Significance
Protection of freshwater ecosystems from organic pollutants is important to preserve biodiversity and the goods they provide to society, such as clean drinking water and recreation. Organic chemicals have been shown to adversely impact freshwater ecosystems in local and regional studies. Nevertheless, due to paucity of studies on larger spatial scales, it remains unknown how widespread the risk from organic chemicals is. For the first time, to our knowledge, we provide strong evidence that chemicals threaten the ecological integrity and consequently the biodiversity of almost half of the water bodies on a continental scale, based on the analysis of governmental monitoring data from 4,000 European sites. Due to limitations associated with the monitoring programs, our results are likely to underestimate the actual risks.
Keywords: toxicity, effect thresholds, streams, river basins, ecological data
Abstract
Organic chemicals can contribute to local and regional losses of freshwater biodiversity and ecosystem services. However, their overall relevance regarding larger spatial scales remains unknown. Here, we present, to our knowledge, the first risk assessment of organic chemicals on the continental scale comprising 4,000 European monitoring sites. Organic chemicals were likely to exert acute lethal and chronic long-term effects on sensitive fish, invertebrate, or algae species in 14% and 42% of the sites, respectively. Of the 223 chemicals monitored, pesticides, tributyltin, polycyclic aromatic hydrocarbons, and brominated flame retardants were the major contributors to the chemical risk. Their presence was related to agricultural and urban areas in the upstream catchment. The risk of potential acute lethal and chronic long-term effects increased with the number of ecotoxicologically relevant chemicals analyzed at each site. As most monitoring programs considered in this study only included a subset of these chemicals, our assessment likely underestimates the actual risk. Increasing chemical risk was associated with deterioration in the quality status of fish and invertebrate communities. Our results clearly indicate that chemical pollution is a large-scale environmental problem and requires far-reaching, holistic mitigation measures to preserve and restore ecosystem health.
The majority of streams and rivers are ecologically impaired or threatened with high losses in biodiversity, which compromise the future provisioning of vital ecosystem services (1, 2). Understanding the causes of these impairments is crucial to inform freshwater management and for directing restoration efforts (3). Despite their ubiquitous global use, organic chemicals have only been shown to affect aquatic communities locally or regionally, whereas the overall extent of their impact is largely unknown (4, 5). Previous studies on the risk assessment of organic chemicals have been limited to a few sites (6), regions (7), or compounds (8) rendering the extrapolation to larger spatial scales questionable. To date, large-scale analyses have been hindered by the lack of large-scale monitoring databases for organic chemicals and by the scarcity of empirical toxicity data (9). Gaps in missing experimental toxicity data can be filled by modeled or predicted toxicity data from read-across methods (10) or quantitative structure–activity relationship approaches (11), which serve as surrogates for experimental data. Once toxicity data are compiled, the availability of chemical datasets such as Waterbase (12), which accommodates information on the chemical concentrations for more than 8,200 European sites, allows chemical risk (CR) assessment to be conducted on large spatial scales.
Chemical risk assessment is typically conducted by comparing measured or predicted environmental concentrations with the respective risk thresholds, which are usually derived from ecotoxicological tests in the laboratory, at or above which effects on aquatic organisms cannot be excluded (13). In particular, when data on acute toxicity of species are the only data available, safety factors (e.g., 100–1,000; ref. 14) are applied to the lowest median lethal concentration (LC50) from three representative taxonomic groups (usually a crustacean, a fish, and an algae). These safety factors are supposed to protect the nontarget species from the likely effects of chemicals. More sophisticated approaches such as species sensitivity distributions (SSDs), which allow for derivation of thresholds that are assumed to protect a distinct percentage of species (usually 95%), are generally not applicable for datasets with a high number of chemicals, due to a paucity of toxicity data (15). However, establishing effect thresholds that are protective for the entire ecosystem is an on-going challenge, due to difficulties with addressing the inherent differences between laboratory test systems and field situations and due to the required balance of ecosystem protection and economic development (13). The plausibility of the chemical risk assessment can, however, be determined by comparison with ecological endpoints from real ecosystems, if ecological data are available.
In this study, we present, to our knowledge, the first comprehensive chemical risk assessment on a continental scale encompassing three major organism groups in freshwater ecosystems (fish, invertebrates, and algae, represented by Pimephales promelas, Daphnia magna, and Pseudokirchneriella subcapitata, respectively). We combined measured concentrations of 223 chemicals for 4,001 sites distributed over 91 European river basins with their respective toxicity information to determine the spatial distribution of chemical risk on the continental scale. For this purpose, the CR per river basin was calculated using two risk thresholds, the acute risk threshold (ART) and the chronic risk threshold (CRT) for each organism group (SI Appendix, SI Methods for rationale). Compounds whose concentrations exceeded the ART at any site were considered as the most relevant compounds for risk assessment and classified as acute-risk chemicals (ARCs). We checked if the CR increased with the number of ARCs analyzed and which compounds contributed most. Furthermore, we identified to what extent different land use types drove the chemical risk. Finally, we compared the chemical risk to the ecological status of fish, invertebrate, and diatom communities at selected sites.
Results and Discussion
Chemical Risk.
