Significance
Curative treatment modalities for erectile dysfunction (ED) are not available. Penile erection is a neurovascular phenomenon, and ED is caused mainly by vascular and neurologic disturbances. Here we demonstrate that inhibition of nerve injury-induced protein 1 promotes penile angiogenesis and neural regeneration through angiopoietin-1–Tie2 signaling and rescues erectile function in diabetic mice. Our preclinical work shed light on the application of therapeutic angiogenesis and neural regeneration for the treatment of human ED.
Keywords: diabetes mellitus, male sexual dysfunction, peripheral neuropathy
Abstract
Penile erection is a neurovascular phenomenon, and erectile dysfunction (ED) is caused mainly by vascular risk factors or diseases, neurologic abnormalities, and hormonal disturbances. Men with diabetic ED often have severe endothelial dysfunction and peripheral nerve damage, which result in poor response to oral phosphodiesterase-5 inhibitors. Nerve injury-induced protein 1 (Ninjurin 1, Ninj1) is known to be involved in neuroinflammatory processes and to be related to vascular regression during the embryonic period. Here, we demonstrate in streptozotocin-induced diabetic mice that inhibition of the Ninj1 pathway by administering Ninj1-neutralizing antibody (Ninj1-Ab) or by using Ninj1-knockout mice successfully restored erectile function through enhanced penile angiogenesis and neural regeneration. Angiopoietin-1 (Ang1) expression was down-regulated and angiopoietin-2 expression was up-regulated in the diabetic penis compared with that in controls, and these changes were reversed by treatment with Ninj1-Ab. Ninj1 blockade-mediated penile angiogenesis and neural regeneration as well as recovery of erectile function were abolished by inhibition of Ang1–Tie2 (tyrosine kinase with Ig and epidermal growth factor homology domain-2) signaling with soluble Tie2 antibody or Ang1 siRNA. The present results suggest that inhibition of the Ninj1 pathway will be a novel therapeutic strategy for treating ED.
Erectile dysfunction (ED), which is defined as an inability to attain or maintain penile erection sufficient for satisfactory sexual intercourse (1), is caused by a variety of pathologic conditions including vascular risk factors or diseases, neurologic abnormalities, and hormonal disturbances (2, 3). Diabetes mellitus is one of the most common causes of ED, and about 50–75% of male diabetic patients have ED (4, 5). Multiple pathogenetic factors, such as endothelial dysfunction, atherosclerosis, autonomic neuropathy, inflammation, fibrosis, and hypogonadism, are involved in diabetic ED (4–6). The multiple factors causing diabetic ED contribute to reduced responsiveness to currently available oral phosphodiesterase-5 (PDE5) inhibitors, which enhance the nitric oxide (NO)–cGMP pathway by inhibiting the breakdown of cGMP (7). The severity of endothelial dysfunction and peripheral neuropathy are mainly responsible for the poor responsiveness of diabetic patients to PDE5 inhibitors (8, 9). Because the effects of PDE5 inhibitors depend on endogenous NO formation, PDE5 inhibitors fail to increase the cGMP level above the threshold required for penile erection if bioavailable NO is insufficient as the result of severe endothelial dysfunction or peripheral neuropathy (9). Therefore, a new treatment strategy that corrects both endothelial dysfunction and peripheral neuropathy is required for men with diabetic ED.
A variety of strategies targeting therapeutic angiogenesis and neural regeneration have been introduced to restore erectile function at the preclinical level. Angiopoietins, the ligands for tyrosine kinase with Ig and epidermal growth factor homology domain-2 (Tie2, also called Tek), are a family of angiogenic growth factors that play a crucial role in blood vessel remodeling, maturation, and stabilization (10–12). We recently reported in mouse models of type I and type II diabetic ED that local delivery of synthetic angiopoietin-1 (Ang1) or angiopoietin-4 protein into the penis restores erectile function by enhancing endothelial cell regeneration (13–15). Bennett et al. (16) demonstrated in a rat model of type I diabetic ED that replication-defective herpes simplex virus vector encoding neurotrophin-3 (NT3) gene induces partial recovery of erectile function by restoring penile neuronal nitric oxide synthase-positive (nNOS+) neurons (16). However, a treatment strategy targeting both endothelial and neural regeneration in diabetic ED has not yet been explored.
Nerve injury-induced protein 1, or Ninjurin-1 (Ninj1), is a cell-surface protein and an adhesion molecule. Ninj1 was discovered during the identification of molecules related to nerve injury and is known to be up-regulated in neuronal and Schwann cells after sciatic nerve injury (17). It also was reported that Ninj1 is responsible for the progression of multiple sclerosis, an autoimmune inflammatory disease of the CNS characterized by demyelination and axonal damage (18). The expression of Ninj1 is significantly increased in CNS lesions of patients with multiple sclerosis, and Ninj1-neutralizing antibody suppresses neuroinflammatory responses in an experimental model of allergic encephalomyelitis that mimics multiple sclerosis (18). Moreover, Ninj1 is known to induce the regression of hyaloid blood vessels, a transiently existing vascular system involved in maturation of the lens during the embryonic period, and inhibition of Ninj1 with neutralizing antibody delays regression of the hyaloid blood vessels (19). These findings suggest that Ninj1 has a functional role in the regulation of nervous and vascular systems. Therefore, inhibition of the Ninj1 pathway might be efficacious for the treatment of diabetic ED, which is characterized by both endothelial dysfunction and peripheral neuropathy.
Here, we studied the effectiveness of Ninj1-neutralizing antibody in promoting regeneration of penile endothelial and neuronal cells and restoration of erectile function in a mouse model of diabetic ED. Our results show that a single injection of Ninj1-neutralizing antibody into the penis induced significant recovery of erectile function in diabetic mice; this recovery was accompanied by enhanced cavernous endothelial cell proliferation and successive phosphorylation of Akt protein kinase B and endothelial nitric oxide synthase (eNOS), decreased production of reactive oxygen species (ROS), decreased endothelial cell apoptosis, and restoration of penile nNOS+ through the secretion of neurotrophic factors. The Ang1 expression was down-regulated, and the expression of angiopoietin-2 (Ang2), an endogenous antagonist of Ang1, was up-regulated in the diabetic penis compared with their expression in controls; this effect was reversed by treatment with Ninj1-neutralizing antibody. The endothelial and neural regeneration as well as the recovery of erectile function mediated by Ninj1 blockade were abolished by inhibition of Ang1–Tie2 signaling with soluble Tie2 antibody (sTie2-Fc) or siRNA for Ang1. Ninj1-KO mice were resistant to diabetes-induced cavernous endothelial and neuronal cell damage, and erectile function was rescued in these mice.
Results
Metabolic Variables.
The fasting and postprandial blood glucose concentrations were significantly higher in the diabetic mice than in control mice. Also, body weight was significantly lower in the diabetic mice than in the controls. The body weight and blood glucose levels of the diabetic mice did not differ significantly regardless of the treatment given (SI Appendix, Tables S1–S4).
Increase in Ninj1 Expression in the Penis of Diabetic Mice and in Mouse Cavernous Endothelial Cells Exposed to the High-Glucose Condition.
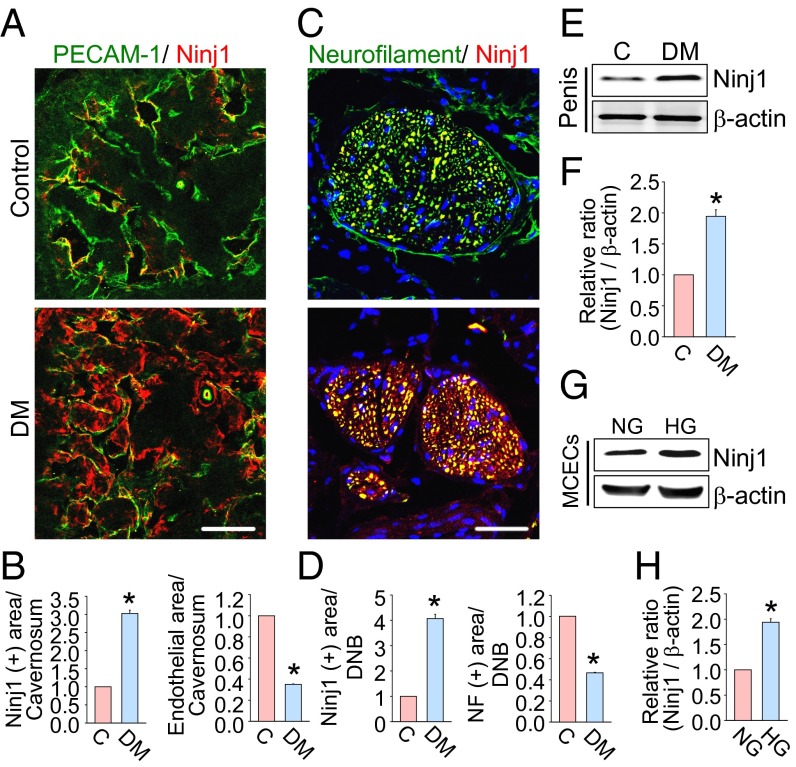
Immunofluorescent staining revealed that the expression of Ninj1 protein was significantly higher in cavernous endothelial cells and in the dorsal nerve bundle of the diabetic mice by approximately three- or fourfold, respectively, than in control mice (Fig. 1 A–D). The contents of endothelial cells and neurofilament were significantly lower in the penis of diabetic mice than in controls (Fig. 1 A–D). Western blot analysis also revealed increased Ninj1 expression in the penis of diabetic mice both in vivo and in primary cultured mouse cavernous endothelial cells (MCECs) exposed to the high-glucose condition in vitro (Fig. 1 E–H). These findings gave us a rationale to use Ninj1 blockade for the treatment of diabetic ED.
