Significance
We investigate selective agents acting on serotiny, a polygenic trait with high heritability and well-documented effects on community and ecosystem structure and function. We show that the frequency of serotiny in lodgepole pine across Yellowstone National Park, and likely over large portions of the Rocky Mountains, represents the balance of selection by two agents: fire and a seed predator, the American red squirrel. Thus, ecosystem structure and function in these ecosystems likely varies as a result of spatial variation in these two selective agents.
Keywords: Greater Yellowstone Ecosystem, geographic selection mosaics, genes to ecosystems
Abstract
Recent work has demonstrated that evolutionary processes shape ecological dynamics on relatively short timescales (eco-evolutionary dynamics), but demonstrating these effects at large spatial scales in natural landscapes has proven difficult. We used empirical studies and modeling to investigate how selective pressures from fire and predispersal seed predation affect the evolution of serotiny, an ecologically important trait. Serotiny is a highly heritable key reproductive trait in Rocky Mountain lodgepole pine (Pinus contorta subsp. latifolia), a conifer that dominates millions of hectares in western North America. In these forests, the frequency of serotiny determines postfire seedling density with corresponding community- and ecosystem-level effects. We found that serotinous individuals have a selective advantage at high fire frequencies and low predation pressure; however, very high seed predation shifted the selective advantage to nonserotinous individuals even at high fire frequencies. Simulation modeling suggests that spatial variation in the frequency of serotiny results from heterogeneity in these two selective agents. These results, combined with previous findings showing a negative association between the density of seed predators and the frequency of serotiny at both landscape and continental scales, demonstrate that contemporary patterns in serotiny reflect an evolutionary response to conflicting selection pressures from fire and seed predation. Thus, we show that variation in the frequency of a heritable polygenic trait depends on spatial variation in two dominant selective agents, and, importantly, the effects of the local trait variation propagate with profound consequences to the structure and function of communities and ecosystems across a large landscape.
Recent work has attempted to unify processes acting across spatiotemporal scales by connecting smaller-scale (e.g., individuals, patches) ecological processes to large-scale (e.g., ecosystems, landscapes) patterns via evolutionary mechanisms (1, 2). When heritable traits in foundation species have important community and ecosystem effects, ecological drivers that exert natural selection on these traits can propagate to large-scale patterns in community and ecosystem function, resulting in landscape heterogeneity driven by small-scale ecological variation (1, 3). Although there is a clear conceptual link between selection acting on individuals and landscape-scale patterns, making mechanistic connections across these very different scales has proven challenging (refs. 4 and 5; but see ref. 6). Forested ecosystems in particular are difficult to study, because long generation times prevent direct observations of any response to selection. Nevertheless, attempts to elucidate the connections between individual-level genetic variation and ecosystem and landscape structure are imperative, especially given the unprecedented pace of global change.
Here, we integrate empirical studies and simulation modeling to show that landscape structure in Rocky Mountain lodgepole pine (Pinus contorta subsp. latifolia) forests results from the combination of opposing selective pressures from fire and a seed predator. We present empirical data relating reproductive success of individual trees to variation in selection exerted by a seed predator, as well as both empirical data and process-based simulations linking local selective pressure from fire and seed predation to landscape-scale variability in phenotype frequencies. We focus on serotiny, which is the retention of seeds in a canopy seed bank until high temperatures (typically stand-replacing fire) triggers seed release (7). Serotiny is an adaptive trait that increases regeneration following stand-replacing fires, and it is common among woody plants in fire-prone ecosystems (7). Lodgepole pine dominates millions of hectares in western North America, and serotiny in lodgepole pine is highly heritable (8) and has far-reaching ecological effects due to a strong correlation between prefire frequency of serotiny and postfire seedling density at local (i.e., patch) scales (9–11). The community- and ecosystem-level effects of variable seedling density are considerable in this system and include competitive exclusion of other trees (12), succession (10), and annual net primary production (13).
Most studies of the evolution of serotiny have focused on the relationship between fire regime and serotiny (7, 14), and both empirical and theoretical work suggest that serotiny is most common where stand-replacing fires occur predictably within the lifetime of the species (14–16). It follows that much geographical variation in serotiny is due to variation in fire regimes (17–19). Postdispersal seed predation may be another mechanism favoring the evolution of serotiny if delayed and synchronous seed release limits the abundance of postdispersal predators (7, 20). However, escape from postdispersal predation may come at the expense of extreme losses to predispersal predators that can easily access the canopy seed bank (21, 22), potentially exerting selection favoring increased allocation to seed defense (21, 23–25).
Previously, we demonstrated that the frequency of serotiny in lodgepole pine in the Greater Yellowstone Ecosystem (GYE) is negatively correlated with the abundance of a predispersal seed predator, the American red squirrel Tamiasciurus hudsonicus (26). In lodgepole pine forests, red squirrels are territorial central-place seed predators (27). Squirrels rely on large caches of lodgepole pine cones to survive the winter, and because entire cones are removed and stored intact, seed dispersal as a result of squirrel foraging is negligible (28). Previous work has shown that energy intake rates on nonserotinous cones are slightly higher than on serotinous cones, implying that preferential feeding on nonserotinous cones is a more profitable strategy (21). However, red squirrels cache nonserotinous cones almost exclusively during several weeks in the fall between seed maturation and when cones open and seeds are dispersed. Serotinous cones, however, are available year-round. This increased availability may result in greater numbers of serotinous cones being removed by squirrels and driving selection against serotiny. However, we lacked data linking this mechanism to the correlations between the frequency of serotiny and squirrel density observed in our field studies (26).