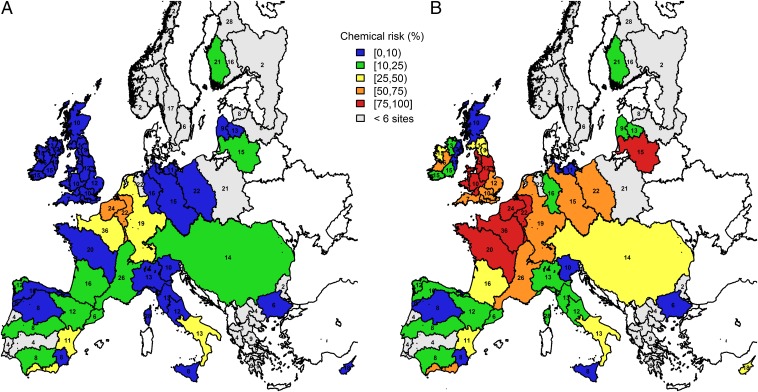
On the continental scale, 14% of the monitoring sites were likely to be acutely affected by organic chemicals (Fig. 1A) and 42% were likely to be chronically affected by organic chemicals for at least one organism group (Fig. 1B). For each organism group, at 3%, 6%, and 9% of sites, the maximum chemical concentrations exceeded the ART for fish, invertebrates, and algae, respectively, and at 6%, 38%, and 13% of sites, the mean chemical concentrations exceeded the CRT (SI Appendix, Fig. S4). Note that the differences in CRT exceedances between organism groups may partly be attributed to the different sources of effect thresholds, which were field based for invertebrates, and extrapolated from laboratory-based acute toxicity data for fish and algae (SI Appendix, SI Methods). In general, these results suggest that organic chemical pollution is an important large-scale pressure.
Fig. 1.
Chemical risk (by percentage range) in European river basins. The map displays the fraction of sites where the maximum chemical concentration exceeds the acute risk threshold (A) and the mean chemical concentration exceeds the chronic risk threshold (B) for any organism group. The color code shows the level of chemical risk, from low chemical risk (blue) to high chemical risk (red). River basins with up to six sites are displayed in gray (SI Appendix, Table S5), whereas river basins without data are displayed in white. The numbers denote the median of the acute-risk chemicals analyzed at the monitoring sites of each river basin. Direct comparisons between river systems are potentially biased by the ecotoxicologically relevant compounds analyzed and the limit of quantification of the compounds (SI Appendix, Fig. S2 and Table S2).
On the regional level, river basins in the north of Europe had higher chemical risks than those situated in the south. For the northwestern river basins, the acute and chronic CR reached high (50–75%) to very high (>75%) levels, respectively. This is in agreement with other studies that predicted loads of chemicals in European rivers [e.g., perfluorinated compounds (16) and insecticides (17)]. In Southern Europe, the low chronic and acute CRs (<25%) were presumably due to the low number of ecotoxicologically relevant chemicals measured and the result of unreliable limits of quantifications for part of the data (e.g., Spain; SI Appendix, SI Methods). On the contrary, the high acute and chronic risks in the French river basins probably resulted from good monitoring practices, such as a dense monitoring network and the inclusion of most ecotoxicologically relevant chemicals (i.e., the ARCs). Hence, comparisons between river basins are potentially biased by spatial and temporal sampling density and the number of ARCs analyzed. One example is the generally poor spatial sampling density for river basins in the Scandinavian and Baltic countries (fewer than six sites), hampering a reliable CR estimation. Furthermore, only 5% of the sites were regularly monitored every year, whereas 53% of the sites were sampled only once in the 5-y interval investigated. Nevertheless, we found only a weak relationship between the number of sampling sites and CR (ART: Kendall τ = 0.42; CRT: Kendall τ = 0.33), and no significant difference between the CR from different years (one-way analysis of variance (ANOVA), ART: F4,41 = 1.37, P = 0.26; CRT: F4,44 = 0.47, P = 0.75). Overall, standardized monitoring programs with regard to spatial and temporal sampling density, as well as the inclusion of ARCs in monitoring schemes (see below for discussion on ARCs), would enhance the comparability of individual basins on large scales. Note that deficiencies in monitoring programs can only result in underestimation of risk, never in overestimation.
Contributors to Chemical Risk.