Fig. 1.
Increase in Ninj1 expression in the diabetic condition. (A) PECAM-1 (green) and Ninj1 (red) immunostaining in penis tissue from age-matched control (C) and diabetic (DM) mice. (Scale bar: 100 µm.) (B) The Ninj1-immunopositive area or endothelial cell content in cavernous tissue was quantified by ImageJ (n = 6). *P < 0.001 vs. control group. (C) Neurofilament (green) and Ninj1 (red) immunostaining in each group. Nuclei were labeled with DAPI (blue). (Scale bar: 25 µm.) (D) The Ninj1-immunopositive area or neurofilament content in the dorsal nerve bundle was quantified by ImageJ (n = 6). *P < 0.001 vs. control group. (E) Representative Western blots for Ninj1 in mouse penis. (F) Normalized band intensity values (n = 4). *P < 0.001 vs. control group. (G) Representative Western blot for Ninj1 in MCECs after exposure to the normal-glucose (NG) or high-glucose (HG) condition for 48 h. (H) Normalized band intensity values (n = 4). *P < 0.001 vs. NG group (Mann–Whitney u test). Data are mean ± SE. The relative ratio of control or NG group was arbitrarily set equivalent to 1. DNB, dorsal nerve bundle; MCECs, mouse cavernous endothelial cells; NF, neurofilament.
In Vivo Detection of Ninj1-Neutralizing Antibody in the Penis of Diabetic Mice.
To determine the distribution of exogenously injected Ninj1-neutralizing antibody in the penis and to distinguish it from endogenous Ninj1 expression, immunofluorescent staining was done with TRITC-conjugated rabbit secondary antibody (Zymed Laboratories) at 1, 3, and 6 h after intracavernous injection of rabbit polyclonal antibody against Ninj1 (2.5 µg/20 µL; provided by K.-W.K.) into the diabetic mice. Ninj1 antibody was detected in both the corpus cavernosum tissue and in the dorsal nerve bundle of the diabetic mice (SI Appendix, Fig. S1).
Ninj1-Neutralizing Antibody Restores Erectile Function in Diabetic Mice.
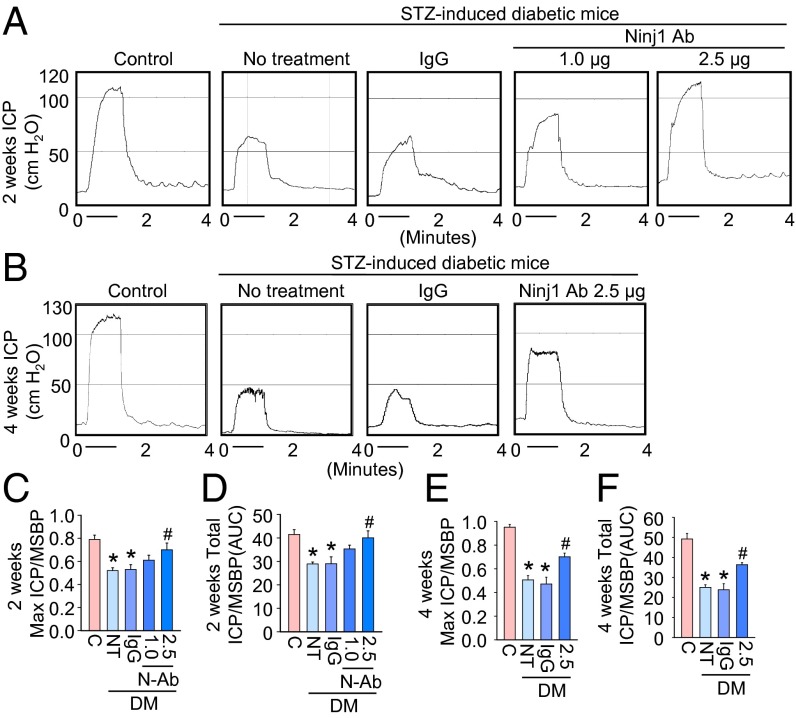
To determine the physiological relevance of intracavernous injection of Ninj1-neutralizing antibody, we evaluated erectile function during electrical stimulation of the cavernous nerve in vivo. A representative intracavernous tracing after stimulation of the cavernous nerve (5 V, 12 Hz, 1 ms) for 1 min in age-matched control and diabetic mice 2 and 4 wk after treatment is shown in Fig. 2 A and B. During electrical stimulation of the cavernous nerve, the ratios of maximal intracavernous pressure (ICP) and total ICP to mean systolic blood pressure (MSBP) were significantly lower in untreated and IgG-treated diabetic mice than in age-matched controls. At 2 wk after treatment, a single intracavernous injection of high-dose Ninj1-neutralizing antibody (2.5 µg/20 µL) induced recovery of erection parameters, which reached up to 90% (maximal ICP) or 96% (total ICP) of control values, whereas low-dose Ninj1-neutralizing antibody (1.0 µg/20 µL) partially restored erectile function (Fig. 2 C and D). At 4 wk after administration of high-dose Ninj1-neutralizing antibody (2.5 µg/20 µL), erectile function recovery was not as great as observed at 2 wk after treatment but still was significantly higher than in the untreated or IgG-treated diabetic groups (Fig. 2 E and F). No detectable differences in MSBP were found among the experimental groups (SI Appendix, Tables S1 and S2).
Fig. 2.
Ninj1-neutralizing antibody restores erectile function. (A and B) Representative ICP responses for the age-matched control (C), untreated diabetic mice, or diabetic mice stimulated at 2 wk (A) or 4 wk (B) after a single intracavernous injection of IgG (2.5 µg) or Ninj1-neutralizing antibody (Ninj1 Ab, 1.0 µg or 2.5 µg). The cavernous nerve was stimulated at 5 V. The stimulus interval is indicated by a solid bar. (C and D) Ratios of mean maximal ICP and total ICP (area under the curve) to MSBP at 2 wk after treatment (n = 8). *P < 0.01 vs. control and Ninj1 antibody (2.5 µg) groups; #P < 0.01 vs. NT and IgG groups. (E and F) Ratios of mean maximal ICP and total ICP (area under the curve) to MSBP at 4 wk after treatment (n = 6). *P < 0.01 vs. control and Ninj1 antibody (2.5 µg) groups; #P < 0.01 vs. NT and IgG groups. P values were determined by one-way ANOVA. Data are mean ± SE. AUC, area under curve; DM, diabetes mellitus; N-Ab, Ninj1 Ab; NT, no treatment; STZ, streptozotocin.
Ninj1-Neutralizing Antibody Induces Proliferation of Cavernous Endothelial Cells in Diabetic Mice.
