Significance
Understanding the intrinsic mechanisms underlying formation of humoral memory during infection and the ability of the humoral memory population to persist for long periods of time is important for identifying potential targets for improving the efficacy of vaccines. Here we demonstrate that the chromatin modifier MOZ regulates B-cell memory formation, controlling memory compartment composition. This activity of MOZ is B cell-intrinsic and is required for establishing the germinal center gene expression program. IgM memory B cells have been implicated in maintaining the memory B-cell population over time, whereas isotype-switched memory B cells rapidly differentiate into plasmablasts upon secondary infection. Therefore, identifying potential chromatin changes that may induce differentiation into one subset over another makes MOZ a viable target for clinical translation.
Abstract
Memory B cells and long-lived bone marrow-resident plasma cells maintain humoral immunity. Little is known about the intrinsic mechanisms that are essential for forming memory B cells or endowing them with the ability to rapidly differentiate upon reexposure while maintaining the population over time. Histone modifications have been shown to regulate lymphocyte development, but their role in regulating differentiation and maintenance of B-cell subsets during an immune response is unclear. Using stage-specific deletion of monocytic leukemia zinc finger protein (MOZ), a histone acetyltransferase, we demonstrate that mutation of this chromatin modifier alters fate decisions in both primary and secondary responses. In the absence of MOZ, germinal center B cells were significantly impaired in their ability to generate dark zone centroblasts, with a concomitant decrease in both cell-cycle progression and BCL-6 expression. In contrast, there was increased differentiation to IgM and low-affinity IgG1+ memory B cells. The lack of MOZ affected the functional outcome of humoral immune responses, with an increase in secondary germinal centers and a corresponding decrease in secondary high-affinity antibody-secreting cell formation. Therefore, these data provide strong evidence that manipulating epigenetic modifiers can regulate fate decisions during humoral responses, and thus could be targeted for therapeutic intervention.
The foundation for vaccine success is the ability of the immune system to provide heightened responses to pathogens if the host has been infected prior—this is termed immune memory. Humoral memory incorporates two populations of cells: long-lived antibody (Ab)-secreting cells (ASCs) and memory B cells. In a T cell-dependent response, humoral memory is mainly produced within germinal centers (GCs) (1), transient sites within lymphoid follicles in which antigen-specific B cells undergo iterative rounds of proliferation and affinity maturation (2–5). The GC can be divided into dark and light zones (DZs and LZs, respectively) in which specialized functions occur (6). Within the DZ, B cells undergo rounds of division and can isotype-switch. Affinity maturation occurs through somatic hypermutation (SHM) of the B-cell receptor, which modulates receptor affinity for the antigen and selection of high-affinity mutants in the LZ. Although memory B cells and ASCs can arise throughout the response, it is within the GC that the quality, and thus the success of these populations to mediate long-term protection, is determined.
Cell proliferation, migration, and differentiation during an immune response are modulated by the integration of extrinsic signals from the microenvironment, together with intrinsic mediators that activate or repress gene expression (7). Transcription factors are often associated with the maintenance of cellular identity, such as BCL-6 for GC B cells (8) and BLIMP-1 for ASCs (9). Other intrinsic factors, such as cell-cycle regulators, are differentially expressed between naïve and memory B cells, thus potentiating the enhanced swiftness of secondary responses (10).
Enzymes known as epigenetic modifiers can also modulate gene expression during an immune response by altering the structure of histones. The N-terminal tails of histones are modified by different enzymes, which affect chromatin conformation to induce or inhibit transcription at particular loci (11–13). There is increasing evidence that epigenetic modifications by histone acetyltransferases, deacetylases, and methyltransferases regulate lymphocyte development and responses. For example, the methyltransferase EZH2, a member of the polycomb repressive complex, is vital for Igh rearrangement (and thus B-cell development) by methylation of histone H3 (14). Polycomb group proteins are differentially expressed in the LZ or/and DZ (15, 16). Accordingly, EZH2 has been found to play a role in GC formation by regulation of cell-cycle checkpoints (17, 18). Furthermore, epigenetic modifiers regulate the stability of T-cell subsets during T-cell development (19). Both T-bet and Gata-3, and thus IFN-γ and IL-4, are regulated by histone modifications in such a way that T helper cells are intrinsically wired to have the flexibility to alter their transcriptional pathway depending on the situation (20). An equivalent plasticity in B cells has not yet been identified.
Differentiation into GC B cells or ASCs is associated with activation of distinct transcriptional networks. In contrast, naïve and memory B cells have very similar transcriptional profiles (10, 21–23). Despite this, there are several key differences between naïve and memory B-cell populations: the ability to persist without continual input from precursors, as well as the rapid and robust response of memory B cells compared with naïve B cells. Memory B cells, however, are antigen-experienced and thus they may retain epigenetic “memory” of their previous involvement in an immune response. The involvement of epigenetic changes in the formation and maintenance of B-cell memory has yet to be investigated.
To test whether chromatin modifiers regulate B-cell differentiation and the stability of memory B-cell subsets, we investigated the role of a chromatin modifier in these processes. Memory B cells have properties that are reminiscent of stem cell populations: They can undergo self-renewal to maintain the population in both the absence and presence of antigen. Although memory B cells are not stem cells, they may contain some of the properties that allow stem cells to self-renew. MOZ (MYST3), a member of the MYST family of histone acetyltransferases, is essential for long-term repopulation of hematopoietic stem cells (24, 25), and translocations of Moz promote continual division of leukemic cells (26). MOZ is part of a transcription regulatory complex analogous to the polycomb repressor complex, but with differing enzymatic activity and is activating rather than repressing. It is expressed in both human and murine B cells, and thus is a candidate regulator of B-cell differentiation. Here we show, using stage-specific deletion, that MOZ regulates the behavior and fate decision of B cells in both primary and secondary responses, demonstrating for the first time, to our knowledge, the requirement of a chromatin activator complex in the formation of humoral memory B cells.
Results
GC Formation Requires the Histone Acetyltransferase MOZ.
