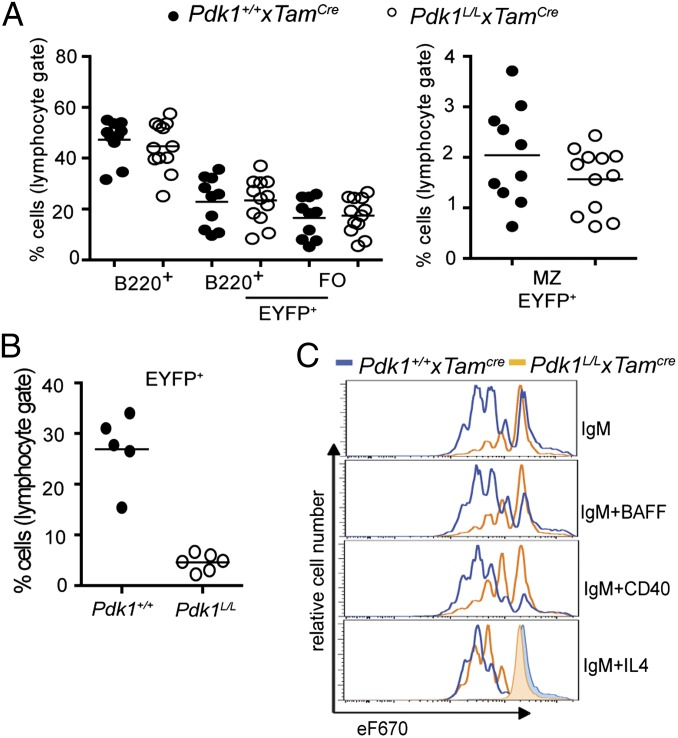
The residual PDK1 expression noted in B cells from Pdk1L/L × Cd21Cre mice (Fig. 4D) raised the possibility that B cells that retain some level of PDK1 expression have a survival advantage. Thus, we established a system to examine the acute effects of Pdk1 inactivation in mature B cells by intercrossing Pdk1L/L mice with the recently described hCD20TamCre strain (17), as well as with the Cre-induced Rosa26eyfp reporter strain (18). In Pdk1L/L × Rosa26eyfp × hCD20TamCre (hereinafter Pdk1L/L × TamCre) mice, Pdk1 deletion was induced in B cells by tamoxifen injection and could be monitored by coinduction of EYFP expression. Tamoxifen was administered for 3 consecutive days, and flow cytometric analysis was performed at 5 d after the final dose. Interestingly, we found a modest reduction in the percentage of EYFP+ MZB cells, suggesting that PDK1 is required for the maintenance of the population, but no significant alteration in the percentage of EYFP+ FOB cells in Pdk1L/L × TamCre versus control mice over this 8-d period (Fig. 5A); however, analysis at several weeks after tamoxifen treatment revealed a dramatic reduction in the number of EYFP+ B cells in the Pdk1L/L × TamCre mice (Fig. 5B). Moreover, induced deletion of Pdk1 at days 3–5 postimmunization with SRBCs resulted in a strong block in germinal center B cell differentiation (Fig. S5A) These findings are consistent with those obtained in the Pdk1L/L × Cd21Cre system, indicating that PDK1 is required for the growth and survival of B cells representing all peripheral subsets.
Fig. 5.
Effect of acute deletion of PDK1 on peripheral B cell homeostasis and activation. (A) Flow cytometry analyses of the percentages of B cells and B cell subsets in the spleens at 5 d after tamoxifen injection. (B) Analysis of spleens at 4–5 wk after tamoxifen injection. (C) B cell division as measured by flow cytometry analysis of eF670 partitioning after 3 d of culture. Data represent mean ± SD of three independent experiments. *P < 0.05, Mann–Whitney U test.