In the present study, we expand our previous work to investigate how hypothesized evolutionary mechanisms (stand-replacing fire and predispersal seed predation) drive landscape-scale variation in the frequency of serotiny in lodgepole pine in the GYE. We used natural variation in the abundance of seed predators to test the hypothesis that predispersal seed predation exerts selection against serotiny and to quantify predation rates. We then developed a stand-level process-based model to explore how the fitness of serotinous and nonserotinous trees varied in relation to variation in both fire frequency and seed predation. The use of simulation modeling allowed us to address evolutionary questions despite the long life span of lodgepole pine, and allowed us to directly compare the response to selection from processes operating on vastly different spatial and temporal scales.
Results
Selection from Seed Predation.
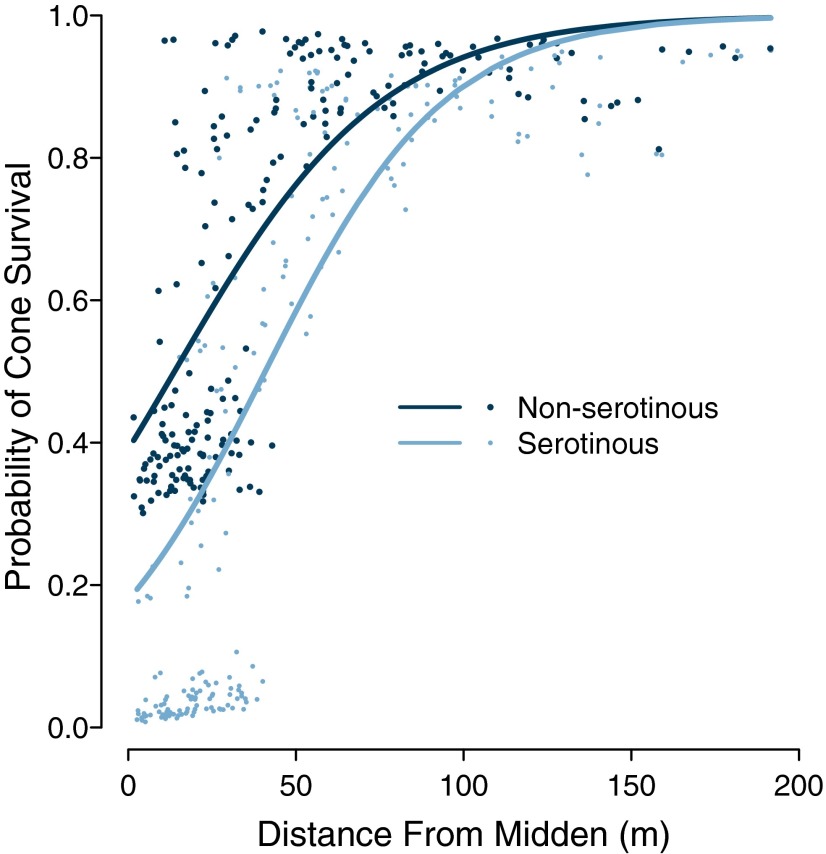
We measured cone removal rates as an assay of seed losses due to predispersal predation for 216 serotinous and 207 nonserotinous trees at five sites. To address local (i.e., per-tree) squirrel density, we used the distance to the nearest squirrel territory center (identifiable by the presence of a large midden of chewed and cached cones) as a proxy for density. Because red squirrels are strongly territorial central-place foragers (27), the average distance to a midden decreases with increasing squirrel density (see Materials and Methods for additional details). We then used a generalized linear mixed model (GLMM) to evaluate cone survival in relation to cone type (first-year serotinous, >1-y-old serotinous, or nonserotinous) and distance to the nearest midden. To avoid confounding selection for cone type with selection based on tree size or age, we included diameter at breast height (DBH) as a covariate. Mean survival was lowest for first-year serotinous cones (0.81), followed by nonserotinous (0.87) and >1-y-old serotinous (0.97) cones. Survival increased with increasing distance from the nearest midden, and the slopes varied with cone type (Fig. 1). The slope of the relationship for serotinous cones was significantly greater than the slope for nonserotinous cones (P < 0.05), indicating that the difference in predation rates between serotinous and nonserotinous cones increased with increasing squirrel density (see Table S1 for full model formulation and parameter estimates). Furthermore, at <50 m from the midden [where most seed predation occurs (27)], when accounting for all predation (i.e., from both first-year and older cones; see Materials and Methods), serotinous trees consistently experienced greater losses from predation than nonserotinous trees, and the difference increased with increasing squirrel density (Fig. 1).
Fig. 1.