To investigate the role of MOZ in B cells, the Cre-loxP system was used. Mice with exons 3–7 of the Moz gene flanked by loxP sites (27) were crossed with Mb-1Cre/+ mice, thereby deleting Moz in B cells when Igα is expressed during development (28). B-cell development in Mozfl/flMb-1Cre/+ mice was comparable to Moz+/+Mb-1Cre/+ (herein called Mb-1Cre/+) control mice (Fig. S1).
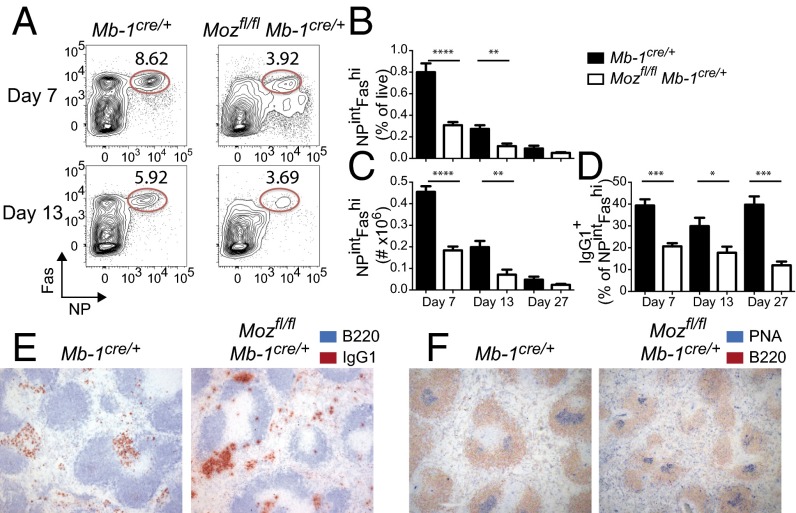
To assess T cell-dependent responses, Mozfl/flMb-1Cre/+ and Mb-1Cre/+ mice were immunized with alum-precipitated (4-hydroxy-3-nitrophenyl)acetyl conjugated to keyhole limpet hemocyanin (NP–KLH). GC formation was defective in the absence of MOZ (Fig. 1A). NP-specific GC B cells were decreased by more than twofold 7 d postimmunization (Fig. 1 A–C), with the frequency and number of NPintFashi B cells both significantly reduced in Mozfl/flMb-1Cre/+ (Fig. 1 A–C). This defect was sustained over time (Fig. 1 A–C). Within the GC population, the frequency of cells that had isotype-switched to IgG1, the predominant isotype in humoral responses to NP–KLH in alum, was decreased in the absence of MOZ at all time points measured (Fig. 1D). In contrast, histological analysis of splenic ASCs demonstrated a comparable presence of IgG1+ ASCs, suggesting there was not an intrinsic defect in isotype switching to IgG1 (Fig. 1E). There was a decrease in GC size (Fig. 1F) but not frequency of GCs per follicle. Therefore, GC expansion was defective in the absence of Moz.
Fig. 1.
Histone acetyltransferase MOZ is important for GC formation. Mb-1Cre/+ (WT control) and Mozfl/flMb-1Cre/+ mice were immunized with NP–KLH/alum. (A and B) B cells (CD19+IgDlo) were stained for antigen-specific GCs (NPintFashi) at multiple time points postimmunization. (A) Representative flow cytometric plot of gating profiles for GC B cells. (B–D) Frequency (B) and number (C) of GC B cells and (D) frequency of GC B cells that are IgG1+. Mb-1Cre/+, black bars; Mozfl/flMb-1Cre/+, white bars. Data are combined from three experiments (day 7) and two per time point (day 13 and day 27); n = 5–10 per genotype. Error bars indicate ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (E and F) Immunohistological analysis of splenic sections stained with (E) B220 (blue) and IgG1 (red) or (F) B220 (red) and PNA (blue) day 7 postimmunization; representative of three spleens per genotype. Original magnification: 5×.
GC Dynamics Are Regulated by Moz.
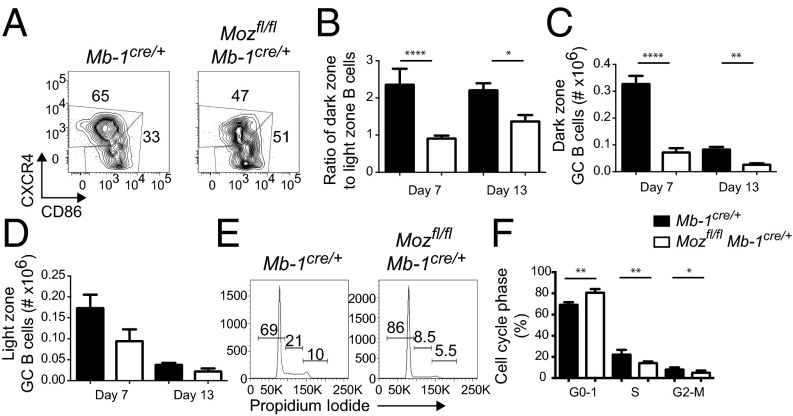
The nature of the GC defect was investigated by assessing multiple characteristics of GC B-cell biology. LZ and DZ representation can be assessed by flow cytometry using the markers CXCR4 and CD86 (6), with CD86loCXCR4hi identifying DZ B cells and CD86hiCXCR4lo identifying LZ B cells (Fig. 2A). In immunized Mozfl/flMb-1Cre/+, the ratio of LZ to DZ cells was skewed significantly toward the LZ, compared with control mice (Fig. 2 A and B). DZ B cells were decreased by approximately fivefold at day 7 postimmunization (Fig. 2C). In contrast, the number of LZ B cells was not significantly different (Fig. 2D).
Fig. 2.