To evaluate the role of PDK1 in peripheral B cells responding to mitogens and growth factors, we purified splenic B cells from Pdk1L/L × TamCre mice at 5 d after tamoxifen injection. At this time point, the Pdk+/+ × TamCre and Pdk1L/L × TamCre mice have similar proportions of MZB cells. Purified B cells were then incubated with anti-IgM F(ab′)2, LPS, BAFF, IL-4, or anti-CD40 for 3 d. Pdk1L/L × TamCre B cells survived poorly in response to anti-IgM F(ab′)2, BAFF, and, to a lesser extent, anti-CD40 or IL-4, but responded normally to LPS (Fig. S5B). In vitro B cell proliferation experiments, as measured by dilution of the eF670 dye, corroborated the survival data, showing that Pdk1L/L × TamCre B cells were hyporesponsive to all stimuli except LPS. Moreover, IL-4 costimulation rescued the proliferative defects seen in Pdk1L/L × TamCre B cells stimulated with anti-IgM F(ab′)2 alone (Fig. 5C). These findings raised the possibility that the elevated basal IgA and IgE observed in vivo (Fig. S4A) may be a result of an augmented class-switch recombination. Indeed, we found that costimulation with IL-4 resulted in sustained proliferation and normalized switch to IgG1 (Fig. S5C). Taken together, these results suggest that IL-4 can function in a PDK1-independent manner to promote B cell survival and function.
PDK1-Regulated Signaling Pathways in Activated B Cells.
To elucidate the PDK1-regulated signaling pathways that impact B cell proliferation and survival downstream of the BCR, whole-cell lysates were prepared from Pdk1L/L × TamCre and control B cells for immunoblot analysis. Interestingly, Pdk1L/L × TamCre cells, similar to Pdk1L/L × CD21Cre cells, did not exhibit a significant reduction in pAktT308, but did display an increase in pAktS473 (Fig. 6A). Nonetheless, phosphorylation of the Akt substrate pFoxo1S256 was reduced in Pdk1-deficient cells, suggesting compromised Akt activity in these cells. As observed in early B cells from Pdk1L/L × mb1Cre mice (Fig. 3B), Pdk1L/L × TamCre cells expressed elevated levels of active caspase 3 (Fig. 6A).
Fig. 6.
PDK1-regulated signaling pathways in activated B cells. Shown are immunoblots of total cell lysates of B cells purified at 5 d after the final injection of tamoxifen and stimulated under different conditions. (A) B cells were stimulated with 10 μg/mL of anti-IgM (Fab′)2 for the indicated period. (B) B cells were cultured for 24 h in medium alone (lanes 1 and 8), BAFF (lanes 2 and 9), IL-4 (lanes 3 and 10), IgM (lanes 4 and 11), IgM and BAFF (lanes 5 and 12), IgM and IL-4 (lanes 6 and 13), and BAFF and IL-4 (lanes 7 and 14). (C) B cells were cultured for 24 h in medium alone (lanes 1 and 5), IL-4 (lanes 2 and 6), IgM (lanes 3 and 7), and IgM and IL-4 (lanes 4 and 8). Data are representative of at least three experiments.
To elucidate upstream defects in survival pathways that would contribute to caspase-3 activation, we prepared whole-cell lysates from Pdk1L/L × TamCre and control B cells that had been stimulated overnight with various stimuli. Consistent with previous work implicating Akt in promoting Mcl-1 stability by inhibiting GSK3α/β activity (13), Pdk1L/L × TamCre B cells showed some diminution in Mcl-1 expression on costimulation with anti-IgM F(ab′)2 (Fig. 6B). Expression of other proapoptotic or antiapoptotic factors, including Bcl-2, Bcl-xL, and Bim, was unchanged in the Pdk1L/L × TamCre cells (Fig. 6B).
