Significance
The inhibitory neurotransmitter GABA plays an important role in many brain circuits involved in anxiety, depression, epilepsy, and sleep disorders. GABA is critical for maintaining the balance between excitatory and inhibitory transmission, thereby ensuring proper neuronal network function. Here, we report that entraining mice to a long-day photoperiod can modify the excitatory/inhibitory balance in the suprachiasmatic neuronal network by increasing GABAergic excitation. These data suggest that day length affects the role of GABA in the circadian clock. This finding is important given the widespread application of GABAergic drugs and the general increase in the prevalence of sleep disorders.
Keywords: circadian, excitation/inhibition balance, calcium, chloride, NKCC1
Abstract
The balance between excitation and inhibition is essential for the proper function of neuronal networks in the brain. The inhibitory neurotransmitter γ-aminobutyric acid (GABA) contributes to the network dynamics within the suprachiasmatic nucleus (SCN), which is involved in seasonal encoding. We investigated GABAergic activity and observed mainly inhibitory action in SCN neurons of mice exposed to a short-day photoperiod. Remarkably, the GABAergic activity in a long-day photoperiod shifts from inhibition toward excitation. The mechanistic basis for this appears to be a change in the equilibrium potential of GABA-evoked current. These results emphasize that environmental conditions can have substantial effects on the function of a key neurotransmitter in the central nervous system.
Seasonal changes in the photoperiod of the Earth’s temperate zones affect the behavior and physiology of many organisms (1). The central circadian clock, located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, can adapt to changes in day length and displays a compressed circadian pattern of electrical activity in short winter days and a decompressed pattern in long summer days (2). This pattern is based on a change in the phase distribution of the activity patterns of individual neurons, which becomes broad in the summer and narrow in the winter (3). The mechanisms that mediate and regulate photoperiod-induced phase distributions are currently not known.
The neurotransmitter γ-aminobutyric acid (GABA) is believed to be involved in the phase adjustment and synchronization of the SCN neuronal network (4, 5). GABA and its receptors are expressed in most SCN neurons (6). GABAergic inhibition has been indicated to be important in normal physiological function within the brain. Alterations in this system (i.e., less inhibition) are shown to cause neurological disorders, such as epilepsy and autism (7, 8). In addition to its classical inhibitory function within the SCN network, GABA has more recently been shown to also act as an excitatory transmitter, although its exact role is uncertain (4, 9–13). To understand the influence of photoperiod on GABAergic function, we studied synaptic activity, using patch clamp, and GABAergic responses, using Ca2+ imaging techniques, in the SCN of mice adjusted to long-day and short-day photoperiods. We hypothesized that the narrow, synchronized phase distribution of active neurons during short-day photoperiods would result in increased synaptic activity during the day. Surprisingly, however, exposure to a short-day photoperiod decreased the frequency of spontaneous GABAergic synaptic events compared with the long-day photoperiod.
Subsequently, we tested the effect of photoperiod on GABA-induced excitation in the SCN neuronal network. Ca2+ transients were measured in response to GABA stimulation in long-day and short-day photoperiods. Interestingly, of all cells from the long-day photoperiod, 40% were excitatory and 36% were inhibitory. In contrast, in the short-day photoperiod, 28% of the cells were excitatory and 52% were inhibitory. Using perforated patch recordings, we demonstrate that the underlying mechanism for long-day-induced GABAergic excitation is a depolarizing shift in chloride equilibrium potential.
We suggest that changes in the environment, such as day length, can change the balance between GABAergic excitation and inhibition, which may contribute to photoperiod-induced phase adjustments within the SCN network.
Results
Synaptic Activity Varied Between Different Photoperiods.