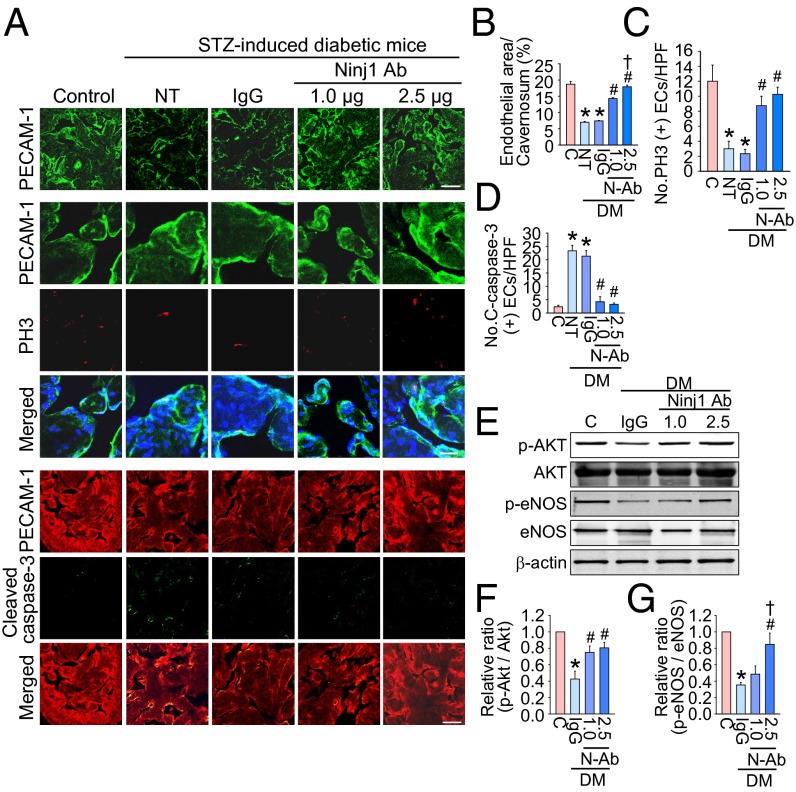
Immunofluorescent staining of cavernous tissue with an antibody to platelet/endothelial adhesion molecule 1 (PECAM-1) was performed in age-matched control and diabetic mice 2 wk after treatment. We found significantly lower cavernous endothelial cell contents in the untreated and IgG-treated diabetic mice than in the control mice. Intracavernous injection of high-dose Ninj1-neutralizing antibody (2.5 µg/20 µL) completely restored cavernous endothelial cell content (Fig. 3 A and B). To test whether the increase in cavernous endothelial cell content was the result of endothelial cell proliferation, we assessed the number of endothelial cells staining positive for phosphohistone H3 (a nuclear protein indicative of cell proliferation). We noted significant increases in phosphohistone H3+ endothelial cells in diabetic mice 2 wk after injection of high-dose Ninj1-neutralizing antibody (2.5 µg/20 µL) (Fig. 3 A and C). Intracavernous injection of low-dose Ninj1-neutralizing antibody (1.0 µg/20 µL) also induced recovery of endothelial content through endothelial cell proliferation but not at the level of the control group (Fig. 3 A–C).
Fig. 3.
Ninj1-neutralizing antibody restores cavernous endothelial content. (A) PECAM-1 (green) and phosphohistone H3 (PH3; red) or PECAM-1 (red) and cleaved caspase-3 (green) immunostaining in penis tissue from age-matched control (C), untreated diabetic mice (NT), or diabetic mice 2 wk after receiving a single intracavernous injection of IgG (2.5 µg) or Ninj1-neutralizing antibody (Ninj1 Ab, 1.0 µg or 2.5 µg). Nuclei were labeled with DAPI (blue). (Scale bars: Top Row, 100 µm; Middle Row, 25 µm; Bottom Row, 50 µm) (B) Quantification of cavernous endothelial cell content by ImageJ (n = 6). (C) Number of PH3-immunopositive endothelial cells per high-power field (n = 6). (D) Number of apoptotic cells in endothelium per high-power field (n = 6). In B–D, *P < 0.001 vs. C, Ninj1 antibody (1.0 µg), and Ninj1 antibody (2.5 µg) groups; #P < 0.001 vs. N and IgG groups; †P < 0.05 vs. Ninj1 antibody (1.0 µg) group. (E) Representative Western blots for phospho-Akt (p-Akt)/Akt and phospho-eNOS (p-eNOS)/eNOS at 2 wk after treatment. (F and G) Normalized band intensity values for p-Akt/Akt (n = 4) (F) and p-eNOS/eNOS (G). *P < 0.001 vs. C, Ninj1 antibody (1.0 µg), and Ninj1 antibody (2.5 µg) groups; #P < 0.001 vs. IgG group; †P < 0.001 vs. Ninj1 antibody (1.0 µg) group. The P values were determined by one-way ANOVA. Data are mean ± SE. C-caspase-3, cleaved caspase-3; DM, diabetes mellitus; ECs, endothelial cells; N-Ab, Ninj1 Ab; STZ, streptozotocin.
Ninj1-Neutralizing Antibody Decreases Cavernous Endothelial Cell Apoptosis in Diabetic Mice.
Double labeling of cavernous tissue with antibodies to PECAM-1 and cleaved caspase-3 showed that the number of apoptotic cells in cavernous endothelial cells was significantly greater in the untreated and IgG-treated diabetic groups than in the control group. Intracavernous injections of both low-dose (1.0 µg/20 µL) and high-dose (2.5 µg/20 µL) Ninj1-neutralizing antibody decreased apoptosis in the cavernous endothelial cells of the diabetic mice significantly, to a level comparable to that found in the age-matched controls (Fig. 3 A and D).
Ninj1-Neutralizing Antibody Induces Cavernous Akt and eNOS Phosphorylation in Diabetic Mice.
Western blot analysis showed that the expression of cavernous phospho-Akt (Ser473) and phospho-eNOS (Ser1177) was significantly lower in the IgG-treated diabetic mice than in the age-matched controls. High-dose Ninj1-neutralizing antibody (2.5 µg/20 µL) significantly induced phosphorylation of Akt and eNOS in the diabetic mice as compared with IgG-treated diabetic mice, but no significant difference in total Akt and total eNOS expression was noted among the four experimental groups (Fig. 3 E–G).
Ninj1 Regulates Expression of Ang1 and Ang2 in Diabetic Mice in Vivo and in MCECs in Vitro.
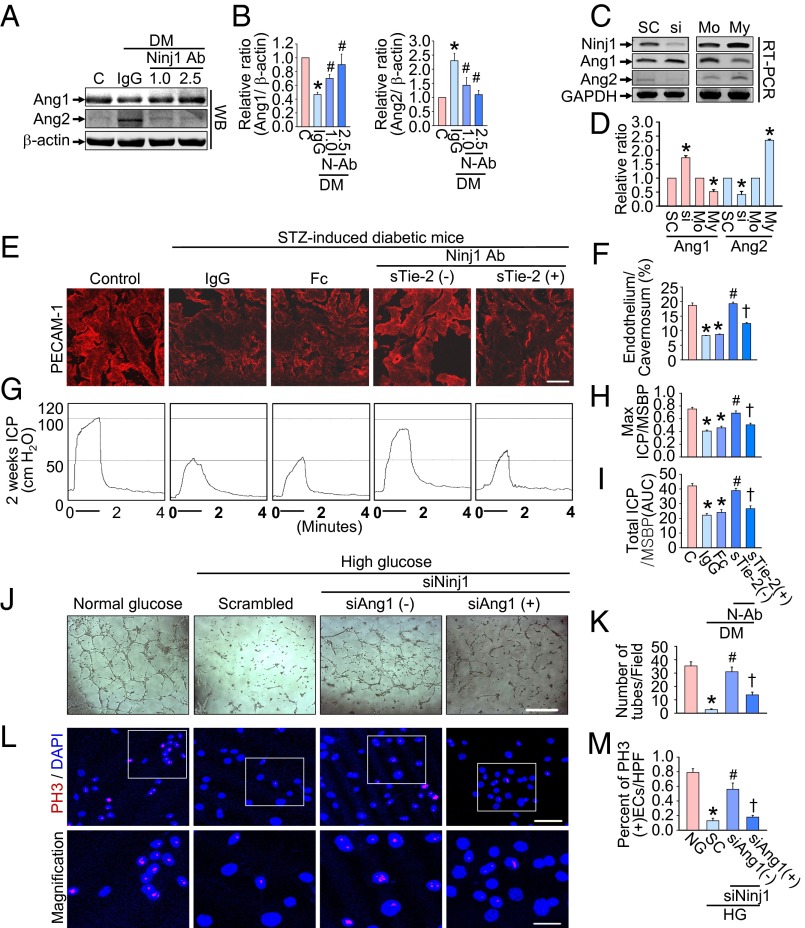
Cavernous Ang1 protein expression was significantly lower and Ang2 protein expression was significantly higher in the IgG-treated diabetic mice than in the age-matched controls. The expression of both Ang1 and Ang2 proteins returned to control values after treatment with Ninj1-neutralizing antibody (Fig. 4 A and B). To confirm further that Ninj1 is involved in the regulation of Ang1 and Ang2, the MCECs were treated with Ninj1 siRNA or plasmid encoding the Ninj1 gene. Knockdown of the Ninj1 gene with siRNA increased Ang1 mRNA expression and decreased Ang2 mRNA expression, whereas overexpression of the Ninj1 gene decreased Ang1 mRNA and increased Ang2 mRNA expression (Fig. 4 C and D).
Fig. 4.