Cone survival decreased more rapidly for serotinous (n = 216) than for nonserotinous (n = 207) trees with decreasing distance from the nearest midden (i.e., increasing squirrel density). Survival probabilities for serotinous cones include the effects of multiple years of predation. The fitted lines are predictions from a binomial generalized linear model.
Simulation Results.
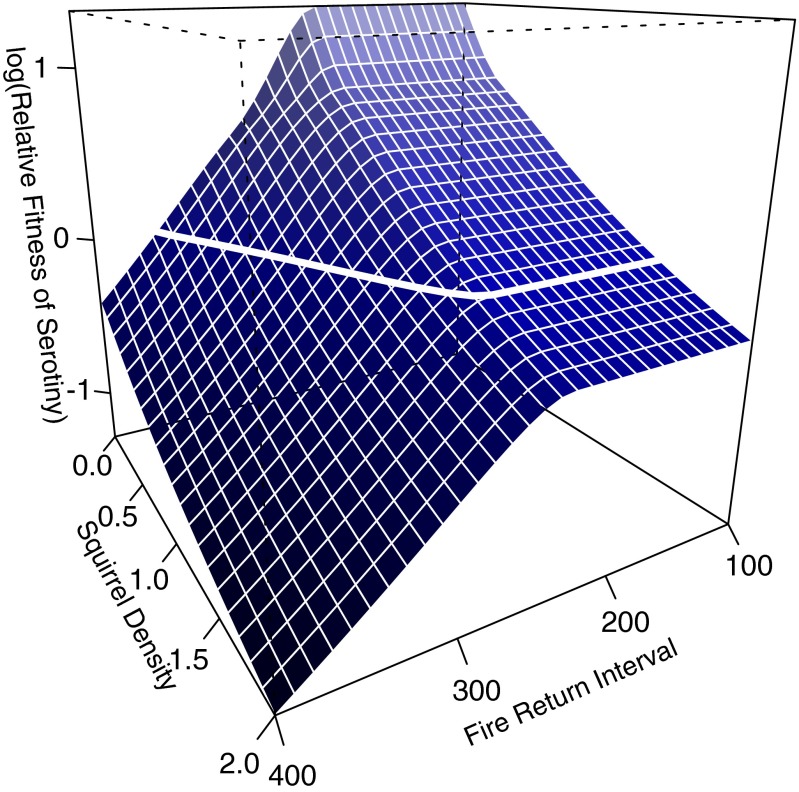
We developed a simulation model, based on a logistic population growth model, that integrated selection from both stand-replacing fire and seed predation by squirrels. Fires were implemented as either deterministic, recurring events or as a stochastic process, with return intervals varying within a realistic range for the GYE [100–400 y (29)]. Squirrel densities ranged from 0 to 1.5 individuals per ha (26) and were assumed to be constant within individual simulation runs (see Discussion for information about this assumption) (28, 30–32). In deterministic simulations, serotinous individuals were favored when squirrels were absent and the fire return interval was <350 y. Increasing selection from seed predation decreased the relative fitness of the serotinous genotype (defined here as the mean population fitness of serotinous individuals/the mean population fitness of nonserotinous individuals) and resulted in higher fire frequencies being required before serotiny was favored (i.e., relative fitness, ≥1; Fig. 2).