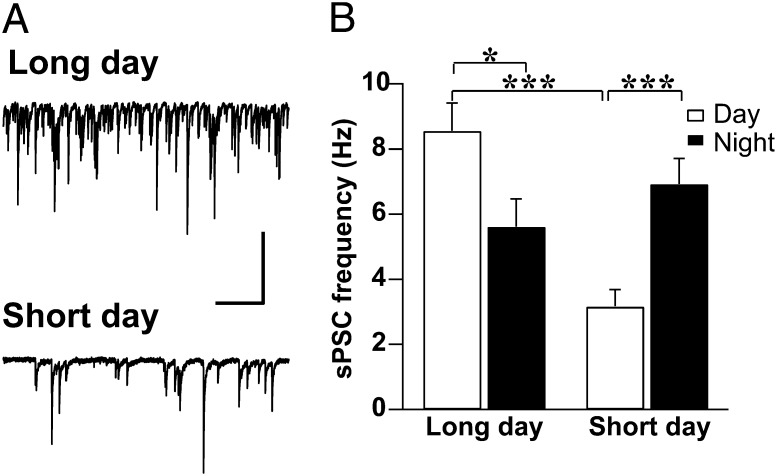
In the present study, whole-cell patch clamp recordings were performed in SCN neurons of mice exposed to various photoperiods to estimate the effect of day length on spontaneous postsynaptic GABAergic currents (sPSC; see Materials and Methods for the details of isolating GABAergic inputs from glutamatergic currents). Exposure to a short-day photoperiod [8 h light, 16 h darkness (LD8:16)] decreased the frequency of GABAergic sPSCs (3.16 ± 0.52 Hz) compared with exposure to a long-day photoperiod (LD16:8; 8.56 ± 0.86 Hz; P = 0.0000005; Fig. 1 A and B) and compared with LD12:12 conditions (7.7 Hz) (14). In a long-day photoperiod, the sPSC frequency was decreased during the night (5.61 ± 0.86 Hz) compared with during the day (P = 0.018). In contrast, neurons recorded from animals adjusted to short-day photoperiod showed an increase in frequency at night (6.92 ± 0.79 Hz) compared with in the daytime (P = 0.0002; Fig. 1B). The amplitude of sPSCs did not exhibit significant differences between and within any of the groups.
Fig. 1.
Long-day photoperiod increases frequency of GABAergic sPSCs. (A) Examples of sPSC daytime recordings from SCN neurons of mice entrained to a long-day or short-day photoperiod. The neurons were voltage-clamped at −70 mV. (Scale bars, 50 pA, 1 s.) (B) Mean ± SEM of the frequency of GABAergic sPSCs recorded during the day and night for long-day (8.56 ± 0.86 Hz, n = 63; 5.61 ± 0.86 Hz, n = 50) and short-day (3.16 ± 0.52 Hz, n = 52; 6.92 ± 0.79 Hz, n = 48) photoperiods. GABAergic events were selected from the raw dataset exemplified in A based on their characteristic decay time (see Materials and Methods). *P < 0.05, **P < 0.01, ***P < 0.001, independent Student's t test.
Excitatory Responses to GABA Require Activation of NKCC1.
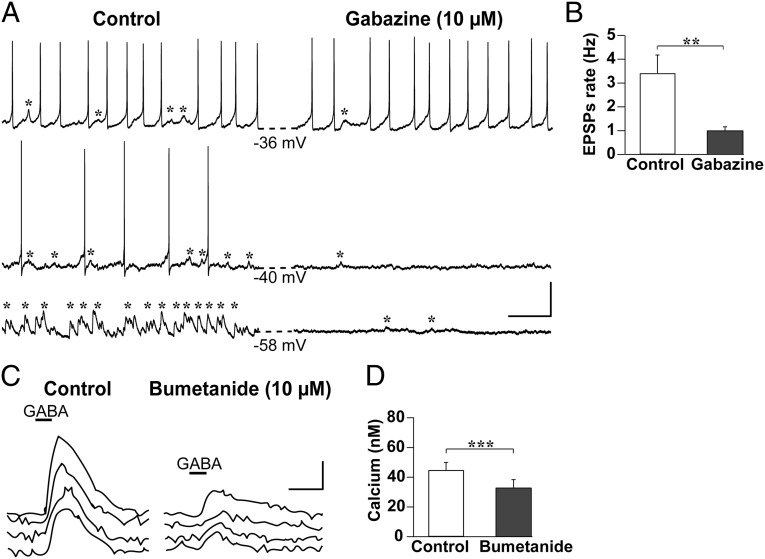
In whole-cell configuration, the pipette filling solution clamps the intracellular chloride (Cl−) concentration, which correlates to a calculated Cl− equilibrium potential of (ECl) = −41.25 mV. As a consequence, spontaneous excitatory or inhibitory actions of the synaptic events cannot be measured (9). Therefore, we performed gramicidin perforated patch recordings to measure postsynaptic potentials in SCN neurons of long-day-entrained mice without manipulating ECl (Fig. 2A). The majority of neurons recorded (92%, n = 12 cells) generated excitatory postsynaptic potentials (EPSPs), and the frequency of these EPSPs was reduced by 71% in the presence of the GABAA receptor blocker gabazine (P = 0.004; Fig. 2B). Similar recordings in animals entrained to LD12:12 revealed that only 21% of the neurons had EPSPs (n = 14 cells). Likewise, neuronal activity was affected by gabazine. On blocking GABAA-mediated transmission in long-day-entrained animals, action potential frequency was increased in 2 of 12 neurons; five neurons had no change in action potential frequency, and the remaining five cells had a significant decrease in frequency (60%; P = 0.043). These findings suggest that GABAergic transmission can be excitatory in the SCN and seems to be increased after entrainment to long days.