MOZ regulates proliferation and zonal formation within the GC. (A–D) GC B cells were assessed for DZ and LZ proportions using CD86 and CXCR4 to differentiate these zones by flow cytometry. (A) NP+FashiIgDlo B cells were assessed for zonal formation at day 7 postimmunization in Mb-1Cre/+ and Mozfl/flMb-1Cre/+. (B–D) Ratio of DZ to LZ GC B cells (B) and number of DZ (C) and LZ (D) GC B cells. Mb-1Cre/+, black bars; Mozfl/flMb-1Cre/+, white bars. Data are combined from three (day 7) and two (day 13) experiments; n = 5–10 per genotype. Error bars indicate ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001. (E and F) Cell-cycle analysis of day 7 sort-purified GC B cells from Mb-1Cre/+ (black bars) and Mozfl/flMb-1Cre/+ (white bars). Data are combined from two experiments; n = 6 per genotype. Error bars indicate ± SEM. *P < 0.05, **P < 0.01.
The DZ is the predominant site of centroblast proliferation. To assess whether the decrease in GC B cells was due to inadequate proliferation in the DZ, cell-cycle analysis of sort-purified GC B cells was assessed (Fig. 2 E and F). At day 7 postimmunization, there was a significantly higher frequency of cells in G0/G1 in the absence of MOZ (Fig. 2 E and F). Thus, it appears that expansion in the DZ requires MOZ activity.
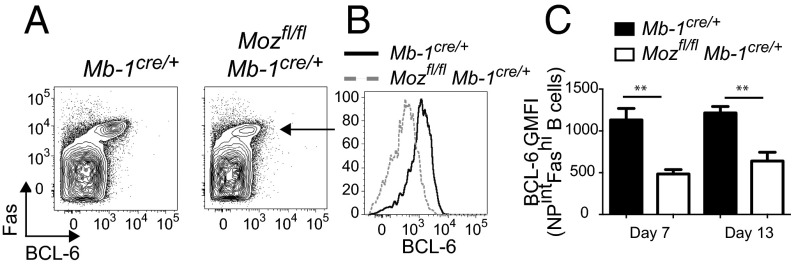
BCL-6 is a transcriptional repressor required for GC formation via regulating the expression of a network of genes (8, 29). We speculated that the inability of MOZ-deficient B cells to form a normal DZ reflected inadequate expression of BCL-6. Assessment of GC B cells 7 d postimmunization demonstrated a significant decrease in BCL-6 protein (Fig. 3A). This was not simply due to decreased numbers of GC B cells, because BCL-6 was significantly decreased within the MOZ-deficient GC population (Fig. 3 B and C). Defective BCL-6 expression was apparent at all time points examined (Fig. 3C). Taken together, formation of the DZ and appropriate BCL-6 expression were dependent on MOZ activity in B cells.
Fig. 3.
Reduced BCL-6 expression in the absence of MOZ. (A and B) Representative plots of BCL-6 expression versus Fas (arrow; A) in CD19+IgDlo cells, and BCL-6 expression in NP+FashiCD19+IgDlo cells (B), day 7 postimmunization. (C) Geometric mean fluorescence intensity (GMFI) of BCL-6 in NP+FashiCD19+IgDlo cells in Mb-1Cre/+ (black bars) and Mozfl/flMb-1Cre/+ (white bars). Data are combined from three (day 7) and two (day 13) experiments; n = 5–10 per genotype. Error bars indicate ± SEM. **P < 0.01.
Moz Activity Is Required After B Cells Are Activated by Antigen.
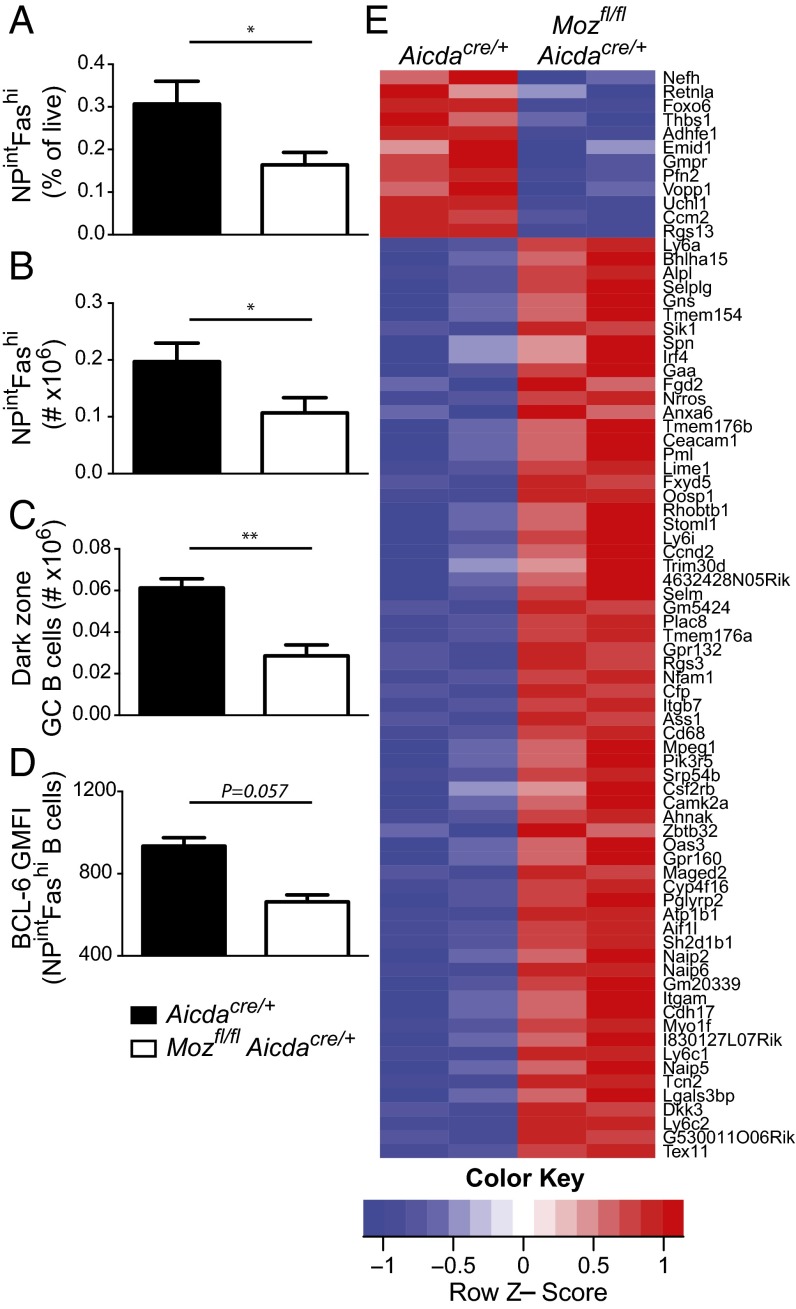
Deleting Moz during B-cell development using Mozfl/flMb-1Cre/+ mice suggested that chromatin-mediated regulation of transcription shaped B-cell responses to immunization. It was unclear, however, whether the GC B-cell defect was due to MOZ activity on the transcriptional program during B-cell development or after activation with antigen. Deleting Moz within antigen-activated B cells would resolve this issue by revealing any requirement for epigenetic reprogramming of gene transcription upon activation. As such, Mozfl/fl mice were crossed with AicdaCre/+ mice. Aicda encodes activation-induced cytidine deaminase, which is expressed by B cells shortly after activation by antigen and is required for SHM and class-switch recombination (30). GC formation in Mozfl/flAicdaCre/+ mice was decreased by more than twofold compared with controls (Fig. 4 A and B), consistent with Mozfl/flMb-1Cre/+ mice. DZ B-cell formation and BCL-6 expression were defective when MOZ was deleted in B cells after activation (Fig. 4 C and D).