Fig. 2.
Simulation results showing how the relative fitness of the serotinous genotype varied with fire return interval (years) and squirrel density (individuals per hectare). Serotiny was favored (above the white line; log relative fitness, >0) with short fire return intervals and low squirrel densities, whereas nonserotinous individuals were increasingly favored even at high fire frequencies as squirrel density increased.
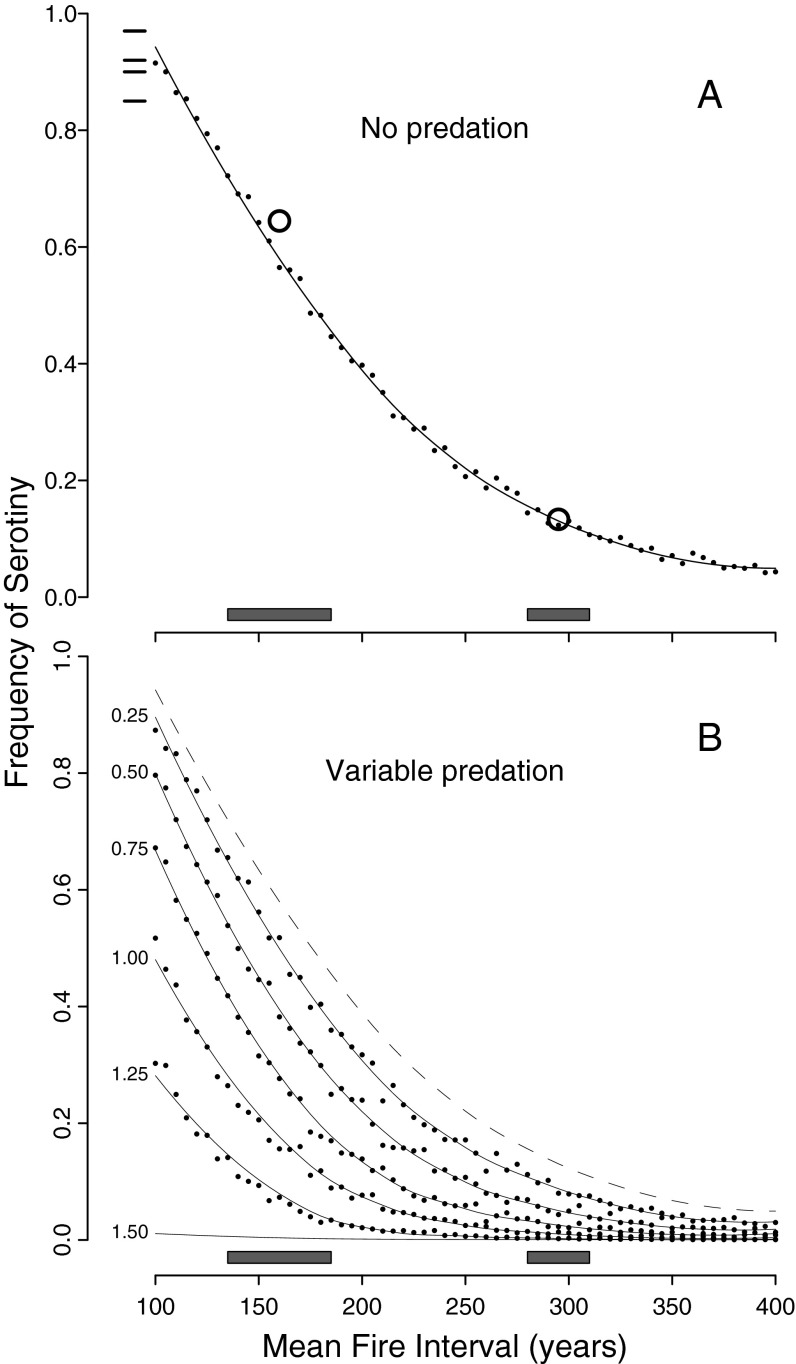
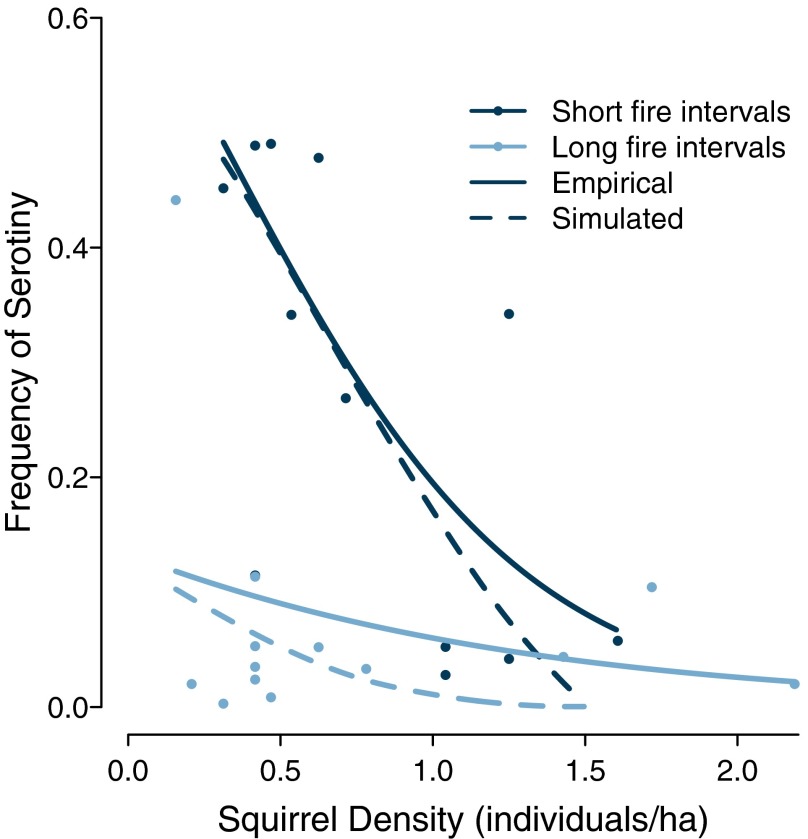
In stochastic simulations with no seed predation, the frequency of serotiny declined monotonically with increasing fire return interval (Fig. 3A). We observed similar patterns after incorporating seed predation, and the predicted frequency of serotiny at a given fire return interval decreased with increasing squirrel density (Fig. 3B). At squirrel densities ≥1.5 individuals per ha, the model predicted no serotiny regardless of the fire regime. Model predictions were nearly identical to landscape-scale patterns we previously reported (26); we observed a steep negative correlation between squirrel density and serotiny at high fire frequencies, and little serotiny regardless of squirrel density at low fire frequencies (Fig. 4).
Fig. 3.