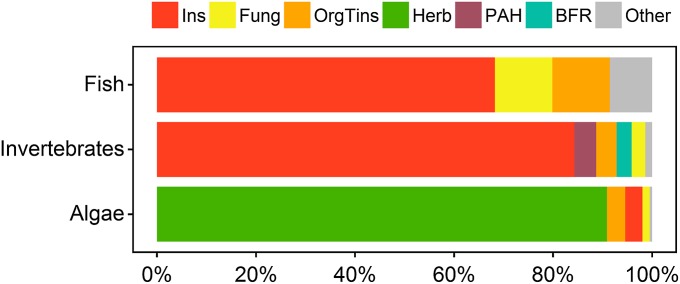
Pesticides were responsible for 81%, 87%, and 96% of the observed exceedances of the ART related to fish, invertebrates, and algae, respectively (Fig. 2). Despite extensive regulation and technological advances in terms of specificity and degradability, pesticides continue to threaten nontarget species, especially those groups exhibiting physiological similarity to pest species (18). Herbicides accounted for most of the exceedances in algae, whereas insecticides accounted for most of the exceedances for invertebrates and fish (Fig. 2 and SI Appendix, Table S4). Whereas pesticides are designed to acutely affect invertebrates and algae, fish typically suffer from compounds affecting development, fitness, or reproduction (e.g., by endocrine disruptors), which are not covered here, but might increase the risk to fish communities (19). Additional ARCs were (i) organotin compounds, mainly the banned biocide tributyltin, which is an antifouling agent that primarily leaches from the hulls of ships; (ii) brominated diphenyl ethers, which are widely used as flame retardants in consumer products; and (iii) polycyclic aromatic hydrocarbons, which are released by petroleum products or by combustion of organic matter. These chemical groups have raised concerns of persistence and biomagnification in the environment (5). For the majority of the ARCs, experimental toxicity data (82% for P. promelas, 89% for D. magna, and 71% for P. subcapitata; SI Appendix, Table S4) were available, reducing the uncertainty related to predicted toxicity values. Here, we frame the chemical risks primarily for the environment, but maintaining environmental integrity is directly and indirectly relevant to human health and welfare (20). Protection of freshwater from pollution safeguards ecosystem services such as water quality, which is pivotal for clean drinking water at an acceptable cost, and recreational values (1).
Fig. 2.
Proportion of sites acutely affected by different chemical groups. The chemical groups analyzed were insecticides (Ins), fungicides (Fung), organotin compounds (OrgTins), herbicides (Herb), polycyclic aromatic hydrocarbons (PAH), brominated flame retardants (BFR), and other compounds (chemical groups with five or fewer sites acutely affected which comprised polychlorinated biphenyls, halogenated alkanes, and phenols). The groups of organisms considered were fish (represented by P. promelas), invertebrates (represented by D. magna), and algae (represented by P. subcapitata). Acutely affected sites were all sites with maximum concentrations exceeding 1/10 of the LC50.
Chemical Risk and Land Use.
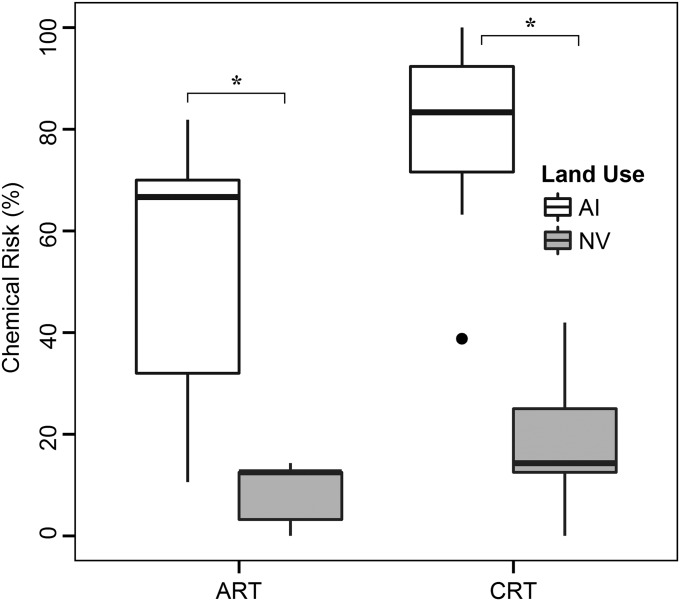
Chemical risks strongly depended on the land use in the upstream catchments of the monitoring sites. We found a significant difference in the chemical risk between sites with intensive agriculture and/or urban practices (>50% land use) and those with natural vegetation (>80% land use) (all, P < 0.05, t test, CRT: t = 5.61, df = 10; ART: t = 4.13, df = 7) (Fig. 3). Adverse effects on the biota of small agricultural streams are well documented (21), but our study suggests that these effects can occur catchment-wide, presumably originating from the interconnectedness of freshwater ecosystems. Hence, management tools such as land sparing, i.e., high-intensity agriculture in defined areas to spare land for conservation in other parts, appear to be less plausible for freshwater biodiversity conservation than land sharing through extensive agriculture (22). Control of diffuse sources of pollution from agriculture remains a challenging task but can, for example, be achieved by implementing riparian buffer strips (especially edge of field), grassed paths, or vegetated treatment systems (23, 24). Risk from other chemicals of concern relates mainly to point source pollution (e.g., input of waste water from households or industry), implying the requirement of optimized treatment technologies (e.g., ozonation; ref. 25) and better source control approaches.
Fig. 3.
Box-and-whisker plots of the chemical risk for different land use categories. The two categories used comprised anthropogenically influenced areas (AI) and natural vegetation (NV) for the acute risk threshold (ART) and chronic risk threshold (CRT). The categories analyzed were significantly different for both thresholds (P < 0.05).
Underestimation of Chemical Risk.