Fig. 2.
GABA-mediated neuronal excitation in the SCN. (A) Example recordings of three SCN neurons adjusted to a long-day photoperiod, depicting EPSPs (marked by asterisks) and action potentials before (Left) and after (Right) the application of the GABAA receptor blocker gabazine (10 µM, 5 min); note that gabazine eliminated the majority of the EPSPs. Dashed lines indicate the resting membrane potential before and after the treatment. (Scale bars, 20 mV, 1 s.) (B) Mean ± SEM of EPSP frequency before and after gabazine application (n = 12). **P < 0.01, Wilcoxon signed-rank test. (C) Example Ca2+-imaging recordings of SCN neurons adjusted to a long-day photoperiod before (left traces) and after (right traces) application of the NKCC1 antagonist bumetanide (10 µM, 5 min). Bumetanide attenuated the GABA-induced transient Ca2+ elevations. (Scale bars, 20 nM, 20 s.) (D) Mean ± SEM of the peak of GABA-induced Ca2+ transients before and after bumetanide application (n = 39). ***P < 0.001, Wilcoxon signed-rank test.
Previous studies showed that Ca2+ transients are a good approximation of neuronal activity (11, 15), with elevations and reductions in intracellular Ca2+ concentration ([Ca2+]i) reflecting excitation and inhibition, respectively. GABA-mediated excitation is based on a change in the activity of the Cl− cotransporter NKCC1, which can be blocked by bumetanide (9). In the majority of slices from mice entrained to either long-day or short-day photoperiods, we recorded a combination of transient increases and decreases in [Ca2+]i in response to GABA (Fig. S1A). To confirm that an increase in [Ca2+]i is based on the excitatory action of GABA, we applied bumetanide (10 µM, 5–7 min) to SCN neurons from mice adjusted to long-day photoperiod. After bumetanide application, the amplitude of GABA-evoked elevation in [Ca2+]i was diminished (P = 0.0003; Fig. 2 C and D). We therefore conclude that the Ca2+ transients we recorded in response to GABA application indeed represent neuronal excitation.
GABAergic Excitations Were Increased in a Long-Day Photoperiod.
As GABA-mediated excitation was previously shown to vary between different SCN areas of the rat (9, 11), we first tested the GABA-evoked excitatory and inhibitory responses for regional differences. The responses we measured in the mouse SCN were not significantly different between anterior, middle, and posterior hypothalamic slices (one-way ANOVA with post hoc Bonferroni test) or between dorsal and ventral SCN (χ2-test, two-tailed), so we pooled the data for further analysis. The lack of spatial differences in GABAergic responses we report may be a result of anatomical differences between species, with a more defined SCN subregion in the rat compared with the mouse.
The GABA-mediated Ca2+ responses revealed a striking difference in the ratio of excitatory to inhibitory GABAergic activity between animals entrained to long-day versus short-day photoperiods (Fig. 3). Compared with a long-day photoperiod, adaptation to a short-day photoperiod led to significantly more inhibitory responses (36% vs. 52%, respectively; P = 0.0002) and fewer excitatory responses (40% vs. 28%, respectively; P = 0.0008). This difference was even greater in a subset of data recorded within 1 h of midday (Fig. 3D). Under LD12:12 conditions, the percentages of GABA-mediated excitation (32%) and inhibition (43%) were intermediate between their respective values for long-day and short-day photoperiods (Fig. 3E and Fig. S1B). Furthermore, this photoperiodic effect was restricted to the day, as GABA-evoked responses during the night did not differ between long-day and short-day photoperiod, either in excitation (50% vs. 40%, respectively; P = 0.071) or in inhibition (20% vs. 25%, respectively; P = 0.121) (Fig. S2).
Fig. 3.
Increased GABA-mediated excitation in the long-day photoperiod. (A) Examples of fura-2-AM loaded SCN neurons from mice entrained to a long-day (Left) or short-day (Right) photoperiod. The numbers indicate the neurons that are shown in B. Color scale indicates fluorescence intensity at 380 nm excitation in arbitrary units. (B) GABA-induced excitatory (orange traces) and inhibitory (blue traces) Ca2+ transients recorded during the day from the cells indicated in A. (Scale bars, 50 nM, 10 s.) (C) Pie charts depicting the distributions of response types of the cells on GABA stimulation for long-day (n = 760) and short-day (n = 680) photoperiod-entrained neurons. (D) Summary of the percentages of excitatory and inhibitory responses in long-day (n = 222) versus short-day (n = 218) photoperiod-entrained neurons recorded within 1 h around midday. *P < 0.05, **P < 0.01, ***P < 0.0001, χ2-test. (E) Summary of the ratios of excitatory to inhibitory GABAergic signaling in different photoperiods.