Simulations predicted a decline in the frequency of serotiny with increasing fire return intervals in the absence of squirrels (A), and a decrease in the frequency of serotiny with increasing squirrel density (B). Points show the mean of 1,000 model runs at a given parameter combination, and the fitted lines are predictions from LOESS models. The gray bars near the x axis show the ranges of fire intervals for low (135–180 y) and high (280–310 y) elevations in the GYE (29). In A, the open circles are the frequencies of serotiny at these elevations in the absence of squirrels predicted from a regression on empirical data (Fig. 4; ref. 26), showing very close correspondence between empirical results and simulation predictions. The small horizontal lines in the Upper Left of A show frequencies of serotiny in isolated mountain ranges that lack squirrels (39). Fire return intervals for these ranges are likely <150 y. The solid lines in B show predictions at different squirrel densities (indicated by small numbers at the left side of the plot); the dashed line shows predictions in the absence of squirrels.
Fig. 4.
Simulation predictions (dashed lines) were qualitatively very similar to regression predictions for empirical data (points and solid lines) from ref. 26. At sites with short mean fire intervals (135–185 y; dark colors), serotiny is variable, and both simulated and empirical data show a negative correlation between the frequency of serotiny and squirrel density. At long fire intervals (280–310 y; light colors), serotiny is uncommon and the relationship between squirrels and the frequency of serotiny is weak. The figure is modified from ref. 26 and used with permission.
Discussion
Our results demonstrate a clear connection between two selective agents (fire and seed predators) and both the relative fitnesses of serotinous and nonserotinous individuals (Figs. 1 and 2) and their frequencies at the stand level (Fig. 4) in lodgepole pine. Combined with previous work showing that serotiny is polygenic and highly heritable (8), that stand-level serotiny covaries with both fire frequency and squirrel density at landscape scales (26), and that serotiny has significant effects on community and ecosystem structure and function (9–11), we argue that the effects of selection on serotiny propagate across multiple scales, driven by forcing mechanisms that are operating at relatively large (i.e., hundreds of hectares for fires) and small (∼1–20 ha for squirrels) scales to influence landscape structure (Fig. S1). Such cross-scale dynamics are well supported by theory but are difficult to demonstrate empirically (5, 33, 34). It is unclear how common such dynamics are in natural systems; however, our results suggest that large-scale “noise” in ecological systems (e.g., variation in the frequency of serotiny) may be attributable to complex interactions at multiple spatial scales, and that evolutionary change in response to local variation in selective agents can be an important driver of these spatial patterns.
Much recent work has focused on investigating how evolutionary processes shape ecological dynamics (1, 5, 35), and on how resulting ecosystem-level dynamics may produce feedbacks and affect the selective environment of foundation species (36). However, demonstrating the complete chain of eco-evolutionary relationships in natural ecosystems has proven challenging (5, 37). In lodgepole pine, serotiny has important ramifications from individual to ecosystem levels (Fig. S1). At the individual level, serotiny determines when seeds are dispersed and the exposure to predispersal seed predators, and thus is tightly associated with fitness. Serotiny is also highly heritable; although exact estimates of heritability are unavailable for this species, a recent association mapping study demonstrated that 11 genomic regions accounted for ∼50% of the variation in serotiny in lodgepole pine (8).
The combination of high heritability and tight coupling to fitness suggests that serotiny is likely to be a target of selection and to respond rapidly to changes in selection with resulting shifts in the frequency of serotiny in the population. At the community and ecosystem levels, the degree of prefire serotiny affects postfire seedling density. Following widespread fires in the GYE, lodgepole pine sapling density varied over several orders of magnitude (0–500,000+ seedlings per ha), and this variation was most strongly explained by the prefire frequency of serotiny (9–11, 13). Unsurprisingly, this extreme variation in sapling density had significant community- and ecosystem-level consequences as well (9, 11–13). Sparse recovery may allow for the colonization of other ecosystem types that would otherwise be outcompeted, increasing landscape heterogeneity (12). Recovery density may also affect successional processes (10), and postfire annual net primary productivity and total leaf area are strongly associated with lodgepole pine sapling density (13). Thus, in lodgepole pine forests, obtaining a clear understanding of ecosystem dynamics at scales ranging from individuals to landscapes requires analysis of the evolutionary mechanisms driving variation in serotiny.
Our model allows for the manipulation of fire frequency and observation of subsequent stand-level evolutionary responses over long timescales; observations of these dynamics would be difficult or impossible in the field. Given the well-known effects of fire on the evolution of serotiny (7, 14–17), such observations are required to understand the evolutionary effects of seed predation in context, and to demonstrate that selection observed in the field (Fig. 1) results in evolution (Figs. 2 and 3) and drives spatial patterns in serotiny [Fig. 4 (26)]. The model makes the simplifying assumption that squirrel densities are constant in time for a given scenario. Although this is certainly unrealistic, the available data demonstrate that squirrel populations are stable over short to medium timescales (i.e., years to decades). In part, this is driven by stable cone production of lodgepole pine, which is the primary food source for red squirrels in this system (21, 30–32). The strictly territorial habit of red squirrels also promotes population stability, as vacant territories are rapidly reoccupied and overwinter survival of squirrels lacking territories is extremely low (28, 31). Therefore, fluctuations in density are likely to be small; adding such fluctuations to the model would increase the variance, but have little impact on the overall conclusions. However, we lack information on fluctuations over longer timescales (potentially in response to changes in postfire recovery as the stand evolves), and the density of squirrel territories may be more variable in ecosystems with less stable cone production (28). If changes in the frequency of serotiny over time lead to changes to stand structure that ultimately affect squirrel population densities, then there is the potential for feedbacks in the system that could either reinforce or dampen spatial patterns in selection on serotiny. Alternatively, stand structure may be more influenced by factors such as bedrock (38), which could lead to similar stand structure from one tree generation to the next, resulting in consistent spatial variation in both squirrel densities and selection.