Notwithstanding the high-quality data used for this analysis, the retrospective risk assessment presented here most likely underestimated the real risk of chemicals and can be considered as the best-case scenario for the following reasons: First, the significantly increasing trend of the CR with the number of ARCs that were analyzed (Fig. 4) suggested that the acute and chronic risks would be higher if more ARCs were analyzed. River basins with more than 15 ARCs analyzed exhibited generally higher chemical risks (Fig. 1). For a more realistic risk assessment, monitoring programs should be designed to measure at least all ARCs, unless there is strong evidence that a specific ARC is ecotoxicologically irrelevant in a basin. However, emerging chemicals other than those frequently monitored are likely to be present in ecotoxicologically relevant concentrations in water samples (e.g., ref. 26) and should be progressively identified and included in monitoring programs.
Fig. 4.
Mean chemical risk of the river basins to exceed the risk thresholds as a function of the number of acute-risk chemicals (ARCs) analyzed. ARCs are chemicals for which the maximum concentration exceeds 1/10 of the lethal effect concentration at any site. Dots correspond to the acute risk threshold (ART), and triangles are for the chronic risk threshold (CRT). The total number of sites for each ARC interval is given in parentheses on the x axis. For the relationship between the number of acute-risk chemicals analyzed and the chemical risk, a cubic smoothing spline (all, df = 3) was fitted to the data to visualize the significant increasing trend (all, P < 0.05, n = 30; ART, dashed line: Kendall τ = 0.53; CRT, solid line: Kendall τ = 0.74).
Second, potential threshold exceedances would go unnoticed due to high limits of quantifications (LOQs). For 18% of the analyzed chemicals, in the majority of cases (>50%), the reported LOQ values were above the CRT (SI Appendix, Table S2). The LOQs provide the smallest concentrations that can be reliably quantified by the analytical method used and should be substantially lower than the risk threshold (27). Thus, analytical measurements with higher sensitivity are required.
Third, other considerations, not addressed here, could exacerbate the chemical risk: (i) chemicals usually occur in mixtures, which have been shown to exhibit stronger combined adverse effects than single compounds, especially for chemicals with similar modes of action (11); (ii) transformation products may be more ecotoxicologically potent than their parent compounds (28); and (iii) current monitoring relies on point grab water samples at monthly or quarterly intervals, which are very likely to underestimate the real maximum concentrations (29). Moreover, very hydrophobic chemicals were omitted from the analysis due to uncertainty with regard to the effect concentrations derived from experiments exceeding the water solubility. Nevertheless, these compounds may bioaccumulate, as well as have other ecological effects such as endocrine disruption, which have been shown to impact ecosystems on large spacial scales (19) (for details, SI Appendix, SI Discussion).
Ecological Status and the Relationship with Chemical Risk.
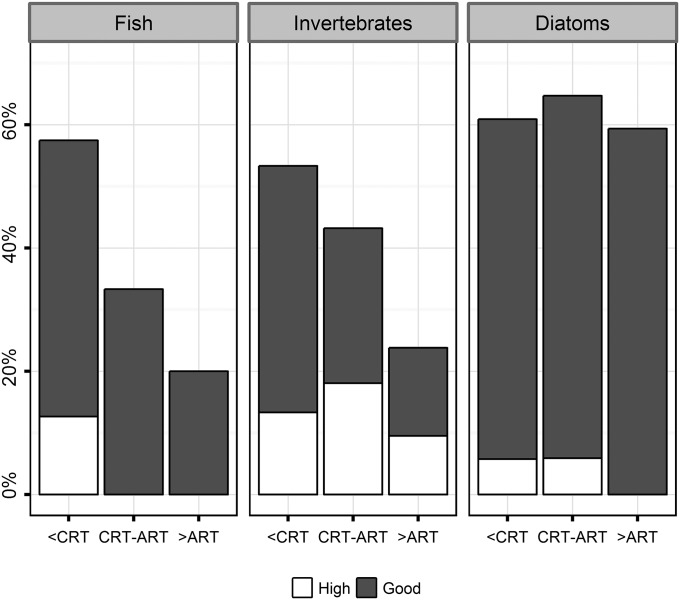
The ecological status decreased strongly with increasing chemical risk for fish and invertebrates, whereas no clear trend was observed for diatoms (Fig. 5). Similarly, a recent study found losses of invertebrate biodiversity above the CRT (30). However, these results should be interpreted with caution, because European streams are subject to multiple stresses (e.g., >90% of lowland streams; ref. 31) and the indices used in our study (Methods) indicate general ecological degradation of a site and are not toxicant specific. The invertebrate (MultiMetric Invertebrate Index; I2M2; ref. 32) and fish (Indice Poisson Rivière; IPR+; ref. 33) indices are multimetric, hence they are designed to respond to a large range of stressors (e.g., nutrients, hydromorphological alterations, and land use; refs. 32 and 34) including toxic chemicals. Therefore, they may be more suitable to detect chemical risk than the diatom index (Indice Biologique Diatomées, IBD) that was tailored to detect the effects of eutrophication (35). With respect to diatoms, confounding factors such as the light regime, turbulence, or current velocity may also mask chemical effects (36). With respect to fish, the low number of sites impacted by chemicals (SI Appendix, Table S7) could hamper the relationship with ecological status. Furthermore, the difficulty in linking chemical stress to ecological status for fish in a given location likely originates from their high mobility. Therefore, fish indices are primarily regarded as indicators of habitat degradation and flow regulations, rather than as indicators of water pollution (37). By contrast, invertebrates are considered as good site-specific bioindicators, due to their low mobility. Note that the standard test species used for estimating chemical risk may not represent the chemical sensitivity of entire communities (e.g., ref. 18), which may add to inconsistencies between the chemical risk and the ecological status of a site. Finally, toxicant-specific indices (e.g., the invertebrates' Species at Risk for Pesticides; SPEARpesticides index; ref. 38) would be more appropriate for detecting chemical effects. However, its application requires access to raw biological data (e.g., species abundance), whereas governmental agencies only provide ecological status information for the biological quality elements (phytoplankton, aquatic macrophytes, benthic macroinvertebrates, and fish) based on general indices. Providing access to raw data would foster our understanding of the links between anthropogenic stressors and populations or communities.