PDK1 phosphorylates and promotes the activation and stability of PKC isoforms. We examined the levels of PKCβ in Pdk1-deficient B cells, because this isoform controls survival pathways mediated by NFκB in B cells. Although we found no changes in the amount of total PKCβ or other PKC isoforms, the amount of pPKCα/βT638/641 was consistently reduced in resting Pdk1L/L × TamCre and Pdk1L/L × CD21Cre B cells (Fig. 6C and Fig. S6). This threonine residue is located in the turn motif of PKCs and is autophosphorylated after PDK1 phosphorylation of the activation loop. Interestingly, BCR signaling did not alter pPKCα/βT638/641 levels in WT or Pdk1L/L × TamCre B cells after either 24 h or short-term culture with anti-IgM F(ab′)2 (Fig. 6 B and C). In contrast, B cells that had been cultured simultaneously with anti-IgM F(ab′)2 and IL-4 had higher levels of pPKCα/βT638/641 compared with B cells that had received anti-IgM F(ab′)2 or IL-4 alone. This induction was similar in WT and Pdk1L/L × TamCre B cells (Fig. 6C).
It was recently shown that PKCβ plays an important role in the induction of aerobic glycolysis on BCR stimulation, and that blockage of glycolysis results in decreased survival of activated B cells (19). We found that BCR engagement failed to induce normal expression of hexokinase II (HKII), a glycolytic enzyme, in Pdk1L/L × TamCre B cells (Fig. 6C). In contrast, the combination of anti-IgM F(ab′)2 and IL-4 induced similar levels of HKII in WT and Pdk1L/L × TamCre cells (Fig. 6C). Thus, induction of HKII by IL-4 contributes to the recovery of B cell growth, proliferation, and survival in a PDK1-independent manner.
Discussion
Here we focused on the role of PDK1 as a critical and nonredundant factor that parses signals downstream of PI3K and has PtdIns(3,4,5)P3-independent functions as well. We found that Pdk1-deficient pro-B cells largely failed to produce μHC and complete their differentiation into pre-B cells. An explanation for this finding may come from the recent findings of Venigalla et al. (20), who reported that PDK1 supports the expression of Pax5, which is required for efficient rearrangement of distal VH gene segments. Once assembled, the pre-BCR can attenuate IL-7R/PI3K/Akt signaling, abetting Foxo1 activity in the nucleus and ultimately limiting cell proliferation, Rag gene transcription, and light chain gene rearrangement (3). Using Cd19Cre mice to inactivate Pdk1, Park et al. (21) recently reported that although the pre-B cell compartment was intact in these mice, PDK1 was required for the generation of immature B cells in the BM. Thus, PDK1 is necessary for pre-BCR assembly, and also appears to be necessary for the positive selection of immature B cells expressing a functional BCR.
PDK1-deficient pro-B cells were more susceptible to cell death. Although Bcl-xL, Bax, and Bim expression was unaffected by PDK1 loss, Mcl-1 expression was modestly reduced, and BadS136 phosphorylation was strongly reduced. These findings are consistent with reports that activated Akt enhances cell survival via inactivation of BAD and stabilization of Mcl-1. Phosphorylation of Bad inhibits apoptosis by preventing dimerization with Bcl-2 or Bcl-xL (22). Phosphorylation of GSK3 by Akt inhibits kinase activity, preventing phosphorylation-dependent ubiquitylation and degradation of Mcl-1 (13, 23). Mcl-1 is indispensable for the survival and development of B cell progenitors (24), and its expression level appears to be regulated primarily by post-translational mechanisms. That said, in pro-B cells, STAT5 is known to drive Mcl1 transcription (25). Because IL-7R/STAT5 activation was normal in Pdk1L/L × mb1Cre B cells, this pathway likely contributed to the steady-state levels of Mcl-1 in these cells.