Fig. 4.
MOZ is required in activated B cells during a humoral response. (A–D) Mozfl/flAicdaCre/+ (white bars) and AicdaCre/+ (WT control; black bars) were immunized with NP–KLH/alum. (A) GC B-cell frequency, (B) GC B-cell number, (C) DZ GC B-cell number, and (D) GMFI of BCL-6 in GC B cells were assessed at day 7 postimmunization. Data are combined from two experiments; n = 7 per genotype. Error bars indicate ± SEM. *P < 0.05, **P < 0.01. (E) Heatmap of RNA-sequencing data displayed in Table S1; each column is an independent sample obtained from pooled sort-purified day 7 GC B cells from either AicdaCre/+ or Mozfl/flAicdaCre/+ mice.
Given the reported activities of MOZ, we hypothesized that in its absence, activated B cells would change their gene expression programs. Microarray analysis of resting Mozfl/flAicdaCre/+ or AicdaCre/+ naïve B cells from mice showed no differential gene expression. To investigate gene expression changes upon activation, we sort-purified day 7 GC B cells from Mozfl/flAicdaCre/+ or AicdaCre/+ mice and performed RNA-sequencing analysis. The majority of genes differentially expressed were up-regulated in the absence of MOZ (Fig. 4E and Table S1); 12 genes were down-regulated in MOZ-deficient GC B cells, whereas 66 genes were up-regulated. There was no substantial overlap between the genes regulated in the absence of MOZ and those that are differentially expressed in the DZ and LZ (6), demonstrating that these changes were not caused by changes in zonal ratio.
Regulation of Memory B-Cell Formation by Moz.
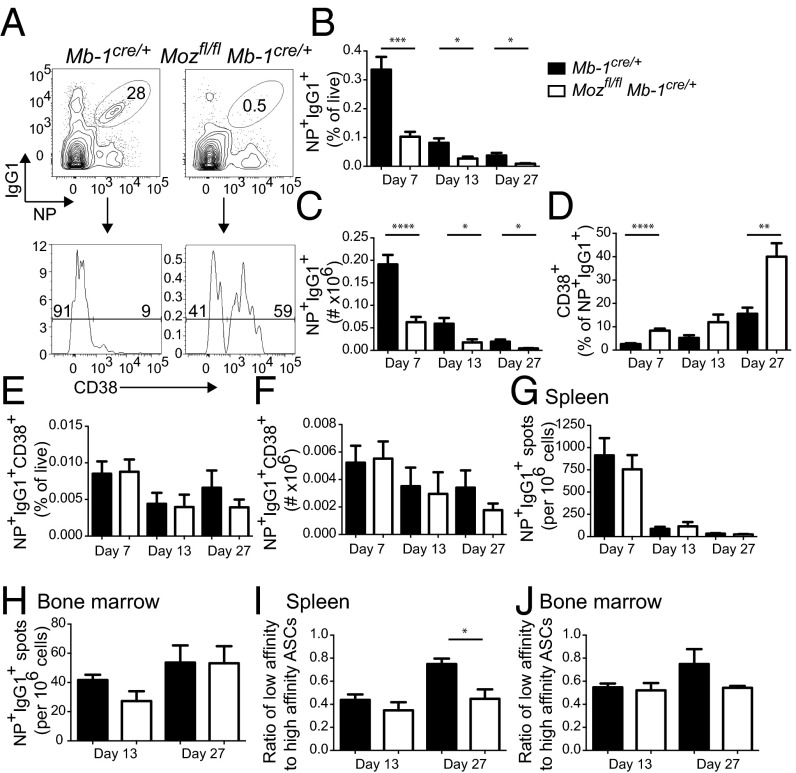
Although GC formation was defective in the absence of MOZ, GC B cells were still detected (Fig. 1). To investigate whether the residual GCs were functional, the quality and quantity of memory B cells and ASCs were assessed (Fig. 5). Strikingly, NP+IgG1+ B cells were almost absent in Mozfl/flMb-1Cre/+, with the few cells detected being mostly CD38+ and therefore memory B cells (Fig. 5 A–C). In contrast, the majority of NP+IgG1+ B cells in immunized Mb-1Cre/+ controls were CD38− GC B cells (Fig. 5D). The increased proportion of CD38+ memory B cells within the diminished NP+IgG1+ population in Mozfl/flMb-1Cre/+ mice resulted in no difference in the frequency or number of the NP+IgG1+CD38+ population compared with Mb-1Cre/+ controls (Fig. 5 E and F).
Fig. 5.