Ninj1-neutralizing antibody–induced cavernous angiogenesis and recovery of erectile function are dependent on the Ang1–Tie2 pathway. (A) Representative Western blots for Ang1 and Ang2 in penis tissue from age-matched control mice (C) or diabetic mice (DM) 2 wk after receiving a single intracavernous injection of IgG (2.5 µg) or Ninj1-neutralizing antibody (Ninj1 Ab, 1.0 µg or 2.5 µg). (B) Normalized band intensity values (n = 4). *P < 0.05 vs. C, Ninj1 Ab (2.5 µg) groups; #P < 0.05 vs. IgG group (Kruskal–Wallis test). (C) Representative RT-PCR for Ninj1, Ang1, and Ang2 in MCECs transfected with Ninj1 siRNA (si) or plasmid encoding Ninj1 gene (My). (D) Normalized band intensity values (n = 4). *P < 0.05 vs. scrambled siRNA (SC) and myc-tagged mock-treated (Mo) (Mann–Whitney u test). (E–I) PECAM-1 immunostaining (E) and quantification (F) and representative ICP responses (G–I) in each group at 2 wk after treatment (n = 6). (Scale bar: 50 µm.) Fc, dimeric-Fc (4 µg); sTie2-Fc, soluble Tie2 antibody (4 µg). *P < 0.001 vs. C and Ninj1 antibody (2.5 µg) + sTie2 (−) groups; #P < 0.001 vs. IgG and Fc groups; †P < 0.001 vs. Ninj1 antibody (2.5 µg) + sTie2 (−) group. DM, diabetes mellitus; STZ, streptozotocin. (J–M) Tube formation assay (J), quantification (number of tubes per field; screen magnification, 40×; n = 4) (K), phosphohistone H3 (PH3; red) staining (L) and (quantification (percent of PH3-immunopositive endothelial cells per high-power field; n = 4) (M) in MCECs exposed to the normal-glucose (NG) or high glucose (HG) condition, which were transfected with scrambled (SC), Ninj1, or Ang1 siRNA. Nuclei were labeled with DAPI (blue). (Scale bars: J, 500 µm; L, Upper, 50 µm; L, Lower, 25 µm.) In K and M, *P < 0.001 vs. NG and Ninj1 siRNA + Ang1 siRNA (−) groups; #P < 0.001 vs. SC group; †P < 0.001 vs. Ninj1 siRNA + Ang1 siRNA (−) group. P values in F, H, I, K, and M were determined by one-way ANOVA. Data are mean ± SE. AUC, area under curve; N-Ab, Ninj1 Ab; STZ, streptozotocin; WB, Western blot.
Cavernous Angiogenesis and Recovery of Erectile Function by Ninj1-Neutralizing Antibody Are Dependent on the Ang1–Tie2 Signaling Pathway.
We determined whether the Ang1–Tie2 signaling pathway participated in the cavernous angiogenesis induced by Ninj1-neutralizing antibody and subsequent restoration of the erectile responses in diabetic mice treated with sTie2-Fc (4 µg/20 µL). Immunofluorescent staining of cavernous tissue with an antibody to PECAM-1 was performed in age-matched control and diabetic mice 2 wk after treatment. Enhancement of cavernous angiogenesis by Ninj1-neutralizing antibody was profoundly diminished in diabetic mice treated with sTie2-Fc (Fig. 4 E and F). A BrdU incorporation assay and cleaved caspase-3 analysis revealed that the increase in endothelial cell proliferation and decrease in endothelial cell apoptosis mediated by Ninj1-neutralizing antibody also was significantly attenuated in diabetic mice treated with sTie2-Fc (SI Appendix, Fig. S2). Moreover, physiologic erection studies revealed that erectile function was not recovered after administration of Ninj1-neutralizing antibody in diabetic mice treated with sTie2-Fc (Fig. 4 G–I). No detectable differences in MSBP were found among the five experimental groups (SI Appendix, Table S3).
We further examined the role of the Ang1–Tie2 pathway in Ninj1 siRNA-mediated angiogenesis in MCECs. An in vitro Matrigel assay revealed impairments in tube formation in MCECs exposed to high-glucose conditions, and these impairments were completely restored by treatment with Ninj1 siRNA. Cotransfection of MCECs with Ang1 siRNA diminished Ninj1 siRNA-mediated enhancement of tube formation (Fig. 4 J and K). Moreover, Ninj1 siRNA-mediated endothelial cell proliferation also was abolished by Ang1 siRNA in MCECs exposed to the high-glucose condition (Fig. 4 L and M).
Ninj1-Neutralizing Antibody Decreases Cavernous ROS Production in Diabetic Mice.
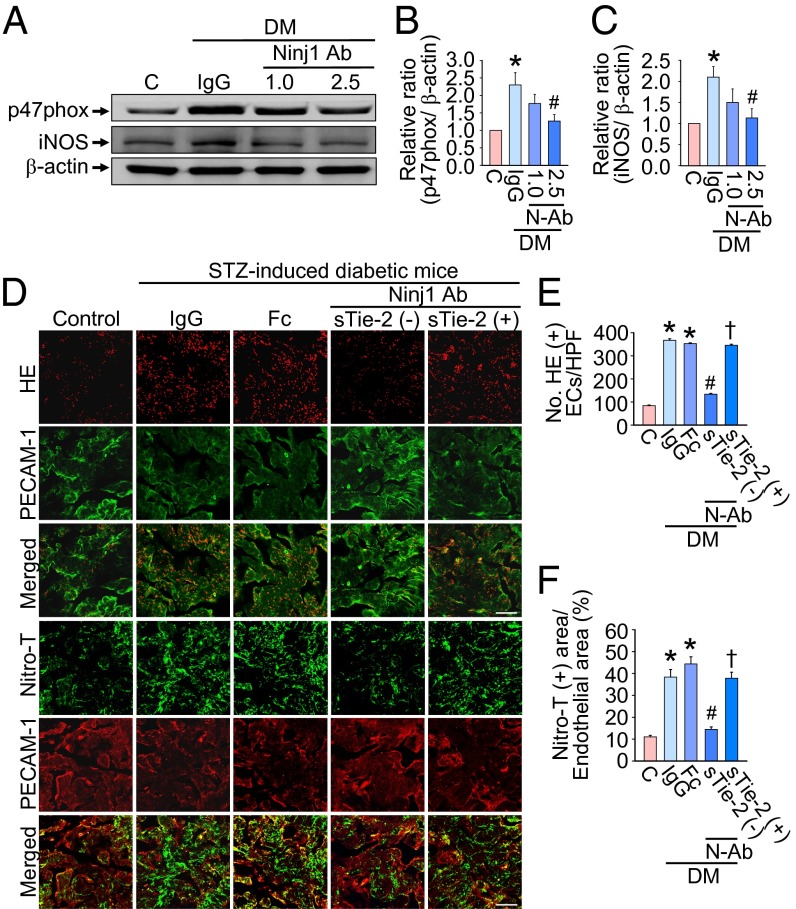
We performed Western blots to evaluate the cavernous tissue expression of p47phox (an active catalytic subunit of NADPH oxidase) and inducible NOS (iNOS) in age-matched control and diabetic mice 2 wk after treatment. Cavernous p47phox and iNOS protein expression was significantly higher in the IgG-treated diabetic mice than in the age-matched controls. Intracavernous injection of Ninj1-neutralizing antibody (2.5 µg/20 µL) significantly decreased cavernous p47phox and iNOS expression in the diabetic mice (Fig. 5 A–C).
Fig. 5.
Ninj1-neutralizing antibody decreases cavernous ROS production in a Tie2-dependent manner. (A) Representative Western blot for p47phox and iNOS in penis tissue from age-matched control (C) or diabetic (DM) mice 2 wk after a single intracavernous injection of IgG (2.5 µg) or Ninj1-neutralizing antibody (Ninj1 Ab; 1.0 µg or 2.5 µg). (B and C) Normalized band intensity values (n = 4). *P < 0.05 vs. C and Ninj1 Ab (2.5 µg) groups; #P < 0.05 vs. IgG group (Kruskal–Wallis test). (D) In situ detection of superoxide anion and nitrotyrosine production in each group at 2 wk after treatment. (Scale bars: 100 µm.) Fc, dimeric-Fc (4 µg); sTie2-Fc, soluble Tie2 antibody (4 µg). (E and F) Number of ethidium bromide fluorescence-positive endothelial cells per high-power field (n = 6) (E) and the quantification of nitrotyrosine-immunopositive endothelial area (n = 6) (F). *P < 0.001 vs. C, Ninj1 antibody (2.5 µg) + sTie2 (-) groups; #P < 0.001 vs. IgG and Fc groups; †P < 0.001 vs. Ninj1 antibody (2.5 µg) + sTie2 (−) group (one-way ANOVA). Data are mean ± SE. DM, diabetes mellitus; ECs, endothelial cells; HE, hydroethidine; iNOS, inducible nitric oxide synthase; p47phox, an active catalytic subunit of NADPH oxidase; N-Ab, Ninj1 Ab; Nitro-T, nitrotyrosine; STZ, streptozotocin.
The generation of superoxide anion and peroxynitrite (which is derived from NO and superoxide anion) was determined by hydroethidine staining and immunohistochemical staining of nitrotyrosine, respectively. The fluorescent products of oxidized hydroethidine and nitrotyrosine expression in cavernous endothelial cells of the corpus cavernosum were higher in the IgG- and Fc-treated diabetic groups than in the control group. Intracavernous administration of Ninj1-neutralizing antibody (2.5 µg/20 µL) significantly decreased the generation of superoxide anion and peroxynitrite. Furthermore, the decrease in cavernous ROS production mediated by Ninj1-neutralizing antibody was blocked completely in the presence of sTie2-Fc (Fig. 5 D–F).