Fig. 5.
Proportion of sites in high and good ecological status for fish, invertebrates, and diatoms. Sites were classified as acutely affected by chemicals (>ART), chronically affected by chemicals (CRT-ART) and not affected by chemicals (<CRT; Methods for details; SI Appendix, Table S7 for the number of sites).
Chemical and ecological data were matched on the basis of sampling years (Methods), because precise sampling dates were unavailable. Hence, the temporal lags between the chemical and the ecological sampling sites may have allowed for recovery, if effects occurred, which is especially relevant for diatoms that have reproduction times of a few hours to days. A harmonization of biological and chemical monitoring schemes would reduce the temporal and spatial bias in estimating ecological effects from chemicals.
Overall, we suggest that the decrease in ecological status for fish and invertebrates with increased chemical risk is an indication of water quality deterioration in aquatic ecosystems in response to chemicals.
Conclusions and Prospective.
Our study suggests that chemical pollution is a continental-scale problem and as such requires large-scale integrated solutions, which are not always provided by end-of-pipe technologies. New frontiers in pollution prevention, such as designing chemicals according to the principles of green chemistry and substitution of hazardous chemicals preferably by nonchemical solutions, closed cycles of chemicals, specific treatment of unavoidable effluents at the source, innovative take-back systems from consumers, as well as new approaches in communication and education, should be promoted (5, 39). Furthermore, considering that ∼100,000 organic chemicals are currently in daily use and may enter freshwater ecosystems via different routes (5), the success of mitigation measures obviously cannot be based on chemical monitoring of a limited set of target chemicals only, but requires a smart combination of stressor-specific indices, effect-based monitoring tools, and chemical screening (40). Holistic basin-scale assessments (e.g., European Water Framework Directive; ref. 41) and chemical regulations [e.g., Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH); ref. 42] are good starting points for addressing large spatial-scale pollution problems. However, more effort is necessary to integrate and advance these regulations toward the reduction of toxic pollution. Our study suggests that a paradigm change in chemical regulation and management is required to achieve a holistic approach, which assesses the toxic pressure as a whole rather than from individual chemicals, and complements specific case studies by large-scale analyses.
Methods
Data Mining.
Chemical concentrations were retrieved from the Waterbase (version 12) dataset of the European Environmental Agency (12). The database quality control comprised (i) removal of duplicate entries, entries with missing concentrations, and entries with missing coordinates or with coordinates outside of Europe; (ii) treatment for concentrations reported as below LOQ; (iii) treatment of sites spatially autocorrelated; and (iv) restriction of the dataset to the most recent data available (2006–2010) for organic chemicals (SI Appendix, Fig. S1 and SI Methods). The chemical concentrations (in micrograms per liter) for each monitoring site were reported as mean (Cmean), and maximum (Cmax) annual values, typically used to characterize chronic and acute exposure, respectively. For sites with few measurements per year (e.g., n ≤ 12), the Cmean can be potentially influenced by the Cmax and/or nondetects (reported as a fraction of LOQ). To account for this bias, we adjusted the reported Cmean as three times lower for n ≤ 12 (Cc-mean) based on the Cmax/Cmean relationship for the sites with n > 12, for which Cmean values were considered as representative of chronic exposure (SI Appendix, SI Methods).
Short-term toxicity values (i.e., LC50) were collected for each chemical and each of the three test species: (i) the fish P. promelas (96 h); (ii) the invertebrate D. magna (48 h); and (iii) the green algae P. subcapitata (formerly known as Selenastrum capricornutum; 48–96 h). In a sequential order, LC50 values were compiled by using experimental, predicted, or baseline (from the octanol–water partitioning coefficient) toxicity data. Toxicity values were excluded when (i) they exceeded 10-fold the water solubility, and (ii) the application domain for baseline toxicity was violated (SI Appendix, Table S1 for sources of toxicity data, and SI Appendix, SI Methods for details). Finally, 223 compounds were considered in this analysis.
Threshold Selection.