Although GABA-induced Ca2+ transients can depend on baseline [Ca2+]i (11), we found no difference in baseline [Ca2+]i of SCN neurons during the day between long-day and short-day photoperiods (Fig. S3A). Moreover, we found no correlation between the response type (i.e., excitatory or inhibitory) and baseline [Ca2+]i in either long-day or short-day photoperiods (Fig. S3B, Left). During the night, baseline [Ca2+]i was higher in inhibitory versus excitatory cells only in a long-day photoperiod (P = 0.001). However, no difference was found in baseline [Ca2+]i of inhibitory and excitatory cells between long-day and short-day photoperiods (Fig. S3B, Right). In conclusion, our data on photoperiodic modulation of the GABAergic responses were not confounded by a change in baseline [Ca2+]i.
Long Photoperiod Shifts Equilibrium Potential of GABA-Evoked Current.
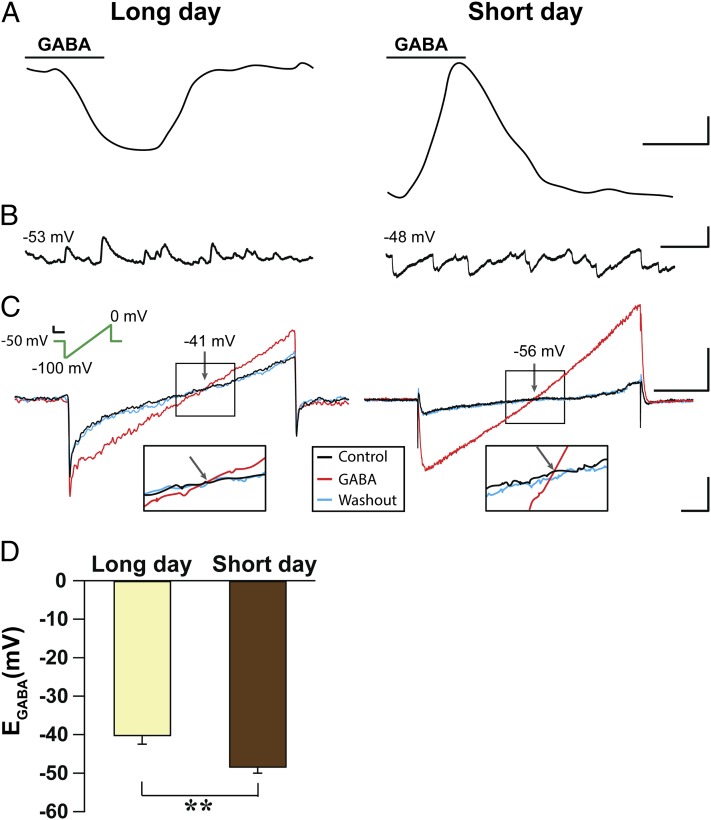
To further investigate the mechanisms underlying these photoperiodic changes in GABAergic signaling, we recorded GABA-evoked currents using the gramicidin perforated patch technique in the presence of blockers for glutamatergic receptors, calcium channels, and action potentials (Fig. 4A). The majority of long-day adapted cells displayed GABA-induced inward currents (eight of nine cells, four animals), whereas more than half of the short-day adapted cells responded with outward current to GABA application (9 of 16 cells, four animals). Current-clamp recordings in the same cells showed that inward currents were always correlated to spontaneous EPSPs, confirming the excitatory nature of endogenous GABAergic activity (Fig. 4B). Because excitatory GABAergic responses seem to depend on activation of NKCC1 (Fig. 2C) (9, 11), we predicted a photoperiod-induced change in intracellular Cl− concentration and a shift in the equilibrium potential of GABA-evoked current (EGABA). Using voltage ramp protocols in the same cells, we recorded GABA-mediated currents and found that EGABA was significantly depolarized in long-day (−40.3 mV) compared with short-day (−48.5 mV; P = 0.002) adapted cells (Fig. 4 C and D). On the basis of these values, the estimated intracellular Cl− concentration in the SCN neurons recorded was higher in the long-day compared with the short-day photoperiod (25 and 18 mM, respectively).
Fig. 4.
EGABA is more depolarized in long-day photoperiod. (A) Example traces of GABA-evoked currents at holding voltage of −50 mV recorded as inward currents in cells adjusted to long-day and as outward currents in cells adjusted to short-day photoperiod. (Scale bar, 5 pA, 5 s.) (B) Examples of excitatory and inhibitory postsynaptic potentials recorded from long-day and short-day photoperiod, respectively, in current clamp configuration. Numbers indicate the resting membrane potential. (Scale bars, 10 mV, 500 ms.) (C) Examples of current responses to a voltage ramp (green line) recorded from cells adjusted to long- and short-day photoperiods in the absence and presence of GABA (Scale bars, 20 mV, 50 ms). The value of ramp potential at which the control and GABA current traces cross (arrow) is equivalent to EGABA. (Scale bar, Left, 20 pA 50 ms; Right, 80 pA, 50 ms.) Inset shows expanded traces of boxed regions within the crossing area. (Scale bars, 10 pA, 10 ms.) (D) Mean EGABA ± SEM recorded from long-day (n = 12) and short-day photoperiod (n = 16). **P < 0.01, independent Student's t test.