Evolutionary Mechanisms.
Previous studies have focused on the role of fire in driving the evolution of serotiny (7, 17–19), although few have addressed seed predators as alternative selective agents (but see refs. 7, 19, and 39), and ours is the first (to our knowledge) to demonstrate strong selection from seed predation driving local-scale (i.e., stand-level) variation in serotiny. In opposition to these processes are potentially high rates of gene flow in lodgepole pine that may have a homogenizing effect on spatial variation in serotiny (40); however, strong selection can drive local adaptation despite the presence of gene flow (41–45), and even at small spatial scales, local adaptation is possible in the presence of gene flow (46). It is likely that gene flow could explain some of the variation in serotiny in this system. However, our simulations predicted strong selection and evolutionary responses to both fire and seed predation in the absence of gene flow, resulting in less serotiny when fires were uncommon or seed predation was more intense (Figs. 2 and 3). Furthermore, the predictions of the model were very similar to landscape-scale patterns we observed in the GYE (Fig. 4), suggesting that selection is the dominant force affecting the local frequency of serotiny in this system. It is likely that a spatially explicit version of the model that directly simulated gene flow would demonstrate weaker evolutionary responses overall, with more stable coexistence of the two types due to the continuous dispersal of maladapted individuals into a given region. In contrast, our spatially implicit model showed a tendency toward fixation of either the serotinous or nonserotinous type when selection was strong, a situation that is rarely observed in the field (9, 26).
Muir and Lotan (47) found that the frequency of serotiny in lodgepole pine was most strongly related to the type of disturbance that initiated a stand, with fire-initiated stands being much more serotinous than other stands. This suggests the potential for very rapid change in the frequency of serotiny (i.e., a single fire interval), and that high frequencies of stand-replacing fire should result in high frequencies of serotiny (because most stands will have initiated due to a stand-replacing fire). Our simulation results generally support this interpretation. In very old stands with dynamics dominated by density-independent mortality, the frequency of serotiny was reduced compared with postfire stands. However, our simulations also suggest that the legacy of long fire return intervals persists even if stands were initiated by fire; i.e., fire-initiated stands that experienced short mean fire return intervals had higher serotiny than fire-initiated stands that experienced long fire return intervals (Fig. 3). Furthermore, the effects of seed predation were completely dominant at very high squirrel densities (>1.5 individuals per ha). Many trees show local adaptation and rapid (per-generation) responses to selection (45, 48); our results in lodgepole pine are consistent with these observations. Lodgepole pine forests have probably been present in the GYE for ∼10,000 y (49). Our simulations, which were run for 2,000 y, suggest that this is sufficient time for consistent and sometimes strong divergent selection to cause local adaptation and spatial structure in serotiny across the landscape.
Implications for Global Change.
The rapid pace of global change is likely to have widespread evolutionary effects due to changing strength and direction of biotic and abiotic interactions (50). In addition to relatively predictable dynamics (e.g., selection favoring greater dispersal or greater tolerance to novel local conditions), these changes may be complex and nonlinear, in part due to interactions between ecological and evolutionary processes (2, 51). For example, in lodgepole pine forests, increasing fire frequency due to forecasted increases in the incidence of fire weather (52) may result in serotiny being increasingly favored regardless of squirrel density, or may result in complex changes due to nonlinear responses to repeated burning (53). Mechanistic models incorporating eco-evolutionary dynamics will be increasingly important in predicting these responses, especially when global change results in combinations of selective agents (e.g., fire frequency and squirrel density in our system) that are outside the scope of current knowledge.
Conclusions
Our results demonstrate that squirrels select against serotiny and that the strength of selection increases with increasing squirrel density, supporting previous results showing negative associations between red squirrel density and the frequency of serotiny in lodgepole pine at landscape and regional scales (26, 39). We have also shown that a simple population model incorporating selection from fire and seed predation reproduces empirical observations (Figs. 3 and 4), suggesting that stand-level frequencies of serotiny in lodgepole pine represent the outcome of selection from fire (favoring serotinous trees) and seed predation (favoring nonserotinous trees). Although gene flow is extensive in lodgepole pine (40), strong selection can overwhelm gene flow and cause local divergence (41–45). In lodgepole pine forests in the GYE, we found little evidence that gene flow prevents local adaptation; rather, our results suggest that divergent selection is the dominant factor affecting spatial variation in the frequency of serotiny. These findings have implications for ecosystem processes due to the influence of serotiny on stand-level responses to fire (9, 10), as well as for global change in a variety of ecosystems, because changes in selective regimes may produce unexpected effects to ecosystem structure and function.
Materials and Methods
Study Area.