We further examined whether macrophages participated in the decrease in ROS production mediated by Ninj1 blockade. Immunofluorescent staining and hydroethidine staining revealed increased infiltration of Ninj1+ macrophages as well as an increase in oxidized hydroethidine and nitrotyrosine expression in the macrophages of diabetic mice, whereas the infiltration of macrophages and the generation of ROS in macrophages were profoundly decreased in Ninj1-KO mice that received streptozotocin (STZ) (SI Appendix, Fig. S3 A–C). Ninj1 protein expression also was significantly higher in RAW 264.7 macrophages exposed to the high-glucose condition than in the cells exposed to the normal-glucose condition (SI Appendix, Fig. S3 D and E). Western blot analysis revealed an increase in the expression of p47phox, iNOS, and nitrotyrosine in RAW 264.7 cells exposed to the high-glucose condition. These levels returned to baseline after treatment with Ninj1 siRNA (SI Appendix, Fig. S3 D and F–H). Similar to the results seen with MCECs, Ang2 protein expression was significantly higher in RAW 264.7 cells exposed to the high-glucose condition than in the cells exposed to the normal-glucose condition, and this elevated expression of Ang 2 protein returned to baseline level after treatment with Ninj1 siRNA (SI Appendix, Fig. S3 D and I). Almost no Ang1 protein expression was observed in RAW 264.7 cells (SI Appendix, Fig. S3D).
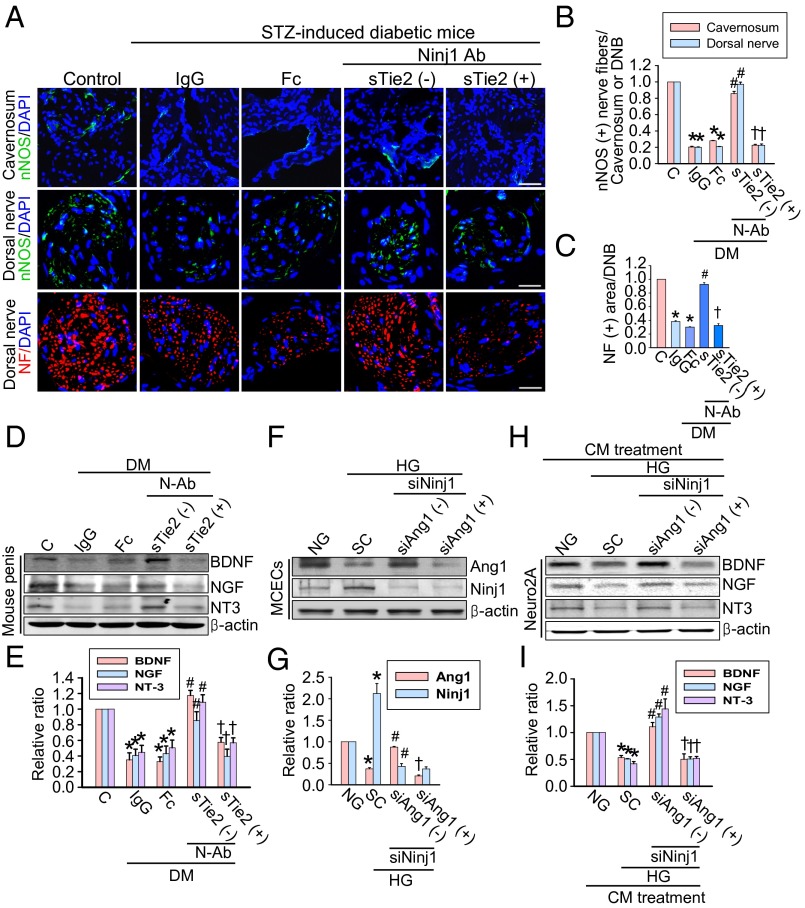
Ninj1-Neutralizing Antibody Restores Penile Nerve Content by Promoting the Secretion of Neurotrophic Factors in Diabetic Mice.
The expression of nNOS and neurofilament in both the corpus cavernosum tissue and the dorsal nerve bundle was significantly lower in the IgG- and Fc-treated diabetic mice than in age-matched controls. Intracavernous administration of Ninj1-neutralizing antibody (2.5 µg/20 µL) completely restored penile nNOS-containing nerve fiber and axonal contents (neurofilament) in diabetic mice (Fig. 6 A–C). To test whether the effects of Ninj1-neutralizing antibody were mediated by the production of neurotrophic factors, we performed Western blot analysis for BDNF, NGF, and NT3. The cavernous expression of neurotrophic factors was significantly higher in diabetic mice receiving Ninj1-neutralizing antibody (2.5 µg/20 µL) than in IgG- or Fc-treated diabetic mice, which was comparable to the levels found in age-matched controls (Fig. 6 D and E). However, the Ninj1-neutralizing antibody-mediated increase in the expression of neurotrophic factors and subsequent restoration of penile nerve content was remarkably diminished by pretreatment with sTie2-Fc (Fig. 6 A–E).
Fig. 6.
Ninj1-neutralizing antibody induces neural regeneration. (A) nNOS (green) and neurofilament (red) immunostaining in penis tissue from age-matched control (C) or diabetic mice 2 wk after a single intracavernous injection of IgG (2.5 µg), dimeric-Fc (Fc, 4 µg), Ninj1-neutralizing antibody (Ninj1 Ab, 2.5 µg), or Ninj1-neutralizing antibody (2.5 µg) + soluble Tie2 antibody (sTie2-Fc, 4 µg). Nuclei were labeled with DAPI (blue). (Scale bars: corpus cavernosum, 50 μm; dorsal nerve bundle, 25 μm.) (B and C) Quantification of the nNOS- (B) or neurofilament (NF)-immunopositive area (C) in cavernous tissue by ImageJ (n = 6). (D) Western blot for neurotrophic factors. (E) Normalized band intensity values (n = 4). In C and E, *P < 0.001 vs. C and Ninj1 antibody (2.5 µg) + sTie2 (−) groups; #P < 0.001 vs. IgG and Fc groups; †P < 0.001 vs. Ninj1 antibody (2.5 µg) + sTie2 (−) group. BDNF, brain-derived neurotrophic factor; DM, diabetes mellitus; DNB, dorsal nerve bundle; NGF, nerve growth factor; NT3, neurotrophin-3; NF, neurofilament; N-Ab, Ninj1 Ab; STZ, streptozotocin. (F–I) Conditioned medium (CM) of MCECs regulates the production of neurotrophic factors in neuro2A cells. (F and H) The MCECs transfected with scrambled (SC), Ninj1, or Ang1 siRNA were exposed to the normal-glucose (NG) or high-glucose (HG) condition (F). Then the supernatant was transferred to Neuro2A cells (H). (G and I) Normalized Western blot band intensity values (n = 4). *P < 0.01 vs. NG and Ninj1 siRNA + Ang1 siRNA (-) groups; #P < 0.01 vs. SC group; †P < 0.01 vs. Ninj1 siRNA + Ang1 siRNA (-) group. P values were determined by Kruskal−Wallis test (B, E, G, and I) or one-way ANOVA (C). Data are mean ± SE.
Conditioned Medium Derived from MCECs Regulates the Production of Neurotrophic Factors in Neuro2A Cells.
Because the results of our in vivo study indicated that the Ang1–Tie2 pathway is crucial for Ninj1-neutralizing antibody–mediated secretion of neurotrophic factors and subsequent neural regeneration (Fig. 6 A–E), we further investigated whether conditioned medium derived from MCECs cultured under the high-glucose condition with or without treatment with siRNA for Ninj1 and Ang1 affected the expression of neurotrophic factors in neuro2A cells. Similar to the results from diabetic mice in vivo, Ang1 protein expression was significantly lower in MCECs exposed to the high-glucose condition than in the cells exposed to the normal-glucose condition, whereas Ninj1 protein expression was increased by the high-glucose condition. These levels returned to baseline after treatment with Ninj1 siRNA. However, knockdown of Ang1 with siRNA did not affect Ninj1 expression in MCECs (Fig. 6 F and G). The protein expression of neurotrophic factors was significantly lower in neuro2A cells treated with conditioned medium derived from MCECs exposed to the high-glucose condition than in the cells treated with conditioned medium from MCECs exposed to the normal-glucose condition. Furthermore, the expression of neurotrophic factors was significantly increased by treatment with conditioned medium from Ninj1 siRNA-transfected MCECs exposed to the high-glucose condition. Conditioned medium from both Ninj1 siRNA and Ang1 siRNA cotransfected MCECs completely abolished the effect of the Ninj1 siRNA-mediated increment in neurotrophic factor expression in neuro2A cells (Fig. 6 H and I).