Discussion
Here, we provide compelling evidence that exposure to a long-day photoperiod switches the polarity of GABAergic activity in most SCN neurons from inhibitory to excitatory. Presynaptically, sPSC frequency changes with differing day lengths, whereas postsynaptically, the photoperiod affects GABAergic activity within the SCN by changing the equilibrium potential of GABA-evoked current. The increase in excitatory GABAergic activity was reduced after blocking the Cl− cotransporter NKCC1 using bumetanide, suggesting a modulation of NKCC1 activity or expression. Thus, our data show that environmental conditions affect GABAergic activity by modulating cellular properties on a basic biophysical level.
The key mechanisms that contribute to the degree of synchronization within the SCN, reflected in the photoperiodic-induced changes in phase distribution (3), may depend on the ratio of excitatory to inhibitory GABAergic activity within the SCN, rather than an overall increase in GABAergic tone. The role of inhibition in synchronization has been shown previously in other neuronal networks (16, 17). Whether inhibition would also induce phase synchrony in the SCN remains to be established. In the present study, we found a relatively high percentage of inhibition in the short-day compared with the long-day photoperiod, which could contribute to the phase synchrony seen in short days. Freeman et al., however, suggested that GABAergic activity could be a phase desynchronizer and destabilizer in the SCN (18), but they did not distinguish between GABAergic excitation and inhibition. We suggest that elevated GABA-mediated excitation during the long-day photoperiod could destabilize the phase of neuronal activity and allow phase dispersal. The relation between GABAergic excitation and inhibition may thus determine the photoperiod-induced phase distribution in the SCN network. As such, it is plausible that both actions of GABA [phase-setting (4) and destabilization (18)] can facilitate the adjustment of different phase distributions during various photoperiods.
The contribution of GABAergic signaling to photoperiodic entrainment seems to depend on the state of synchrony within the SCN network and may be restricted to the day. After photoperiodic entrainment to a regime of LD20:4, the SCN neurons require neurotransmission by GABA as well as vasoactive intestinal peptide to readjust the phase of their rhythms in PERIOD2 expression (19). We have shown in the present study that the effect of a long-day photoperiod (LD16:8) on the ratio between excitation and inhibition is restricted to the day. This suggests a differential role of GABA during day and night, with an increased excitatory action during long days. However, the requirement of GABA during photoperiodic entrainment and the role of photoperiod-induced GABAergic excitation in determining the phase of SCN neurons needs to be addressed in future studies.
Changes in the photoperiod were recently reported to affect the relative expression of two neurotransmitters in hypothalamic neurons after exposure to long days (20). Here, we show that the action of a single transmitter system is affected by environmental conditions. On the basis of our findings, it would be interesting to investigate the influence of photoperiod on neurotransmitter systems in other brain regions. It is worth noting that our study has been performed in C57BL/6 mice. Although these mice are not seasonal breeders, they clearly show adaptation of behavior and SCN activity to changes in photoperiod (3). They exhibit after-effects in constant darkness, such as changes in rest and activity duration, and in waveform of the SCN rhythm, indicating a “memory” for day length.
The balance between excitation and inhibition has been proven to be necessary to preserve a normal physiological function in the central nervous system (7, 8). GABA, specifically, plays a pivotal role in the maintenance of this balance, as it is the major inhibitory neurotransmitter in the brain. During early development, GABA is an excitatory transmitter. A disturbance in GABAergic function at this stage causes neurodevelopmental disorders, such as mental retardation, Angelman syndrome, epilepsy, and autism (21). Recently, bumetanide has been shown to improve behavior in children with autism by reducing GABAergic excitation (22). GABA also acts as an excitatory neurotransmitter in the mature but pathological brain (23). Remarkably, in the mature healthy nervous system, GABA can also act as an excitatory transmitter in cortex, hippocampus, and amygdala in addition to the SCN (24). Although the role of GABAergic excitation is not known in the mature brain, it is clear that the balance of excitation and inhibition is regulated within a narrow range, and deviations from this range can lead to neuropsychiatric disorders (25).