To quantify the potential effects of chemicals on ecosystem health, for each site within a river basin, we compared (i) Cmax to the ART, defined as 1/10 of the LC50 values for each of the three standard test organisms; and (ii) Cmean (or Cc-mean) to the CRT, defined as 1/1,000, 1/100, and 1/50 of the LC50 values for invertebrates, fish, and algae, respectively. Concentrations exceeding these thresholds may cause acute and chronic ecological effects, respectively (SI Appendix, SI Methods).
Chemical Risk Calculation.
First, the CR index for each organism group was calculated on the river basin scale as CRj,o,b = Nj,o,b/Ntotal,b, where N represents the number of sites for which one of the chemical concentrations exceeded the respective risk threshold j for each organism group o within a river basin b, and Ntotal represents the total number of sites within that river basin. The risk thresholds j are either the CRT or the ART. Fewer than six monitoring sites were considered as unrepresentative for a river basin, which were subsequently omitted from the analysis (basins in gray in Fig. 1 and SI Appendix, Fig. S4). Furthermore, the chemical risk index for each threshold j was calculated as the (i) aggregation over all organism groups o per basin b: CRj,b = Nj,b/Ntotal,b; (ii) overall chemical risk on the continental scale for each organism group o: CRj,o = Nj,o/Ntotal,o; and (iii) overall chemical risk on the continental scale CRj = Nj/Ntotal.
We created maps on the distribution of the chemical risk in Europe by dividing the CRj,b and CRj,o,b indices into five classes: (i) 0–10% as very low; (ii) 10–25% as low; (iii) 25–50% as moderate; (iv) 50–75% as high; and (v) 75–100% as very high (Fig. 1 and SI Appendix, Fig. S4 for CRj,b and CRj,o,b, respectively). We based the definition of the likelihood of observing acute and chronic effects on a literature review summarized in SI Appendix, Table S3.
Finally, the Kendall τ correlation coefficient was used to check the relationship between the chemical risk in river basins (CRj,b) and the number of sampling sites.
Acute-Risk Chemicals.
Chemicals for which the Cmax exceeded the ART at any site for any organism group were classified as ARCs. We hypothesized that the chemical risk at a monitoring site would be positively correlated with the number of ARCs analyzed. Therefore, we (i) calculated the chemical risk for groups of sites at which a given number of ARCs were analyzed; (ii) fitted a cubic smoothing spline to visualize the trend between the calculated chemical risk and ARCs; and (iii) used the nonparametric rank-based Mann–Kendall test (43) to assess the significance of the trend (P < 0.05; for details on the calculations, SI Appendix, SI Methods).
Temporal Variation.
Large differences in the monitoring frequencies among sites raised the question of whether temporal variability biased the chemical risk. Therefore, the chemical risk was calculated for each year to check for potential differences among the years 2006–2010. The chemical risk was calculated as: CRa,j,b = Na,j,b/Ntotal,a,b, where a represents the year, ranging from 2006 to 2010. Only sites that had data for more than 1 y and basins that had more than six sites were included in the analysis. To test for differences in the chemical risk between years, one-way ANOVA with Welch correction was used (P < 0.05).
Land Use Practices.
To identify the potential origin of pollution, we retrieved the land use information from the same dataset (12), which was reported as the percentage of the upstream catchment land use of each monitoring site (available for 14% of the sites; SI Appendix, Table S5). We investigated the difference in chemical risk between two types of land use categories (i) natural vegetation (NV); and (ii) anthropogenically influenced areas (AI; SI Appendix, Table S6 for subcategories), as we assumed that organic chemicals would originate primarily from agricultural or urban areas. The land use was further restricted to (i) sites with more than 80% natural vegetation (n = 117) and (ii) sites with more than 50% anthropogenically influenced areas (n = 189). Chemical risk for the sites from the two land use categories was calculated as CRu,j,b = Nu,j,b/Ntotal,u,b, where u is NV or AI areas. Basins with fewer than six sites for each land use category were omitted. Chemical risk between the two land use categories was compared with the Student t test (P < 0.05).
Ecological Status.
We compared the chemical risk to the ecological status using sites from the French National Monitoring Program, because this program measured the highest number of ARCs and had the highest match of chemical and ecological data (SI Appendix, Table S7). The ecological data for 2007–2010 were extracted from the French National Network (Réseau de Contrôle de Surveillance) performed by 22 regional environmental agencies (for details, ref. 32). The ecological status classification (high, good, moderate, poor, and bad) was based on biotic indices, namely (i) the multimetric IPR+ (33) for fish, (ii) the I2M2 (32) for invertebrates, and (iii) the IBD (35) for diatoms. To avoid pseudoreplication for sites with multiple years of matching chemical and ecological data, the year with the highest chemical risk was selected and matched with the lower ecological status of the corresponding or following year. The rationale was that the sampling dates were unknown and the ecological data for the same year might have predated the chemical data that drove the chemical risk classification. Furthermore, we selected the ecological data corresponding to small (>90% of the sites between 5 and 15 m width) and lowland (<200 m altitude) streams, to minimize confounding effects from other stressors (e.g., the number of stressors increases with stream size; ref. 31) or from different ecoregions (e.g., alpine streams). Based on the CR thresholds and the chemical concentration, sites with ecological status were divided into three classes: (i) sites with chemical concentrations exceeding ART, which were the sites acutely affected by chemicals; (ii) sites with chemical concentrations exceeding CRT, but not ART, which were the sites chronically affected by chemicals; and (iii) sites with chemical concentrations lower than CRT, which were the sites with no or negligible risk from chemicals. Finally, the frequency of sites with high or good ecological status was calculated per class.