In addition to tonic signaling, the BCR and BAFFR promote mature B cell survival by regulation of metabolic fitness, which depend on PKCβ and PI3K/Akt signals (19, 26). BAFF induces PKCβ translocation to the membrane, followed by Akt activation and changes in cell size (26), whereas BCR engagement causes transcription of glycolytic genes in a PKCβ-dependent manner (19). We found reduced levels of pPKCα/βT638/641, which represents the mature active PKC isoforms, as well diminished amounts of the glycolytic protein HKII in Pdk1-deficient B cells. Curiously, costimulation with anti-BCR and IL-4 restored the levels of HKII and pPKCα/βT638/641 and rescued BCR-activated Pdk1-deficient cells from apoptosis. IL-4 costimulation with anti-CD40 or LPS also allowed for robust proliferation-dependent Ig class-switch recombination in the absence of PDK1. Interestingly, these findings are consistent with the description of an alternative PI3K-independent BCR signaling pathway activated by IL-4 stimulation (27), which induces a STAT6-dependent glycolytic program (28); however, our in vivo studies indicate that this alternate pathway cannot compensate for the loss of PDK1 in promoting B cell homeostasis and function.
Materials and Methods
Mice.
Mice bearing loxP sequences flanking exons 3 and 4 of Pdk1 (Pdk1L/L) have been described previously (10) and were purchased from the University of Dundee. Pdk1L/L mice were crossed with mb1Cre (11) or Cd21Cre (14) mice to delete Pdk1 in B cell progenitors or mature B cells, respectively. Pdk1L/L also was crossed to hCD20TamCre and B6.129 × 1-Gt(ROSA)26Sortm1(EYFP)Cos/J (Rosa26eyfp) mice (18) for B cell-restricted tamoxifen-induced deletion of Pdk1 in mature B cells, as described previously (17). Mice were bred and housed in the animal facility located at Sanford–Burnham Medical Research Institute. All of the crosses and procedures were carried out in accordance with Institutional Animal Care and Use Committee guidelines.
Flow Cytometry.
The following antibodies were purchased from eBiosciences or BD Biosciences (clone numbers in parentheses): B220 (RA3-6B2), CD43 (S7), BP1 (6C3), CD24 (M1/69), CD117 (2B8), CD25 (PC61.5), CD19 (ID3), IgM (II/41), IgD (11-26), μ (II/41), κ (187.1), CD127 (A7R34), CD11b (M1/70), CD3e (145-2C11), CD9 (KM08), CD23 (B3B4), CD21 (7G6), Gr-1 (RB6-8C5), CD49b (DX5), Ter119 (Ter-119), Fas (15A7), and GL-7 (GL7). Apoptotic/necrotic cells were detected by incubation of cells in FACS buffer containing 7AAD. For intracellular staining, cells were initially stained for surface markers as described above, followed by fixation and permeabilization with Cytofix/Cytoperm buffer (BD Biosciences) and 0.1% saponin as recommended by the manufacturer. Data were acquired on a FACSCanto (BD Biosciences) using FACSDiva software and analyzed using FlowJo software (TreeStar).
Immunizations and ELISA.
For analysis of T cell-independent responses, peripheral blood from 8-wk-old mice was collected at day 0 or day 7 after i.p. injection of 10 mg TNP-Ficoll. TNP-coated EIA/RIA plates were used for the detection of Ag-specific IgM and IgG3 (Bethyl Laboratories) in the sera by ELISA. For analysis of T cell-dependent responses, mice were immunized i.p. with 0.2 mL of a 10% SRBC suspension in PBS. Histological analysis and ELISA were performed as described previously (5, 29).
Cell Culture.