One form of depression that is susceptible to changes in day length is seasonal affective disorder, which is at least partially based on photoperiodic alterations of the circadian system (26). A recent study using a day-active rodent model suggests a link between depressive-like behaviors and the photoperiodic responsiveness of the circadian clock (27). Although there is still insufficient evidence on the neurobiological mechanisms underlying seasonal affective disorder, altered GABAergic signaling in the SCN neuronal network should be considered a potential contributing factor. Furthermore, considering that GABA is a target for therapeutic treatments of sleep disorders and the role of the SCN in sleep regulating processes, the seasonal influence on GABAergic function may affect the therapeutic manipulation of this neurotransmitter system differently in summer compared with winter. Together, these findings imply that some crucial neuronal networks within the CNS are sensitive to changes in day length, or even to prolonged artificial light exposure common in modern society (28, 29).
Materials and Methods
Animals and Housing Conditions.
Male C57BL/6 mice (Harlan, Horst, the Netherlands; 8–16 wk old; n = 88) were housed under different light regimes, such as long-day (LD16:8), short-day (LD8:16), or equinoctial (LD12:12) photoperiods, in climate-controlled cabinets with ad libitum access to food and water. Before experimentation, the mice were exposed to their respective photoperiod for a minimum of 4 wk to ensure entrainment to the given light schedule. Experiments were performed within a 3-h interval centered in the middle of the day and the middle of the night. We used external time (ExT) to allow for easier comparison between different photoperiods. ExT 12 is defined as midday (middle of the light period), and ExT 0 as midnight (middle of the dark period) (30). All experimental procedures were approved by the Committee on Animal Health and Care of the Dutch government (no. 11010).
Slice Preparation.
The mice were killed between ExT 8–9 for the daytime recordings. For nighttime recordings, animals adjusted to short-day photoperiod were killed between ExT 11–12, whereas animals adjusted to long-day photoperiod were killed between ExT 15–16. Animals entrained to LD12:12 were killed between ExT 6–7.
Hypothalamic slices containing the SCN were prepared as described previously (31). In brief, brains were quickly removed and submerged into modified ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 116.4, KCl 5.4, NaH2PO4 1.0, MgSO4 0.8, CaCl2 1.0, MgCl2 4.0, NaHCO3 23.8, glucose 15, and 5 mg/L gentamicin (Sigma Aldrich, Munich, Germany) saturated with 95% O2-5% CO2 (pH, 7.2–7.4; osmolality, 290–310 mOsm). Coronal slices were cut using a vibratome (VT 1000S, Leica Microsystems, Wetzlar, Germany) and subsequently maintained in regular ASCF (CaCl2 increased to 2 mM and MgCl2 decreased to 0 mM) for at least 1 h before recordings. Slices containing the SCN were transferred to a recording chamber (RC-26G, Warner Instruments, Hamden, CT) mounted on the fixed stage of an upright fluorescence microscope (Axioskop 2-FS Plus; Carl Zeiss Microimaging, Oberkochen, Germany) and constantly perfused with oxygenated ACSF (2.5 mL/min).
Patch Clamp Recordings.
Whole-cell voltage-clamp recordings of sPSCs (n = 64 slices from 35 animals) were performed using a patch amplifier (EPC 10–2; HEKA, Lambrecht/Pfalz, Germany), as described previously (31). Micropipettes (with a tip resistance of 5–7 MΩ when filled with internal pipette solution) were fabricated from borosilicate glass capillaries using a PC-10 puller (Narishige, London, United Kingdom) and filled with an internal solution (pH, 7.2–7.3; osmolality, 290–300 mOsm) containing (in mM): 112.5 K-gluconate, 1 EGTA, 10 Hepes (Na+ salt), 5 MgATP, 1 GTP, 0.1 leupeptin, 10 phosphocreatine, 4 NaCl, 17.5 KCl, 0.5 CaCl2, and 1 MgCl2.
Spontaneous postsynaptic currents were analyzed using the MiniAnalysis program (Synaptosoft, Decatur, GA). Events were sorted by their decay time into glutamatergic PSCs [average decay time, 4.6 ms (32)] and GABAergic PSCs [average decay time, 15.4 ms (14)]. The GABA receptor antagonist gabazine (10 µM) was applied after the recording to confirm that the currents were GABAergic. Recordings with series resistance ≥40 MΩ were excluded from the data pool and subsequent analyses.