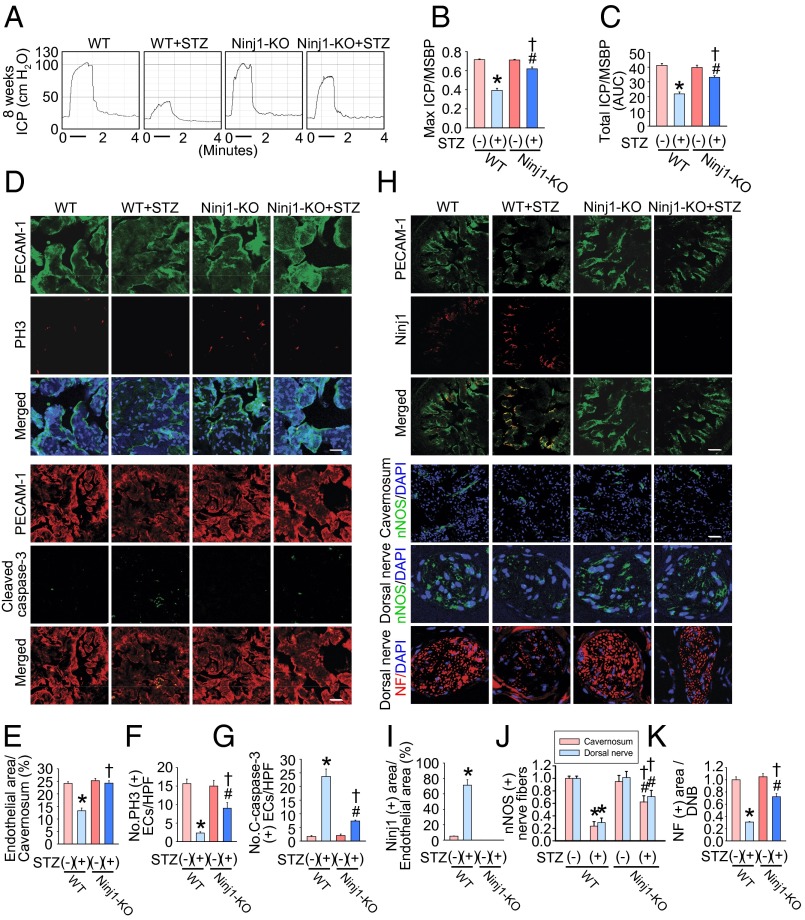
Ninj1-KO Mice Are Resistant to Diabetes-Induced Angiopathy and Neuropathy and Have Restored Erectile Function.
To confirm further the results regarding the role of Ninj1 in the pathogenesis of diabetic ED resulting from angiopathy and neuropathy, we examined the effect of STZ administration in Ninj1-KO mice. At 8 wk after the injection of STZ and induction of diabetes, the ratios of maximal ICP and total ICP to MSBP were significantly lower in WT mice that received STZ than in untreated WT mice, whereas erectile function was relatively preserved in Ninj1-KO mice that received STZ (Fig. 7 A–C). In accordance with this finding, targeted depletion of the Ninj1 gene rescued the diabetes-induced decrease in cavernous endothelial and neuronal cell contents as well as the increase in endothelial cell apoptosis (Fig. 7 D–K).
Fig. 7.
Ninj1-KO mice are resistant to diabetes-induced angiopathy and neuropathy and have restored erectile function. (A) Representative ICP responses for WT mice, WT mice receiving STZ, Ninj1-KO mice (Ninj1-KO), or Ninj1-KO mice receiving STZ. (B and C) Ratios of mean maximal ICP (B) and total ICP (C) (area under the curve) to MSBP were calculated for each group at 8 wk after the induction of diabetes (n = 6). *P < 0.001 vs. WT + STZ (−) group; #P < 0.01 vs. Ninj1-KO + STZ (−) group; †P < 0.001 vs. WT + STZ (+) group. (D) PECAM-1 (green) and phosphohistone H3 (PH3; red) or PECAM-1 (red) and cleaved caspase-3 immunostaining in each group. Nuclei were labeled with DAPI (blue). (Scale bars: PECAM-1-PH3 images, 25 µm; PECAM-1–cleaved caspase-3 images, 50 µm.) (E) Endothelial cell content in cavernous tissue was quantified using ImageJ. (F) Number of PH3-immunopositive endothelial cells per high-power field. (G) Number of apoptotic cells in endothelium per high-power field. *P < 0.001 vs. WT + STZ (-) group; #P < 0.001 vs. Ninj1-KO + STZ (−) group; †P < 0.001 vs. WT + STZ (+) group. (H) PECAM-1 (green), Ninj1 (red), nNOS (green), or neurofilament (red) immunostaining in each group. Nuclei were labeled with DAPI (blue). (Scale bars: PECAM-1-Ninj1 images, 100 µm; corpus cavernosum, 50 μm; dorsal nerve bundle, 25 μm. (I–K) Quantification of Ninj1 (I), nNOS (J), and neurofilament (K) in cavernous tissue or dorsal nerve bundle using ImageJ. Note there is no expression of endogenous Ninj1 in Ninj1-KO mice. *P < 0.05 vs. WT + STZ (−) group; #P < 0.05 vs. Ninj1-KO + STZ (−) group; †P < 0.05 vs. WT + STZ (+) group. The P values were determined by one-way ANOVA (B, C, E, F, and K), Kruskal–Wallis test (G and J), or Mann–Whitney u test (I). Data are mean ± SE. AUC, area under curve; C-caspase-3, cleaved caspase-3; DNB, dorsal nerve bundle; ECs, endothelial cells; NF, neurofilament.
Transcriptome Analysis of Ninj1 Target Genes in MCECs.
To identify the genes regulated by Ninj1 siRNA, a microarray analysis was performed (SI Appendix, Fig. S4 A–C). MCECs were exposed to the normal-glucose or high-glucose condition and transfected with scrambled or Ninj1 siRNA. We selected genes that changed more than 1.5-fold at the ratios calculated by both conditions, i.e., MCECs exposed to the normal-glucose condition compared with those exposed to the high-glucose condition + scrambled siRNA, and MCECs exposed to the high-glucose condition + Ninj1 siRNA compared with those exposed to the high-glucose condition + scrambled siRNA. After data filtering, 9,103 genes were changed more than 1.5-fold (SI Appendix, Fig. S4, B and C). The functional clustering analysis on the common genes showed that 6.5%, 6.2%, and 5.9% of these were involved in cell death, apoptosis, and cell proliferation, respectively (SI Appendix, Fig. S4, B and C). The selected genes relevant to apoptosis (47 genes) or proliferation (37 genes) are summarized in SI Appendix, Tables S5 and S6. These genes included previously known Ninj1 targets, such as Angpt1 (Ang1) and Angpt2 (Ang2) (highlighted in SI Appendix, Tables S5 and S6). The expression of Ninj1 mRNA was significantly increased in MCECs exposed to the high-glucose condition as compared with the normal-glucose condition; this level returned to baseline after treatment with Ninj1 siRNA (SI Appendix, Fig. S4 D and E).
Discussion
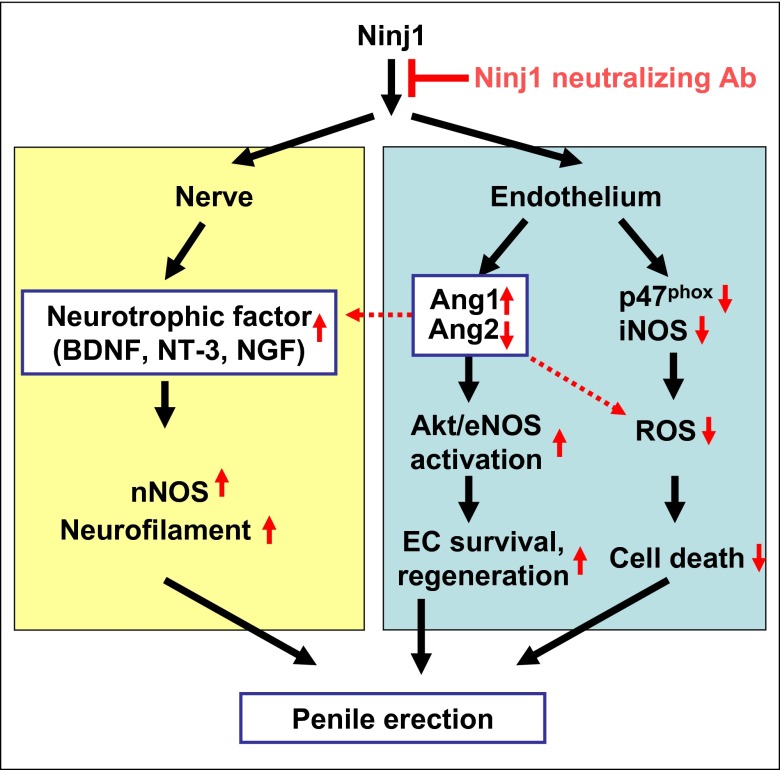
Here, we investigated whether inhibition of the Ninj1 pathway exerts beneficial effects in diabetic ED. In a mouse model of diabetic ED, a single intracavernous injection of Ninj1-neutralizing antibody or targeted depletion of the Ninj1 gene successfully restored erectile function through dual angiogenic and neurotrophic effects. The detailed mechanisms of action by which inhibition of the Ninj1 pathway restores erectile function are summarized in Fig. 8.