MOZ regulates memory formation. (A) Flow cytometric assessment of differentiation in the absence of Moz. NP+IgG1+CD19+Dumplo B cells were assessed for CD38 expression. (B and C) Frequency (B) and number (C) of NP+IgG1+ B cells in Mb-1Cre/+ (black bars) and Mozfl/flMb-1Cre/+ (white bars). (D–F) The frequency of CD38+ memory B cells within NP+IgG1+ (D), NP+IgG1+CD38+ frequency (E), and number (F) were assessed at multiple time points postimmunization. (G–J) ELISPOT analysis of NP+IgG1+ ASCs in the spleen (G and I) and BM (H and J) at multiple time points postimmunization. (I and J) Affinity of ASCs was assessed by calculating the ratio of NP2-binding to NP14–16-binding IgG1 ASCs. Data are combined from three experiments (day 7) and two per time point (day 13 and day 27); n = 5–10 per genotype. Error bars indicate ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The frequencies of splenic (Fig. 5G) and bone marrow (BM) (Fig. 5H) NP+IgG1+ ASCs were comparable in Mozfl/flMb-1Cre/+ and Mb-1Cre/+ mice, and serum Ab was also comparable (Fig. S2). It was possible that these ASCs were formed early in the response and would therefore be expected to be of low affinity. Consistent with this, the ratio of NP2- to NP14-binding IgG1+ ASCs was significantly decreased in MOZ-deficient splenic ASCs (Fig. 5I) and slightly decreased in BM ASCs (Fig. 5J), likely due to the defect in the DZ.
The NP+IgG1+CD38+ B cells detected could represent either GC-independent or GC-derived—and therefore somatically mutated—memory B-cell populations. We investigated this possibility by sequencing VH genes of responding B-cell populations. Single GC and memory B cells were sort-purified 13 d and 4 wk postimmunization, respectively (Table S2). MOZ-deficient GC B cells underwent mutation and affinity-based selection (as indicated by the frequency of the canonical position 33 Trp→Leu exchange at VH33) comparable to controls. However, MOZ-deficient memory B cells had a reduction in the frequency of VH186.2 mutations and Trp→Leu mutation. Therefore, although GC B cells underwent SHM and selection in the absence of MOZ, the memory B-cell population appeared to have undergone insufficient rounds of both. However, the presence of mutations and some Trp→Leu replacements demonstrated that MOZ-deficient memory B cells were in fact GC-derived (Table S2). Taken together with the reduced affinity of the ASC compartment, MOZ deficiency in B cells appeared to affect the quality but not the number of cells differentiating into NP+IgG1+ memory B cells and ASCs.
B Cell-Intrinsic Regulation of Humoral Responses.
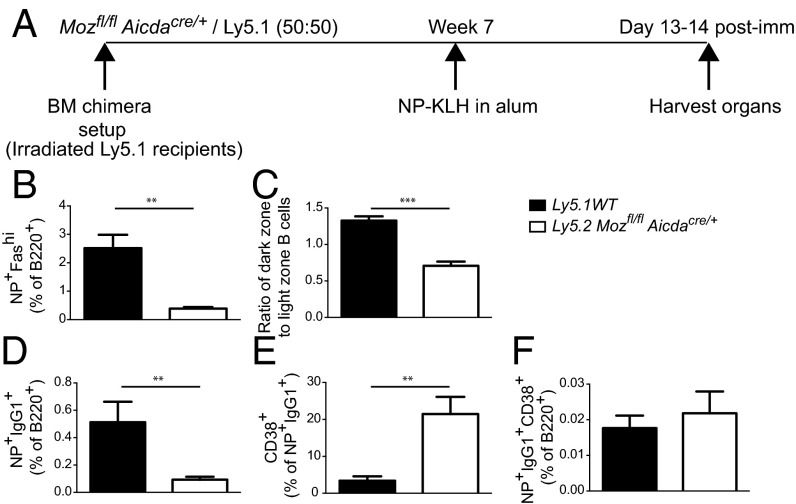
Defective GC development will also impact on T follicular helper cell (TFH) numbers, which can then in turn influence B-cell behavior through alteration of the microenvironment. Thus, it was important to determine whether the defects observed were directly due to the lack of MOZ in B cells and not downstream of the defect in TFH development. To discriminate these possibilities, mixed BM chimeras were used. BM from Ly5.2 Mozfl/flAicdaCre/+ was mixed in a 1:1 ratio with BM from Ly5.1 wild-type mice and used to reconstitute lethally irradiated recipients (Fig. 6A). After full reconstitution, mice were immunized and assessed for humoral responses. Similar to intact mice, frequencies of MOZ-deficient GC B cells were reduced compared with controls (Fig. 6B), and the DZ-to-LZ proportions were skewed (Fig. 6C) due to the reduction of MOZ-deficient, DZ GC B cells. The frequency of NP+IgG1+ B cells was significantly decreased in the absence of MOZ (Fig. 6D). Although the proportion that was CD38+ was significantly increased (Fig. 6D), the total frequency of NP+IgG1+CD38+ B cells was not significantly different (Fig. 6E), similar to intact mice (Fig. 5). Therefore, in the presence of a normal microenvironment, MOZ-deficient B cells had an intrinsic defect in GC formation.
Fig. 6.
B cell-intrinsic defect in the absence of MOZ. (A) Schematic representation of the BM chimera setup as detailed in Materials and Methods. BM chimeras in which irradiated Ly5.1 recipients were reconstituted with 50% Ly5.1 BM and 50% Mozfl/flAicdaCre/+ BM. (B–F) Flow cytometric assessment of the frequency of GC B cells (B), ratio of DZ to LZ within GC B cells (C), NP+IgG1+ B cells (D), CD38+ frequency of NP+IgG1+ B cells (E), and frequency of NP+IgG1+CD38+ B cells (F). Data are combined from two independent experiments; n = 7 mice per genotype. Error bars indicate ± SEM. **P < 0.01, ***P < 0.001.
MOZ Regulates ASCs and GCs in Secondary Responses.