All data analyses, statistical computations, and graphics were generated with the open source software R (44). The R scripts and data are made available to enable reproducibility of our analysis.
Supplementary Material
Acknowledgments
We thank R. Schulz, M. Bundschuh, A. Lorke, V. Dietrich-Bischoff, C. Hug, C. Vogs, S. Steiner, and two anonymous reviewers for comments that improved this manuscript and E. Szöcs for checking the computer code. This work was funded by Electricité de France with financial support from the French National Research Agency (Grant ANR-07-ECOT-013). P.C.v.d.O. was supported through a Deutsche Forschungsgemeinschaft postdoctoral fellowship (Grant PAK 406/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.L.S. is a guest editor invited by the Editorial Board.
Data deposition: Datasets and R code are deposited at www.uni-koblenz-landau.de/campus-landau/faculty7/environmental-sciences/landscape-ecology/publications/Malaj/at_download/file.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321082111/-/DCSupplemental.
References
- 1.Vörösmarty CJ, et al. Global threats to human water security and river biodiversity. Nature. 2010;467(7315):555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 3.Stendera S, et al. Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: A review. Hydrobiologia. 2012;696(1):1–28. [Google Scholar]
- 4.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzenbach RP, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313(5790):1072–1077. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 6.Belden JB, Gilliom RJ, Martin JD, Lydy MJ. Relative toxicity and occurrence patterns of pesticide mixtures in streams draining agricultural watersheds dominated by corn and soybean production. Integr Environ Assess Manag. 2007;3(1):90–100. [PubMed] [Google Scholar]
- 7.Schäfer RB, von der Ohe PC, Kühne R, Schüürmann G, Liess M. Occurrence and toxicity of 331 organic pollutants in large rivers of north Germany over a decade (1994 to 2004) Environ Sci Technol. 2011;45(14):6167–6174. doi: 10.1021/es2013006. [DOI] [PubMed] [Google Scholar]
- 8.de Zwart D, Dyer SD, Posthuma L, Hawkins CP. Predictive models attribute effects on fish assemblages to toxicity and habitat alteration. Ecol Appl. 2006;16(4):1295–1310. doi: 10.1890/1051-0761(2006)016[1295:pmaeof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Strempel S, Scheringer M, Ng CA, Hungerbühler K. Screening for PBT chemicals among the “existing” and “new” chemicals of the EU. Environ Sci Technol. 2012;46(11):5680–5687. doi: 10.1021/es3002713. [DOI] [PubMed] [Google Scholar]
- 10.Schüürmann G, Ebert R-U, Kühne R. Quantitative read-across for predicting the acute fish toxicity of organic compounds. Environ Sci Technol. 2011;45(10):4616–4622. doi: 10.1021/es200361r. [DOI] [PubMed] [Google Scholar]
- 11.Altenburger R, Walter H, Grote M. What contributes to the combined effect of a complex mixture? Environ Sci Technol. 2004;38(23):6353–6362. doi: 10.1021/es049528k. [DOI] [PubMed] [Google Scholar]
- 12.European Environmental Agency 2012. Waterbase - Rivers, v.12. Available at www.eea.europa.eu/data-and-maps/data/waterbase-rivers-9. Accessed August 20, 2012.
- 13.Calow P, Forbes VE. Peer reviewed: Does ecotoxicology inform ecological risk assessment? Environ Sci Technol. 2003;37(7):146–151. [Google Scholar]
- 14.Commission of the European Community 2011. Technical Guidance for Deriving Environmental Quality Standards. Guidance Document No. 27. Common Implementation Strategy for the Water Framework Directive 2000/60/EC. Available at http://ec.europa.eu/environment/water/water-dangersub/lib_pri_substances.htm. Accessed March 12, 2013.