Fig. 8.
Schematic diagram of a proposed mechanism showing how Ninj1 blockade restores erectile function in diabetic mice. Ang1, angiopoietin1; Ang2, angiopoietin-2; BDNF, brain-derived neurotrophic factor; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; NGF, nerve growth factor; Ninj1, nerve injury-induced protein 1; nNOS, neuronal nitric oxide synthase; NT-3, neurotrophin-3; ROS, reactive oxygen species.
To investigate whether the recovery of erectile response was related to an increase in the number of endothelial cells, we evaluated the expression of PECAM-1 by immunohistochemical analysis. In agreement with the results of previous studies in STZ-induced diabetic rats (20–22) and mice (14), the cavernous endothelial area was significantly lower in untreated or IgG-treated diabetic mice than in control mice. Intracavernous injection of high-dose Ninj1-neutralizing antibody (2.5 μg/20 μL) induced complete recovery of the PECAM-1+ endothelial area in the corpus cavernosum of diabetic mice. Immunohistochemical examination of phosphohistone H3 expression showed that the increase in cavernous endothelial content by Ninj1-neutralizing antibody was partly the result of the endothelial proliferation.
In the present study, cavernous expression of Ang1 was down-regulated and that of Ang2 was up-regulated by the diabetic condition, and Ninj1-neutralizing antibody restored the expression of Ang1 and Ang2 to the levels in controls. Similar to this finding, knockdown of the Ninj1 gene increased the Ang1/Ang2 ratio in primary cultured MCECs, whereas overexpression of the Ninj1 gene decreased the Ang1/Ang2 ratio. The Ang1–Tie2 signaling pathway plays an important role in generating a nonleaky, stable, functional vasculature (23) and also is known to be involved in endothelial cell survival through the phosphatidylinositol 3-kinase/Akt pathway (24, 25). A previous study reported that Ninj1 promotes cell–cell adhesion and induces production of Wnt7b and Ang2, which lead to apoptosis and destabilization of endothelial cells in the hyaloid vascular system, while suppressing the expression of Ang1, an endothelial cell survival factor (19). We recently reported that in diabetic mice intracavernous administration of synthetic Ang1 gene or protein restores erectile function by enhancing endothelial cell proliferation and decreasing endothelial cell apoptosis (14, 15). In the present study, we show that inhibition of the Ang1–Tie2 pathway with sTie2-Fc diminished the increase in cavernous endothelial cell proliferation and decrease in endothelial cell apoptosis mediated by Ninj1-neutralizing antibody and failed to restore erectile function in the diabetic mice in vivo. Moreover, Ninj1 siRNA-mediated enhancement of tube formation and endothelial cell proliferation was abolished by cotransfection with Ang1 siRNA in MCECs in vitro. Collectively, these findings suggest that restoration of the Ang1/Ang2 balance is crucial for Ninj1 blockade-mediated cavernous angiogenesis and subsequent recovery of erectile function. However, the exact mechanisms by which Ninj1 regulates the expression of Ang1 and Ang2 are largely unknown and remain to be clarified.
The generation of superoxide anion and peroxynitrite was significantly higher in the penis of diabetic mice than in controls. Endothelial cells generate ROS, which may play a physiologic or pathologic role depending on the level of ROS. Low levels of ROS act as signaling molecules that induce endothelial cell proliferation and migration in response to a variety of stimuli, such as ischemia and angiogenic factors (26, 27). In pathologic conditions in which ROS already are generated at high levels (e.g., in the penis of diabetic mice, as shown in the present study), the high levels of ROS induce apoptosis and cell death (28). Similar to our results showing a decrease in the production of ROS in the penis of diabetic mice treated with synthetic Ang1 (14), Ninj1-neutralizing antibody significantly reduced the generation of superoxide anion and peroxynitrite in endothelial cells of diabetic mice, possibly by inhibiting the expression of cavernous p47phox and iNOS, respectively. In accordance with these results, Ninj1-neutralizing antibody significantly reduced the number of apoptotic cells in the cavernous endothelium of diabetic mice. Pretreatment of diabetic mice with sTie2-Fc completely abolished the effect of Ninj1-neutralizing antibody on the generation of ROS. This finding suggests the importance of the Ang1–Tie2 pathway in the Ninj1 blockade-mediated regulation of ROS production.
Macrophages play an essential role in inflammation and increase oxygen uptake, resulting in the accumulation of ROS during inflammation (29). Similar to a previous study showing that Ninj1 blockade decreased the infiltration of macrophages in an experimental model of allergic encephalomyelitis (18), targeted depletion of the Ninj1 gene significantly reduced diabetes-induced infiltration of macrophages and the generation of ROS in macrophages.
The amount of intact nNOS+ neurons is crucial for proper erectile function, because NO derived from nerve terminals initiates penile erection by inducing the dilatation of cavernous sinusoids, and subsequent shear stress-mediated activation of Akt and eNOS sustains penile erection (30). In the present study, intracavernous injection of high-dose Ninj1-neutralizing antibody (2.5 μg/20 μL) completely restored nNOS+ nerve content in the penis of diabetic mice. We further examined the mechanisms by which Ninj1-neutralizing antibody induces neural regeneration. The expression of neurotrophic factors, such as BDNF, NGF, and NT3, was significantly lower in the penis of diabetic mice than in controls, but the expression was restored remarkably by treatment with Ninj1-neutralizing antibody. Similar to the results in vivo, the expression of neurotrophic factor proteins was down-regulated in neuro2A cells treated with conditioned medium derived from MCECs exposed to the high-glucose condition. Furthermore, the expression was profoundly restored by Ninj1 siRNA-transfected conditioned medium. Inhibition of the Ang1–Tie2 signaling pathway with sTie2-Fc or Ang1 siRNA abolished the Ninj1 blockade-mediated increase in neurotrophic factors and restoration of nNOS-positive neurons in diabetic mice in vivo and in neuro2A cells in vitro. A recent study reported that Ang1 inhibits apoptosis of retinal neurons and prevents neuronal dysfunction in a mouse model of oxygen-induced retinopathy (31). Thus, Ang1 may have a protective effect on ischemia-induced neuronal loss. Further studies are necessary to document how the Ang1–Tie2 signaling pathway regulates the expression of neurotrophic factors.
Finally, we examined the effect of targeted depletion of the Ninj1 gene on diabetes-induced angiopathy and neuropathy. Endothelial cell and nerve content were preserved and erectile function was rescued in Ninj1-KO mice receiving STZ. These results suggest that therapeutic endothelial and neural regeneration may provide a pathway for the treatment of diabetic ED.
However, our study had some limitations. First, this study did not explain how Ninj1 regulates the expression of Ang1 and Ang2 or how the Ang1–Tie2 signaling pathway is involved in the regulation of neurotrophic factor expression mediated by Ninj1-neutralizing antibody or by Ninj1 siRNA. Second, the duration of recovery of erectile function was relatively short. Further studies are needed to test whether repeated intracavernous injections of Ninj1-neutralizing antibody or the development of more potent Ninj1-blocking peptide would induce more durable recovery of erectile function. Finally, our data demonstrating that Ninj1 inhibition plays a positive role in neural regeneration in diabetic penis in vivo are inconsistent with the study by Araki and Milbrandt (17), which showed that Ninj1 facilitates neurite extension in cultured dorsal root ganglion neurons in vitro. This disparity may result from different experimental conditions or from the observation of different organ systems.
In summary, our results indicate that inhibition of the Ninj1 pathway rescues erectile function in diabetic mice by inducing both angiogenesis and neural regeneration in an Ang1-Tie2–dependent manner. The dual angiogenic and neurotrophic effects of Ninj1 blockade, especially local therapy in the form of neutralizing antibody or blocking peptide, opens a new avenue through which to treat diabetic ED.
Materials and Methods
Expanded methods are available in SI Appendix, SI Methods.
Study Design.
The primary aim of the present study was to investigate the mechanisms through which inhibition of the Ninj1 pathway restores diabetes-induced erectile function. To do so, we administered Ninj1-neutralizing antibody into the penis of diabetic mice and also used Ninj1-KO mice. Detailed mechanisms were evaluated with WT or Ninj1-KO mice and primary cultured MCECs and Neuro2A cells. All parameters of genetically modified mice and diabetic mice were compared with those of littermate controls.