To measure GABA-evoked sPSPs, gramicidin perforated patch current-clamp recordings were performed (c.f. ref. 9; n = 16 slices from six animals). GABAergic responses are mediated by transmembrane flux of Cl−, and the polarity depends on the Cl− equilibrium potential (ECl) of the cell. Unlike whole-cell recordings, the gramicidin perforated patch technique does not change the intracellular Cl− concentration, and therefore GABAergic EPSPs can be measured at unaltered ECl. First, the tip of the pipette was filled with gramicidin-free solution containing (in mM): 143 K-gluconate, 2 KCl, 0.5 EGTA, and 10 Hepes, (pH, 7.2; osmolality, 295 mOsm) to facilitate the formation of a giga-Ohm seal. The pipettes were then back-filled with the same solution containing 50 μg/mL gramicidin. Within 6–20 min of forming a giga-Ohm seal, stable access resistance (40–200 MΩ) was achieved and remained throughout the recordings. Gabazine (10 μM) was applied to confirm that recorded EPSPs are evoked by GABA. We measured the liquid junction potential (16 mV) and corrected the values of membrane potential for the current-clamp recordings.
To determine the time course and equilibrium potential of GABA-evoked currents (EGABA), gramicidin perforated patch voltage-clamp recordings were performed (eight slices from eight animals) and GABA (200 μM, 6 s) was applied locally, using a focal application system (ALA-VM8; ALA Scientific Instruments, Farmingdale, NY). AMPA and NMDA receptors and Na+ and Ca2+ channels were blocked using CNQX (25 µM), AP-5 (50 µM), TTX (0.5 μM), and cadmium (25 μM), respectively. Voltage ramps from −100 to 0 mV in 200 ms were applied from a holding potential of −50 mV in the absence and subsequent presence of GABA with 40-s intervals (c.f. refs 33 and 34). In 4 ± 0.31 s (n = 26), the GABA current (either inward or outward) could reach its maximum. Therefore, we applied GABA for 6 s to ensure a proper response during the ramp protocol. Control ramp was repeated 40 s after the GABA ramp to check the reversibility of the GABA-evoked current. The membrane potential at which the current traces recorded in the absence and presence of GABA crossed was considered as EGABA. This value for EGABA was corrected for series resistance voltage error by subtracting the product of the net membrane current flowing at the cross point and the residual series resistance.
Ca2+ Imaging.
Ca2+ measurements were performed as described previously (35). Brain slices that included the SCN were loaded with the ratiometric Ca2+ indicator dye fura-2-acetoxymethyl ester (Fura-2-AM). The slices were loaded with 7 µM Fura-2-AM in ACSF at 37 °C for 10 min. The slices were then rinsed with freshly oxygenated ACSF for 10–30 min before recording. A monochromator (Polychrome V; TILL Photonics, Gräfeling, Germany) was used to deliver paired 50-ms light pulses of two excitation wavelengths (340 and 380 nM). Emitted light (505 nM) was detected by a cooled CCD camera (Sensicam; TILL Photonics), and images were acquired at 2-s intervals (0.5 Hz). Single-wavelength images were background subtracted, and ratio images (340/380) were generated. Region-of-interest-defined cells and mean ratio values were determined, from which the intracellular Ca2+ concentration was calculated. Experiments were controlled by imaging software (TILLvision; TILL Photonics).
GABA (200 μM, 10 s) was applied locally to trigger Ca2+ transients in SCN neurons, using a focal application system (ALA-VM8). In addition, ACSF containing elevated K+ (“High-K,” 20 mM, 10 s) was applied to identify healthy, responding cells (LD16:8: day, n = 17 slices from eight animals; night, n = 11 slices from six animals; LD8:16: day, n = 16 slices from eight animals; night, n = 13 slices from seven animals; LD12:12: day, n = 8 slices from five animals). Additional experiments were performed in slices from mice adjusted to a long-day photoperiod to which bumetanide (10 μM), a pharmacological agent that blocks the activity of the NKCC1 cotransporter, was administered for 5–7 min before GABA application (n = 8 slices from five animals).
Chemicals.
GABA, gramicidin, and all salts were purchased from Sigma-Aldrich. Gabazine, bumetanide, CNQX, AP-5, and TTX were purchased from Tocris Bioscience (Bristol, United Kingdom), and Fura-2-AM was purchased from Teflabs (Austin, TX).
Data Analysis.
Data were collected and analyzed using FitMaster (HEKA), Igor Pro (Wavemetrics, Portland, OR), and MiniAnalysis (Synaptosoft). Ca2+ images were analyzed using TILLvisION, and the responses of neurons were analyzed using IGOR Pro. Ca2+ transients with an increase in amplitude ≥10% from the baseline level were defined as excitatory, and transients with a decrease in amplitude ≥10% were defined as inhibitory. Cells that responded with both excitatory and inhibitory responses after a single GABA pulse were defined as biphasic. Last, cells that responded with a change in amplitude <10% from baseline were defined as nonresponding. The effects of bumetanide were analyzed by inspecting the difference in amplitude of the cell’s response to a GABA pulse before and after bumetanide application. The amplitude of the response was calculated by subtracting the baseline from the peak Ca2+ level achieved during GABA application.