- 15.Schäfer RB, et al. How to characterize chemical exposure to predict ecologic effects on aquatic communities? Environ Sci Technol. 2013;47(14):7996–8004. doi: 10.1021/es4014954. [DOI] [PubMed] [Google Scholar]
- 16.Pistocchi A, Loos R. A map of European emissions and concentrations of PFOS and PFOA. Environ Sci Technol. 2009;43(24):9237–9244. doi: 10.1021/es901246d. [DOI] [PubMed] [Google Scholar]
- 17.Kattwinkel M, Kühne J-V, Foit K, Liess M. Climate change, agricultural insecticide exposure, and risk for freshwater communities. Ecol Appl. 2011;21(6):2068–2081. doi: 10.1890/10-1993.1. [DOI] [PubMed] [Google Scholar]
- 18.Stark JD, Banks JE, Vargas R. How risky is risk assessment: The role that life history strategies play in susceptibility of species to stress. Proc Natl Acad Sci USA. 2004;101(3):732–736. doi: 10.1073/pnas.0304903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998;32(17):2498–2506. [Google Scholar]
- 20.Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ. 2004;328(7437):447–451. doi: 10.1136/bmj.328.7437.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schäfer RB, et al. Thresholds for the effects of pesticides on invertebrate communities and leaf breakdown in stream ecosystems. Environ Sci Technol. 2012;46(9):5134–5142. doi: 10.1021/es2039882. [DOI] [PubMed] [Google Scholar]
- 22.Phalan B, Onial M, Balmford A, Green RE. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science. 2011;333(6047):1289–1291. doi: 10.1126/science.1208742. [DOI] [PubMed] [Google Scholar]
- 23.Stehle S, et al. Pesticide risk mitigation by vegetated treatment systems: A meta-analysis. J Environ Qual. 2011;40(4):1068–1080. doi: 10.2134/jeq2010.0510. [DOI] [PubMed] [Google Scholar]
- 24.Reichenberger S, Bach M, Skitschak A, Frede HG. Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness: A review. Sci Total Environ. 2007;384(1–3):1–35. doi: 10.1016/j.scitotenv.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Hollender J, et al. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ Sci Technol. 2009;43(20):7862–7869. doi: 10.1021/es9014629. [DOI] [PubMed] [Google Scholar]
- 26.Slobodnik J, et al. Identification of river basin specific pollutants and derivation of environmental quality standards: A case study in the Slovak Republic. TrAC Trends Analyt Chem. 2012;41:133–145. [Google Scholar]
- 27.Lepom P, et al. Needs for reliable analytical methods for monitoring chemical pollutants in surface water under the European Water Framework Directive. J Chromatogr A. 2009;1216(3):302–315. doi: 10.1016/j.chroma.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Fenner K, Canonica S, Wackett LP, Elsner M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science. 2013;341(6147):752–758. doi: 10.1126/science.1236281. [DOI] [PubMed] [Google Scholar]
- 29.Stehle S, Knäbel A, Schulz R. Probabilistic risk assessment of insecticide concentrations in agricultural surface waters: A critical appraisal. Environ Monit Assess. 2013;185(8):6295–6310. doi: 10.1007/s10661-012-3026-x. [DOI] [PubMed] [Google Scholar]
- 30.Beketov MA, Kefford BJ, Schäfer RB, Liess M. Pesticides reduce regional biodiversity of stream invertebrates. Proc Natl Acad Sci USA. 2013;110(27):11039–11043. doi: 10.1073/pnas.1305618110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schinegger R, Trautwein C, Melcher A, Schmutz S. Multiple human pressures and their spatial patterns in European running waters. Water Environ J. 2012;26(2):261–273. doi: 10.1111/j.1747-6593.2011.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondy CP, Villeneuve B, Archaimbault V, Usseglio-Polatera P. A new macroinvertebrate-based multimetric index (I2M2) to evaluate ecological quality of French wadeable streams fulfilling the WFD demands: A taxonomical and trait approach. Ecol Indic. 2012;18:452–467. [Google Scholar]
- 33.Marzin A, Delaigue O, Logez M, Belliard J, Pont D. Uncertainty associated with river health assessment in a varying environment: The case of a predictive fish-based index in France. Ecol Indic. 2014;43:195–204. [Google Scholar]
- 34.Mondy CP, Usseglio-Polatera P. Using conditional tree forests and life history traits to assess specific risks of stream degradation under multiple pressure scenario. Sci Total Environ. 2013;461–462(0):750–760. doi: 10.1016/j.scitotenv.2013.05.072. [DOI] [PubMed] [Google Scholar]
- 35.Coste M, Boutry S, Tison-Rosebery J, Delmas F. Improvements of the Biological Diatom Index (BDI): Description and efficiency of the new version (BDI-2006) Ecol Indic. 2009;9(4):621–650. [Google Scholar]
- 36.Marcel R, Bouchez A, Rimet F. Influence of herbicide contamination on diversity and ecological guilds of river diatoms. Cryptogam, Algol. 2013;34(2):169–183. [Google Scholar]
- 37.Hering D, et al. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw Biol. 2006;51(9):1757–1785. [Google Scholar]
- 38.Liess M, Von Der Ohe PC. Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem. 2005;24(4):954–965. doi: 10.1897/03-652.1. [DOI] [PubMed] [Google Scholar]
- 39.Kümmerer K. Sustainable from the very beginning: Rational design of molecules by life cycle engineering as an important approach for green pharmacy and green chemistry. Green Chem. 2007;9(8):899–907. [Google Scholar]
- 40.Brack W, et al. Toward a holistic and risk-based management of European river basins. Integr Environ Assess Manag. 2009;5(1):5–10. doi: 10.1897/IEAM_2008-024.1. [DOI] [PubMed] [Google Scholar]
- 41.Commission of the European Community 2000. Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy. Off J Eur Union L 327:1–73.
- 42. Commission of the European Community (2006) Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006, concerning the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH). Off J Eur Union L 396:1–849.
- 43.McLeod AI. 2011. Kendall: Kendall rank correlation and Mann–Kendall trend test. R package vs. 2.2. Available at http://CRAN.R-project.org/package=Kendall. Accessed May 10, 2013.
- 44.R Core Team 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at www.R-project.org/. Accessed May 10, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.