We performed all field work in mature (i.e., not burned within 100 y) lodgepole pine forests within the GYE, Wyoming, United States. We selected five sites that were known to vary from ∼0.5 to 1.5 squirrels per ha (26). The sites ranged from 25.6 to 45.4 ha and were dominated (>90%) by lodgepole pine. One site was sampled in July 2010 and July 2011, and four sites were sampled in August 2011 and July to August 2012.
Cone Survival.
We estimated survival probabilities of serotinous and nonserotinous cones by repeated observation with a one-year (±1 mo) intervening interval. During the initial visit, we photographed the tops of randomly selected, individually marked trees (n = 216 serotinous and 207 nonserotinous trees). We also measured the DBH of each tree. During subsequent visits, we recreated the initial photograph, using the same position, camera angle, and focal length. We compared these photographs in the laboratory and tabulated the number of cones that had disappeared during the 1-y interval [i.e., individual cones that were present in the first photograph but absent in the second (Dataset S1)]. A cone was counted as surviving if it could be clearly identified (based on position relative to other cones, branch features, etc.) in both photographs. Cones were counted as absent if the cone’s location in the second photograph could be clearly seen (i.e., was identifiable based on nearby cones and branches and not obscured). Cones that were not identifiable in this manner were treated as missing data and ignored for analysis. The rate of disappearance between intervals is an appropriate measure of the predation rate from red squirrels because no other organism removes cones from lodgepole pine (due to extreme defense requiring specialized predators), and cones do not generally fall from branches (even after they have opened) (21, 25). Because survival of serotinous cones is likely to vary as the cone ages, we analyzed survival as a function of the degree of weathering, with separate estimates for green (first-year), brown (likely 1- to 5-y-old), and gray (>5-y-old) cones. Exploratory analysis revealed that survival of gray cones was nearly 100% in all circumstances, so we omitted these cones from our analyses.
To address how squirrel density affected cone survival, we used the distance of each tree to the center of the nearest squirrel territory as a proxy for density. Territory centers were located by an exhaustive ground search by two observers and were identified by the presence of a large, active midden containing burrows and discarded cone remnants (21). Because red squirrels are strictly territorial, the average distance to a midden decreases with increasing density; for four sites where mean squirrel density for the entire site was known (from ref. 26), squirrel density and the distance from trees to the nearest midden were strongly negatively correlated (r = −0.88).
We used package lme4 in R to develop GLMMs to analyze survival probability as a function of distance to the midden (54). All models used the number of surviving cones and the number of cones initially observed as the response variable and assumed binomial errors and a logit link, and all included site as a random variable. Fixed effects consisted of cone type, distance to the midden, and an interaction between the two. For the relationship between distance and survival, we developed three models: fixed slopes and random intercepts, random slopes and intercepts, and no effect of distance. We also tested models including or excluding DBH as a covariate, for a total of six models. We compared all models using the Bayesian information criterion (BIC). The fully parameterized model minimized the BIC. Furthermore, ΔBIC was ≥39 for all other models, indicating little uncertainty in which was the best model (55); therefore, we selected the full model alone for interpretation (see Table S1 for parameter estimates). Because serotinous cones are exposed to predation for multiple years, the overall cone survival is the product of survival probabilities over the years in which cones are exposed to predation. Thus, in addition to the GLMM described above, we calculated for each serotinous tree the probability that a cone survived long enough to enter the “weathered” category (at which point survival was nearly 100%), and then compared this to the nonserotinous survival rate as a function of distance using a logistic regression (Fig. 1).
Simulation Model.
We developed a stand-level simulation model to provide an integrated estimate of the relative fitness of both tree types and to address the potential for the observed predation rates to drive variation in the frequency of serotiny. Our general approach was to use a density-dependent population model (56) to simulate stand development, starting with postfire initiation and proceeding through self-thinning (or infilling, for sparse stands) and stand maturity. The response of lodgepole pine to fire is well known; therefore, we used the literature to parameterize this portion of the model. Fires were initiated at regular intervals (for deterministic scenarios) or probabilistically (for stochastic scenarios), and we varied either the fire return interval or the fire probability to assess the effect of fire frequency on serotiny in the simulated stand. For predation, we used the results presented in this paper to reduce the reproductive success of trees as a function of serotinous/nonserotinous state, and varied the intensity of predation based on the ranges observed in the field. See SI Text and Table S2 for complete details on model specification and parameterization. Sensitivity analysis was conducted by examining how the relationship between serotiny and both fire and seed predaction was affected by perturbations to key model parameters (SI Text, Figs. S2–S7).
Supplementary Material
Acknowledgments
Liz Mandeville, Theresa Laverty, Colin Beech, and Tracey Johnson provided assistance in the field. We thank Andrew Hendry, two anonymous reviewers, and the editor for providing comments on previous versions of the manuscript. We are grateful for assistance received from the US National Park Service and the Yellowstone National Park Research Office. Funding for this study was provided by Program in Ecology, Department of Zoology and Physiology, the Robert B. Berry Chair in Ecology at University of Wyoming, University of Wyoming–National Park Service Research Center, and American Society of Mammalogists.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400944111/-/DCSupplemental.