Statistical analyses were performed using SPSS (IBM, Armonk, NY). The appropriate statistical test was selected after using Shapiro-Wilk and Levene’s tests to evaluate the normality of the data and homogeneity of variances respectively. All values obtained from the whole-cell patch clamp recordings were tested for significance using an independent Student's t test (two-tailed). The gramicidin perforated patch recordings were analyzed using the nonparametric Wilcoxon signed-rank test (two-tailed). The distributions of GABA-induced responses were analyzed using the χ2-test (two-tailed). The effect of bumetanide was measured using the Wilcoxon signed-rank test (two-tailed). The difference in equilibrium potential of GABA-evoked current in various photoperiods was analyzed using independent Student's t test (two-tailed). Differences with P ≤ 0.05 were considered to be significant.
Supplementary Material
Acknowledgments
We thank Heleen Post-van Engeldorp Gastelaars and Kit-Yi Yam for their technical assistance. This work was supported by The Netherlands Organisation for Scientific Research/Netherlands Organisation for Health Research and Development Grant TOPGo 91210064 (to J.H.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319820111/-/DCSupplemental.
References
- 1.Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol. 2011;32(3):303–319. doi: 10.1016/j.yfrne.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mrugala M, Zlomanczuk P, Jagota A, Schwartz WJ. Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R987–R994. doi: 10.1152/ajpregu.2000.278.4.R987. [DOI] [PubMed] [Google Scholar]
- 3.VanderLeest HT, et al. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17(5):468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 4.Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15(10):886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25(1):123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 6.Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309(1):89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 7.Baroncelli L, et al. Brain plasticity and disease: A matter of inhibition. Neural Plast. 2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trevelyan AJ, Schevon CA. How inhibition influences seizure propagation. Neuropharmacology. 2013;69:45–54. doi: 10.1016/j.neuropharm.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Choi HJ, et al. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28(21):5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: Excitatory effects during the night phase. J Neurophysiol. 2002;87(2):834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- 11.Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci. 2009;30(8):1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387(6633):598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- 13.Wagner S, Sagiv N, Yarom Y. GABA-induced current and circadian regulation of chloride in neurones of the rat suprachiasmatic nucleus. J Physiol. 2001;537(Pt 3):853–869. doi: 10.1111/j.1469-7793.2001.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004;92(1):311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin RP, Allen CN. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci. 2007;27(43):11748–11757. doi: 10.1523/JNEUROSCI.1840-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartos M, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA. 2002;99(20):13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci. 1994;1(4):313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- 18.Freeman GM, Jr, Krock RM, Aton SJ, Thaben P, Herzog ED. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron. 2013;78(5):799–806. doi: 10.1016/j.neuron.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron. 2013;80(4):973–983. doi: 10.1016/j.neuron.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340(6131):449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- 21.Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011;2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemonnier E, et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatr. 2012;2:e202. doi: 10.1038/tp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Ari Y, Holmes GL. The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol. 2005;18(2):141–145. doi: 10.1097/01.wco.0000162855.75391.6a. [DOI] [PubMed] [Google Scholar]
- 24.Chung L. Recent progress in GABAergic excitation from mature brain. Arch Pharm Res. 2012;35(12):2035–2044. doi: 10.1007/s12272-012-1202-8. [DOI] [PubMed] [Google Scholar]
- 25.Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci USA. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leach G, Ramanathan C, Langel J, Yan L. Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus) Neuroscience. 2013;234:31–39. doi: 10.1016/j.neuroscience.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage. 2011;92(10):2714–2722. doi: 10.1016/j.jenvman.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Dijk DJ, et al. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS ONE. 2012;7(2):e30037. doi: 10.1371/journal.pone.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daan S, Merrow M, Roenneberg T. External time—internal time. J Biol Rhythms. 2002;17(2):107–109. doi: 10.1177/074873002129002375. [DOI] [PubMed] [Google Scholar]
- 31.Farajnia S, et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci. 2012;32(17):5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel S, Itri J, Colwell CS. Excitatory mechanisms in the suprachiasmatic nucleus: The role of AMPA/KA glutamate receptors. J Neurophysiol. 2002;88(2):817–828. doi: 10.1152/jn.2002.88.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol. 2002;545(Pt 1):183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Proddutur A, Elgammal FS, Ito T, Santhakumar V. Status epilepticus enhances tonic GABA currents and depolarizes GABA reversal potential in dentate fast-spiking basket cells. J Neurophysiol. 2013;109(7):1746–1763. doi: 10.1152/jn.00891.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel S, et al. Mechanism of bilateral communication in the suprachiasmatic nucleus. Eur J Neurosci. 2013;37(6):964–971. doi: 10.1111/ejn.12109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.