Animals and Treatments.
The experiments were approved by the Institutional Animal Care and Use Subcommittee of Inha University. Male 8-wk-old C57BL/6J mice (Orient Bio) and Ninj1-KO mice (B6.129P2-Ninj1TM1GTO/J, provided by G.T.O.) were used in this study. Ninj1-KO mice were backcrossed with C57BL/6 for at least seven generations. The primer sequences for genotyping were as follows: WT (forward): 5′-GAG ATA GAG GGA GCA CGA CG-3′; Neo (forward): 5′-ACG CGT CAC CTT AAT ATG CG-3′; reverse primer: 5′-CGG GTT GTT GAG GTC ATA CTT G-3′. Diabetes was induced by i.p. injections of multiple low doses of STZ (50 mg/kg body weight in 0.1 M citrate buffer, pH 4.5) for 5 d consecutively as previously described (20). Eight weeks after diabetes was induced, the mice were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) i.m. and were placed supine on a thermoregulated surgical table.
To test the efficacy of Ninj1-neutralizing antibody, the mice were distributed into five groups: age-matched controls, diabetic mice without treatment, diabetic mice receiving a single i.v. injection of mouse IgG control antibody, diabetic mice receiving a single i.v. injection of low-dose (1.0 µg/20 µL) mouse neutralizing antibody to Ninj1 (BD Biosciences), and diabetic mice receiving a single intracavernous injection of high-dose (2.5 µg/20 µL) mouse neutralizing antibody to Ninj1. In the pilot study, we determined the optimal concentration to induce maximal recovery of erectile function.
For the inhibition study with sTie2-Fc, the mice were distributed into five groups: age-matched controls, diabetic mice receiving a single intracavernous injection of mouse IgG control antibody, diabetic mice receiving a single intracavernous injection of dimeric-Fc, diabetic mice receiving a single intracavernous injection of high-dose (2.5 µg/20 µL) mouse neutralizing antibody to Ninj1, and diabetic mice receiving a single intracavernous injection of high-dose (2.5 µg/20 µL) mouse neutralizing antibody to Ninj1 and s.c. injection of sTie2-Fc (4 µg/20 µL) (R&D Systems). sTie2-Fc was administered immediately before the injection of Ninj1-neutralizing antibody. We evaluated erectile function by cavernous nerve electrical stimulation 2 or 4 wk after treatment. The penis was harvested for histologic examination and biochemical study.
For the Ninj1-KO study, the mice were distributed into four groups: WT controls, WT mice receiving STZ (50 mg/kg body weight, for 5 d), Ninj1-KO mice, and Ninj1-KO mice receiving STZ (50 mg/kg body weight, for 5 d). At 8 wk after the induction of diabetes, we measured erectile function during electrical stimulation of the cavernous nerve, and then the penis was harvested for histologic examination. Fasting and postprandial blood glucose levels were determined with an Accu-check blood glucose meter (Roche Diagnostics) before the mice were killed.
Measurement of Erectile Function, Histologic Examinations, and In Situ Detection of Superoxide Anion.
Measurement of erectile function, histologic examinations, and in situ detection of superoxide anion were performed as described in SI Appendix, SI Methods.
Cell-Culture Experiments.
Cell culture, transfection assay, RT-PCR, cDNA microarray, and in vitro tube formation assay were performed as described in SI Appendix, SI Methods.
Preparation of Conditioned Medium from MCECs and Transfer to Neuro2A Cells.
To examine the effect of conditioned medium derived from MCECs on Neuro2A cells, the MCECs were cultured and treated under the following conditions: MCECs exposed to normal-glucose condition (5 mmol), MCECs exposed to the high-glucose condition (30 mmol) and transfected with scrambled siRNA (200 pmol), MCECs exposed to the high-glucose condition (30 mmol) and transfected with Ninj1 siRNA (200 pmol), and MCECs exposed to the high-glucose condition (30 mmol) and transfected with both Ninj1 siRNA (200 pmol) and Ang1 siRNA (200 pmol). The MCECs were transfected with Ninj1 siRNA or Ang1 siRNA for 24 h and then exposed to the high-glucose condition for another 48 h. The culture supernatants were collected and centrifuged for 10 min at 200 × g to remove cell debris and then were transferred to Neuro2A cells. After 12 h, Neuro2A cells were harvested for Western blot analysis.
Western Blot.
Western blot analysis was performed as described in SI Appendix, SI Methods.
Statistical Analysis.
The results are expressed as mean ± SE. For parametric data, intergroup comparisons were made by one-way ANOVA followed by Newman–Keuls post hoc tests. We used the Mann–Whitney u test or Kruskal–Wallis test to compare nonparametric data. Probability values less than 5% were considered significant. We used SigmaStat 3.11 software (Systat Software) for statistical analyses.
Supplementary Material
Acknowledgments
This work was supported by Grant A110076 from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (to J.-K.S. and J.-K.R.); by Medical Research Center Grant 2014R1A5A2009392 (to J.-K.R.); and by Grant 2012RIA3A2026454 (to G.T.O.) from National Research Foundation, funded by the Korean government (Ministry of Science, ICT and Future Planning).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: cDNA microarray data have been deposited in the Gene Expression Omnibus database, www.ncbi.nlm.nih.gov/geo (accession no. GSE57525).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403471111/-/DCSupplemental.
References
- 1. National Institutes of Health Consensus Development Panel on Impotence (1993) Impotence. NIH Consensus Conference. JAMA 270(1):83–90. [PubMed]
- 2.Friedewald VE, et al. American Journal of Cardiology Journal of Periodontology The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104(1):59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342(24):1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 4.Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am. 1996;25(2):379–400. doi: 10.1016/s0889-8529(05)70329-7. [DOI] [PubMed] [Google Scholar]
- 5.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232–1247. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 6.Vignozzi L, et al. Testosterone regulates RhoA/Rho-kinase signaling in two distinct animal models of chemical diabetes. J Sex Med. 2007;4(3):620–630, discussion 631–632. doi: 10.1111/j.1743-6109.2007.00440.x. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Jabaloyas JM, et al. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol. 2001;40(6):641–646, discussion 647. doi: 10.1159/000049850. [DOI] [PubMed] [Google Scholar]
- 8.Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19(2):129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- 9.Angulo J, et al. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med. 2010;7(2 Pt 1):758–768. doi: 10.1111/j.1743-6109.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis S, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 11.Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: Adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;13(1):19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- 12.Suri C, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 13.Kwon MH, et al. Effect of intracavernous administration of angiopoietin-4 on erectile function in the streptozotocin-induced diabetic mouse. J Sex Med. 2013;10(12):2912–2927. doi: 10.1111/jsm.12278. [DOI] [PubMed] [Google Scholar]
- 14.Jin HR, et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes. 2011;60(3):969–980. doi: 10.2337/db10-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin HR, et al. Intracavernous delivery of synthetic angiopoietin-1 protein as a novel therapeutic strategy for erectile dysfunction in the type II diabetic db/db mouse. J Sex Med. 2010;7(11):3635–3646. doi: 10.1111/j.1743-6109.2010.01925.x. [DOI] [PubMed] [Google Scholar]
- 16.Bennett NE, et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J Urol. 2005;173(5):1820–1824. doi: 10.1097/01.ju.0000158056.66236.1f. [DOI] [PubMed] [Google Scholar]
- 17.Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17(2):353–361. doi: 10.1016/s0896-6273(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 18.Ifergan I, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70(5):751–763. doi: 10.1002/ana.22519. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, et al. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009;16(10):1395–1407. doi: 10.1038/cdd.2009.78. [DOI] [PubMed] [Google Scholar]
- 20.Jin HR, et al. Functional and morphologic characterizations of the diabetic mouse corpus cavernosum: Comparison of a multiple low-dose and a single high-dose streptozotocin protocols. J Sex Med. 2009;6(12):3289–3304. doi: 10.1111/j.1743-6109.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LW, et al. Role of increased penile expression of transforming growth factor-beta1 and activation of the Smad signaling pathway in erectile dysfunction in streptozotocin-induced diabetic rats. J Sex Med. 2008;5(10):2318–2329. doi: 10.1111/j.1743-6109.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 22.Burchardt T, et al. Reduction of endothelial and smooth muscle density in the corpora cavernosa of the streptozotocin induced diabetic rat. J Urol. 2000;164(5):1807–1811. [PubMed] [Google Scholar]
- 23.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 24.Kim I, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 25.Papapetropoulos A, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275(13):9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 26.Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9(4):231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 27.Łuczak K, Balcerczyk A, Soszyński M, Bartosz G. Low concentration of oxidant and nitric oxide donors stimulate proliferation of human endothelial cells in vitro. Cell Biol Int. 2004;28(6):483–486. doi: 10.1016/j.cellbi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 29.Brüne B, et al. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19(6):595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurt KJ, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99(6):4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, et al. 2013. Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci Transl Med 5(203):203ra127.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.