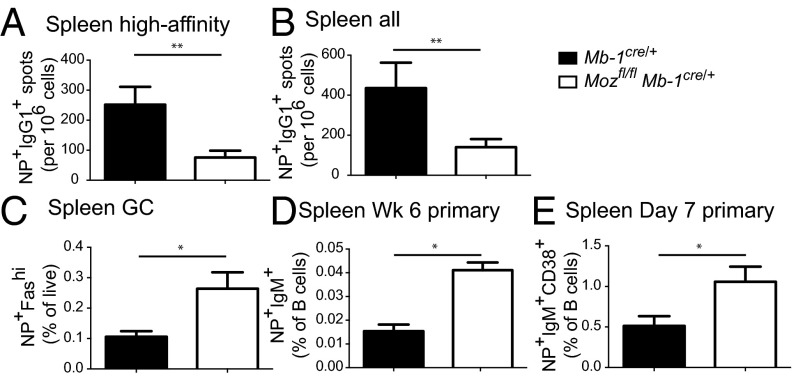
Memory B cells respond rapidly and robustly to infection with quick expansion and differentiation into high-affinity ASCs while not exhausting the memory B-cell pool. It is not known whether epigenetic properties of memory B cells contribute to this outcome. Although our results demonstrated that the quality, but not the quantity, of the memory B-cell pool was altered by the absence of Moz, we assessed whether the memory B-cell pool was functional. Accordingly, Mozfl/flMb-1Cre/+ mice were boosted with soluble antigen at least 6 wk after the primary immunization (Fig. 7). The memory B-cell pool in Mozfl/flMb-1Cre/+ mice was able to produce high-affinity ASCs in the spleen; however, they were significantly reduced compared with controls (Fig. 7 A and B) although starting frequencies were comparable (Fig. S3). In contrast, there was an approximately twofold increase in GC B-cell frequency in Mozfl/flMb-1Cre/+ mice compared with controls 3 d postsecondary immunization (Fig. 7C). It was recently demonstrated that secondary GCs arise from IgM+ memory B cells, whereas switched memory B cells differentiate into ASCs (1). Accordingly, IgM+ antigen-specific memory B cells were also increased in Mozfl/flMb-1Cre/+ mice by approximately twofold preboost (Fig. 7D). This was also confirmed in Mozfl/flAicdaCre/+, demonstrating that Moz deletion in activated B cells also resulted in an increase in IgM memory B cells (Fig. S3). To investigate whether these cells were generated early in the primary response, IgM+ antigen-specific memory B cells were assessed 7 d postprimary immunization. Indeed, IgM+ antigen-specific memory B cells were increased early in the primary response (Fig. 7E). The results suggest that chromatin modification by MOZ during a primary response can alter the composition of the memory B-cell population and therefore the dynamics of a secondary response.
Fig. 7.
Alteration in secondary responses in the absence of MOZ. To assess secondary responses, mice were immunized with NP–KLH precipitated in alum, rested for at least 6 wk, and then boosted with NP–KLH in PBS. Humoral responses were assessed 3 d postboost. (A and B) ELISPOT analysis of secondary NP+IgG1+ ASCs in the spleen in Mb-1Cre/+ (black bars) and Mozfl/flMb-1Cre/+ (white bars). High-affinity NP+IgG1+ ASCs (A) as well as all NP+IgG1+ ASCs (B) were assessed. (C) Flow cytometric assessment of GC B cells generated during a secondary response. (D and E) IgM+IgDlo memory B cells generated during a primary response 6 wk (D) or 7 d (E) postprimary immunization. Data are combined from two experiments; n = 4–7 per genotype. Error bars indicate ± SEM. *P < 0.05, **P < 0.01.
Discussion
The fundamental role of memory B cells is to differentiate rapidly into high-affinity plasmablasts on challenge. However, for long-lived immunity, the memory B-cell pool cannot be depleted completely during secondary responses but rather must be maintained by as yet undetermined mechanisms. Recently, this dichotomy of persistence and differentiation has been ascribed to the IgM+ memory and classically switched memory B-cell subsets (31, 32). Here we demonstrate that the chromatin-modifying enzyme MOZ participates in regulating the formation of these memory B-cell states and thus their function during secondary responses.
Memory B-cell populations have properties reminiscent of stem cell populations in that the population is maintained in the absence of antigen (33) but is also able to produce different types of B cells (ASCs, GCs, and memory B cells) with functionally distinct properties. Murine memory B cells have been found to express LIF-R protein and Bmpr1a transcript, two receptors that have important roles in stem cells (22). We and others have shown that the chromatin modifier MOZ has a role in stem cell self-renewal (24, 25) and specifically targets H3K9 (27, 34). In other situations, where cell development is altered by the absence of MOZ, only a very small number of genes have been identified as being direct targets of histone acetylation by MOZ (27, 34). The small number of gene expression changes revealed in our analysis suggests that a similar phenomenon may be occurring in MOZ-deficient B cells during a humoral response. There was no substantial overlap of the altered gene expression signature with LZ/DZ signatures (6), demonstrating that the changes were not solely due to the changed zonal ratios and point instead to a discreet function. Although there is little current information about epigenetic modifications during humoral responses, an essential role has been identified for the histone methyltransferase EZH2 for GC formation (18, 35) with the consequence that EZH2-deficient mice have decreased memory and ASC populations (35). Here we demonstrate that a chromatin modifier can also modulate GC B-cell differentiation into memory populations to favor one subset of memory B cell over another.
Similar to mice with EZH2 deficiency, GC formation was also defective in the absence of MOZ activity. Previous analysis of expression of EZH2 and other polycomb group proteins demonstrated that these enzymes segregated primarily into either the LZ or DZ (15, 16), suggesting activity is closely associated with particular functions in a specific zone. Decreased GC B-cell proliferation and number of DZ B cells in the absence of MOZ indicated that the consequences of MOZ activity were localized to the DZ of the GC. The use of Aicda-Cre mice in our study revealed that MOZ was active in antigen-activated B cells, suggesting reprogramming of gene transcription in these cells affecting both GC and memory populations. Thus, histone-modifying enzymes program antigen-activated B-cell subsets, subsequently regulating the formation and function of B-cell states. This is reminiscent of what happens in Th1 and Th2 subsets, in which histone modifications are required for the stability of these subsets (20) and alterations to histone-modifying enzymes increased plasticity and thus differentiation potential of these populations.