For expansion of hematopoietic stem cell (HSC)-derived pro-B cells, lineage-positive cells were depleted from BM with anti-Gr1, CD11b, CD3e, CD49b, Ter119, and B220 antibodies. Lineage-depleted cells were cultured in 10 ng/mL recombinant mouse IL-7, Flt3-L, and SCF (Peprotech). Flt3-L and SCF were withdrawn sequentially, and the cells were cultured for up 10 d in IL-7. For analysis of cell proliferation, 10 μM BrdU was added to the cultures, and intracellular cell staining and FACS analysis were performed 24 h later. Alternatively, the cells were resuspended in 500 μL of propidium iodine (PI) hypotonic solution (0.1% sodium citrate, 0.1% Triton X, 100 μg/mL RNase, and 50 μg/mL PI) and incubated at 4 °C overnight before cell cycle analysis by flow cytometry. For detection of active caspase-3, cells were cultured overnight with or without 20 μM pan-caspase inhibitor Q-VD-OPh (R&D Systems) and detected with CaspGLOW FITC-active caspase-3 (eBioscience). In vitro stimulation assays with splenic B cells were performed as described previously (5).
Immunoblot Analysis.
Western blot analysis was performed as described previously (5). All antibodies were purchased from Cell Signaling Technology, except anti-total PKCβ (BD Biosciences), anti–Bcl-xL (BD Biosciences), anti–c-Myc (Santa Cruz Biotechnology), anti–Mcl-1 (Rockland Immunochemicals), and anti-PDK1 (Upstate Biotechnology).
Statistics.
All of the experiments were performed with a minimum of three animals in each group, and two or three similar experiments were combined for statistical analysis. The Mann–Whitney U test was used for all comparisons, and a P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. K. Rajewsky and M. Shlomchik for providing the Cd21Cre and hCD20TamCre mice, respectively, and the Rickert laboratory for discussions. This work was supported by National Institutes of Health Grants AI041649 and AI059447 (to R.C.R.) and German Science Foundation Grants TRR130 and SFB746 (to M.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.C.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314562111/-/DCSupplemental.
References
- 1.Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23(2):178–183. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramadani F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010;3(134):ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol. 2014;14(2):69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miletic AV, et al. Coordinate suppression of B cell lymphoma by PTEN and SHIP phosphatases. J Exp Med. 2010;207(11):2407–2420. doi: 10.1084/jem.20091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MR, et al. The role of 3-phosphoinositide–dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10(8):439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 7.Frödin M, et al. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 2002;21(20):5396–5407. doi: 10.1093/emboj/cdf551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokoe D, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277(5325):567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor MA, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21(14):3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobeika E, et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103(37):13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21(6):749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Ellwood-Yen K, et al. PDK1 attenuation fails to prevent tumor formation in PTEN-deficient transgenic mouse models. Cancer Res. 2011;71(8):3052–3065. doi: 10.1158/0008-5472.CAN-10-2282. [DOI] [PubMed] [Google Scholar]
- 16.Su B, Jacinto E. Mammalian TOR signaling to the AGC kinases. Crit Rev Biochem Mol Biol. 2011;46(6):527–547. doi: 10.3109/10409238.2011.618113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336(6085):1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1(1):4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair D, Dufort FJ, Chiles TC. Protein kinase Cβ is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochem J. 2012;448(1):165–169. doi: 10.1042/BJ20121225. [DOI] [PubMed] [Google Scholar]
- 20.Venigalla RK, et al. PDK1 regulates VDJ recombination, cell-cycle exit and survival during B-cell development. EMBO J. 2013;32(7):1008–1022. doi: 10.1038/emboj.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SG, et al. The kinase PDK1 is essential for B-cell receptor mediated survival signaling. PLoS ONE. 2013;8(2):e55378. doi: 10.1371/journal.pone.0055378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 23.Schwickart M, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463(7277):103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 24.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 25.Malin S, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11(2):171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patke A, Mecklenbräuker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203(11):2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo B, Rothstein TL. B cell receptor (BCR) cross-talk: IL-4 creates an alternate pathway for BCR-induced ERK activation that is phosphatidylinositol 3-kinase independent. J Immunol. 2005;174(9):5375–5381. doi: 10.4049/jimmunol.174.9.5375. [DOI] [PubMed] [Google Scholar]
- 28.Dufort FJ, et al. Cutting edge: IL-4–mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179(8):4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- 29.Omori SA, et al. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25(4):545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.