Recently, Shlomchik and colleagues postulated that production of memory B cells and ASCs is tied to the kinetics of the GC (7, 36). They propose that memory B cells arise during the initial phase of the GC, whereas the majority of ASCs that will populate the long-lived compartments arise later. In addition to the increase in IgM memory B cells early in the response, the MOZ-deficient memory B-cell IgG1+ population also appeared to have not undergone normal affinity maturation, even though SHM (mutation frequency) and selection (Trp→Leu mutation frequency) of GC B cells was comparable to that of control mice. The kinetics of the GC response, in which a decrease in GC B-cell number is evident by day 7 but the difference between MOZ-deficient and control GCs remains similar for the rest of the GC response, as well as the difference in GC size rather than frequency per follicle, demonstrate that the defect can be localized to the early stages of a response postimmunization. Taken together with the sequencing data, the findings suggest that in the absence of MOZ there is inadequate expansion and aberrant differentiation of GC B cells into memory B cells occurring during the early phase of the response.
The regulation of GC B-cell differentiation into ASCs has been an area of intense research for the past decade, resulting in the identification of so-called master regulators of this process including the transcription factors BLIMP-1 and IRF4 (9, 37). In contrast, no such “master transcription factors” have been identified in regulating the formation of memory B cells, suggesting a different mechanism may operate. Previous studies have shown that memory B cells, unlike GCs and ASCs, do not have vastly different transcriptional profiles from naïve B cells (10, 21–23). This should not be surprising, because in essence the function of naïve and memory B cells is quite similar; for example, both are required to recirculate in a quiescent state and then proliferate and differentiate into ASCs following antigen exposure. However, memory B cells (and long-lived ASCs) can persist in the absence of antigen or input from precursors for periods far greater than naïve B cells (33). One possibility is that epigenetic changes enacted during memory B-cell formation are responsible for the longevity and/or enhanced responses of the memory B-cell population. Recently, it was published that discreet memory B-cell functions were mediated by memory B-cell subsets distinguished by whether or not they had undergone isotype switching (31, 32). In these studies, IgG+ memory B cells rapidly differentiated into plasmablasts during a secondary response, whereas IgM+ memory B cells formed GCs (if serum Ab was reduced), thus potentially repopulating the memory compartment. Here we demonstrate that not only can the chromatin modifier MOZ affect differentiation into memory B cells during a humoral response but in doing so it also affects the function of the memory population. Modulation of MOZ expression or activity could regulate the proportion of memory B cells acquiring either of these two important functions and, in its absence, the output tilts in favor of promoting secondary GCs, likely due to an increase in the production of IgM memory B cells. Thus, this study provides strong evidence that histone acetylation during B-cell differentiation is a mechanism for fine-tuning the regulation of memory formation in humoral immunity.
In this study, we have demonstrated that chromatin modifiers are regulators of B-cell differentiation. Thus, there is the potential for mimetics and inhibitors to be used clinically as novel therapies. For example, they could be used to enforce one B-cell fate over another, such as in patients where memory B cells are depleted or plasma cells are overrepresented. Therefore, revealing a role for MOZ in lymphocyte differentiation makes it a viable target for clinical intervention.
Materials and Methods
Mice, Immunizations, and Cell Purification.
Mozfl/fl mice were generated as detailed (27). Mb-1-Cre and Aicda-Cre mice were provided by Michael Reth, Max-Planck Institute of Immunobiology, Freiburg, Germany (28) and Meinrad Busslinger, Research Institute of Molecular Pathology, Vienna (38), respectively. Ly5.1 mice were maintained at The Walter and Eliza Hall Institute of Medical Research (WEHI). Animal procedures were approved by the WEHI Animal Ethics Committee. Primary responses: Mice were injected intraperitoneally with 100 μg of NP conjugated to KLH (molar ratio between 13 and 20) precipitated on alum. Secondary responses: Mice were injected intraperitoneally with 50 μg of NP–KLH in PBS. For some experiments, B cells were preenriched before sort purification using either B220-positive or B cell-negative isolation kits (Miltenyi Biotec). Sort purification: Cells were stained with antibodies and purified by FACSAria or Influx (BD Biosciences), with purity >98%.
Flow Cytometry and Antibodies.
Single-cell suspensions were prepared in PBS, 2% (vol/vol) FCS and stained for flow cytometry; live cells were acquired (propidium iodide-negative) on a FACSCanto (BD Biosciences) and data were analyzed with FlowJo software (TreeStar). The following antibodies were used: BD Biosciences: CD95 (JO2), IgG1 (×56), CD86 (GL1), Ly5.2 (104), CD138 (281); eBioscience: CD19 (ID3), CXCR4 (2B11); Vector Laboratories: PNA; conjugated in-house: CD38 (NIMR5), IgM (331.12), IgD (11.26C), Gr1 (8C5), B220 (RA3-6B2), BCL-6 (7D1-10), NIP. FcγRII/III (24G2; in-house hybridoma supernatant) was used to block nonspecific binding. Intracellular staining: Cells were surface-stained, fixed, permeabilized, and stained with anti–BCL-6 per the manufacturer’s instructions (BD Biosciences FoxP3 Staining Kit). Cell-cycle analysis was performed as previously described (39). To stain for IgM memory B cells, splenocytes were first incubated for 1 min in acid buffer (0.085 M NaCl, 0.005 M KCl, 0.05 M Na acetate) with 1% FCS to reduce nonspecific staining. Cells were washed in PBS, 2% FCS and then stained for flow cytometry.
Bone Marrow Chimeras.
Lethally irradiated Ly5.1 mice (2 × 550 rad) were reconstituted with 50% Ly5.1 BM and 50% MOZ-deficient or Cre-only BM. Mice were rested for 7–8 wk before NP–KLH/alum immunization.
Immunohistology, Enzyme-Linked Immunospot and ELISA, and VH Sequencing.
Immunohistological analysis of frozen spleen sections was performed as previously detailed (39). ASCs or Abs were analyzed by enzyme-linked immunospot (ELISPOT) and ELISA, respectively, as previously described (39). Individual cells were sort-purified on a BD Biosciences FACSAria equipped with a plate sorter and sequenced for VH186.2-Cγ1 as previously detailed (40). Analysis was performed by published methods (41, 42).
RNA Sequencing.
CD19+IgDloCD95hiCD38lo B cells from two independent pools of Mozfl/flAicdaCre/+ mice and two independent pools of AicdaCre/+ mice were sort-purified. RNA was isolated using the Qiagen microkit according to the manufacturer’s guidelines. RNA sequencing was performed on a HiSeq 2000 at the Australian Genome Research Facility (Melbourne) using Illumina CASAVA pipeline version 1.8.2. RNA-sequencing analysis methods are contained in SI Materials and Methods.
Microarrays.
CD19+ B cells from Mozfl/flAicdaCre/+ mice and AicdaCre/+ mice were sort-purified. RNA was isolated using the Qiagen microkit according to the manufacturer’s guidelines. Microarray analysis was performed using an Illumina MouseRef-8 v2.0 Expression BeadChip at the Australian Genome Research Facility (Melbourne).
Statistical Analysis.
The Mann–Whitney nonparametric, two-tailed test was used (GraphPad Prism).
Supplementary Material
Acknowledgments
We thank Ingela Vikstrom and Dimitra Zotos for critical reading of this manuscript, Simon Willis for helpful discussions, and Dana Piovesan and D.T. laboratory members for technical assistance. This work is supported by a National Health and Medical Research Council (NHMRC) Australia Program Grant (to D.T.; 1054925) and Project Grant (to K.L.G.-J.; 1022458). K.L.G.-J. is supported by an NHMRC Biomedical Research Fellowship, and A.K.V., G.K.S., T.T., and D.T. by NHMRC Research Fellowships. This work was made possible through the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC Independent Research Institute Infrastructure Support Scheme.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402485111/-/DCSupplemental.
References
- 1.Tarlinton D, Good-Jacobson K. Diversity among memory B cells: Origin, consequences, and utility. Science. 2013;341(6151):1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- 2.Han S, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155(2):556–567. [PubMed] [Google Scholar]
- 3.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y, et al. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice. J Exp Med. 1999;190(3):399–410. doi: 10.1084/jem.190.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187(6):885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: The influence of germinal center interactions and dynamics. J Immunol. 2010;185(6):3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 8.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276(5312):589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17(1):51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 10.Good KL, Tangye SG. Decreased expression of Kruppel-like factors in memory B cells induces the rapid response typical of secondary antibody responses. Proc Natl Acad Sci USA. 2007;104(33):13420–13425. doi: 10.1073/pnas.0703872104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 12.Tarakhovsky A. Tools and landscapes of epigenetics. Nat Immunol. 2010;11(7):565–568. doi: 10.1038/ni0710-565. [DOI] [PubMed] [Google Scholar]
- 13.Lee KK, Workman JL. Histone acetyltransferase complexes: One size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 14.Su IH, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4(2):124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 15.Raaphorst FM, et al. Cutting edge: Polycomb gene expression patterns reflect distinct B cell differentiation stages in human germinal centers. J Immunol. 2000;164(1):1–4. doi: 10.4049/jimmunol.164.1.1. [DOI] [PubMed] [Google Scholar]
- 16.van Galen JC, et al. Distinct expression patterns of polycomb oncoproteins and their binding partners during the germinal center reaction. Eur J Immunol. 2004;34(7):1870–1881. doi: 10.1002/eji.200424985. [DOI] [PubMed] [Google Scholar]
- 17.Velichutina I, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010;116(24):5247–5255. doi: 10.1182/blood-2010-04-280149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Béguelin W, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23(5):677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149(2):467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allan RS, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487(7406):249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- 21.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182(2):890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 22.Tomayko MM, et al. Systematic comparison of gene expression between murine memory and naive B cells demonstrates that memory B cells have unique signaling capabilities. J Immunol. 2008;181(1):27–38. doi: 10.4049/jimmunol.181.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya D, et al. Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation. J Immunol. 2007;179(10):6808–6819. doi: 10.4049/jimmunol.179.10.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas T, et al. Monocytic leukemia zinc finger protein is essential for the development of long-term reconstituting hematopoietic stem cells. Genes Dev. 2006;20(9):1175–1186. doi: 10.1101/gad.1382606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsumoto T, et al. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20(10):1321–1330. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voss AK, Thomas T. MYST family histone acetyltransferases take center stage in stem cells and development. BioEssays. 2009;31(10):1050–1061. doi: 10.1002/bies.200900051. [DOI] [PubMed] [Google Scholar]
- 27.Voss AK, Collin C, Dixon MP, Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and segment identity. Dev Cell. 2009;17(5):674–686. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Pelanda R, et al. Cre recombinase-controlled expression of the mb-1 allele. Genesis. 2002;32(2):154–157. doi: 10.1002/gene.10070. [DOI] [PubMed] [Google Scholar]
- 29.Shaffer AL, et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13(2):199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102(5):553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 31.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dogan I, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10(12):1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407(6804):636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 34.Voss AK, et al. MOZ regulates the Tbx1 locus, and Moz mutation partially phenocopies DiGeorge syndrome. Dev Cell. 2012;23(3):652–663. doi: 10.1016/j.devcel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caganova M, et al. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J Clin Invest. 2013;123(12):5009–5022. doi: 10.1172/JCI70626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 37.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7(7):773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 38.Kwon K, et al. Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity. 2008;28(6):751–762. doi: 10.1016/j.immuni.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207(2):365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blink EJ, et al. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201(4):545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uduman M, et al. Detecting selection in immunoglobulin sequences. Nucleic Acids Res. 2011;39(Web Server issue):W499–W504. doi: 10.1093/nar/gkr413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hershberg U, Uduman M, Shlomchik MJ, Kleinstein SH. Improved methods for detecting selection by mutation analysis of Ig V region sequences. Int Immunol. 2008;20(5):683–694. doi: 10.1093/intimm/dxn026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.