References
- 1.Whitham TG, et al. A framework for community and ecosystem genetics: From genes to ecosystems. Nat Rev Genet. 2006;7(7):510–523. doi: 10.1038/nrg1877. [DOI] [PubMed] [Google Scholar]
- 2.Thuiller W, et al. A road map for integrating eco-evolutionary processes into biodiversity models. Ecol Lett. 2013;16(Suppl 1):94–105. doi: 10.1111/ele.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wymore AS, et al. Genes to ecosystems: Exploring the frontiers of ecology with one of the smallest biological units. New Phytol. 2011;191(1):19–36. doi: 10.1111/j.1469-8137.2011.03730.x. [DOI] [PubMed] [Google Scholar]
- 4.Hersch-Green EI, Turley NE, Johnson MTJ. Community genetics: What have we accomplished and where should we be going? Philos Trans R Soc Lond B Biol Sci. 2011;366(1569):1453–1460. doi: 10.1098/rstb.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoener TW. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331(6016):426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- 6.Farkas TE, Mononen T, Comeault AA, Hanski I, Nosil P. Evolution of camouflage drives rapid ecological change in an insect community. Curr Biol. 2013;23(19):1835–1843. doi: 10.1016/j.cub.2013.07.067. [DOI] [PubMed] [Google Scholar]
- 7.Lamont BB, Le Maitre DC, Cowling RM, Enright NJ. Canopy seed storage in woody plants. Bot Rev. 1991;57(4):277–317. [Google Scholar]
- 8.Parchman TL, et al. Genome-wide association genetics of an adaptive trait in lodgepole pine. Mol Ecol. 2012;21(12):2991–3005. doi: 10.1111/j.1365-294X.2012.05513.x. [DOI] [PubMed] [Google Scholar]
- 9.Tinker DB, Romme WH, Hargrove WW, Gardner RH, Turner MG. Landscape-scale heterogeneity in lodgepole pine serotiny. Can J For Res. 1994;24(5):897–903. [Google Scholar]
- 10.Turner MG, Romme WH, Gardner RH, Hargrove WW. Effects of fire size and pattern on early succession in Yellowstone National Park. Ecol Monogr. 1997;67(4):411–433. [Google Scholar]
- 11.Turner MG, Romme WH, Tinker DB. Surprises and lessons from the 1988 Yellowstone fires. Front Ecol Environ. 2003;1(7):351–358. [Google Scholar]
- 12.Turner MG, Romme WH, Reed RA, Tuskan GA. Post-fire aspen seedling recruitment across the Yellowstone (USA) landscape. Landscape Ecol. 2003;18(2):127–140. [Google Scholar]
- 13.Turner MG, Tinker DB, Romme WH, Kashian DM, Litton CM. Landscape patterns of sapling density, leaf area, and aboveground net primary production in postfire lodgepole pine forests, Yellowstone National Park (USA) Ecosystems (N Y) 2004;7(7):751–775. [Google Scholar]
- 14.Keeley JE, Zedler PH. In: Ecology and Biogeography of Pinus. Richardson DM, editor. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 15.Enright NJ, Marsula R, Lamont BB, Wissel C. The ecological significance of canopy seed storage in fire-prone environments: A model for non-sprouting shrubs. J Ecol. 1998;86(6):946–959. [Google Scholar]
- 16.Lamont BB, Enright NJ. Adaptive advantages of aerial seed banks. Plant Species Biol. 2000;15(2):157–166. [Google Scholar]
- 17.Gauthier S, Bergeron Y, Simon JP. Effects of fire regime on the serotiny level of jack pine. J Ecol. 1996;84(4):539–548. [Google Scholar]
- 18.Radeloff VC, Mladenoff DJ, Guries RP, Boyce MS. Spatial patterns of cone serotiny in Pinus banksiana in relation to fire disturbance. For Ecol Manage. 2004;189(1–3):133–141. [Google Scholar]
- 19.Clarke PJ, Knox KEJ, Butler D. Fire, soil fertility and delayed seed release: A community analysis of the degree of serotiny. Evol Ecol. 2013;27(2):429–443. [Google Scholar]
- 20.Groom PK, Lamont BB. Fruit-seed relations in Hakea: Serotinous species invest more dry matter in predispersal seed protection. Aust J Ecol. 1997;22(3):352–355. [Google Scholar]
- 21.Smith CC. The coevolution of pine squirrels (Tamiasciurus) and conifers. Ecol Monogr. 1970;40(3):349–371. [Google Scholar]
- 22.Steele MA, Wauters LA, Larsen KW. In: Seed Fate: Predation, Dispersal and Seedling Establishment. Forget PM, Lambert JE, Hulme PE, Vander Wall SB, editors. Wallingford, UK: CABI; 2005. pp. 205–221. [Google Scholar]
- 23.Benkman CW, Parchman TL, Mezquida ET. Patterns of coevolution in the adaptive radiation of crossbills. Ann N Y Acad Sci. 2010;1206(1):1–16. doi: 10.1111/j.1749-6632.2010.05702.x. [DOI] [PubMed] [Google Scholar]
- 24.Leslie AB. Predation and protection in the macroevolutionary history of conifer cones. Proc Biol Sci. 2011;278(1720):3003–3008. doi: 10.1098/rspb.2010.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benkman CW, Smith JW, Maier M, Hansen L, Talluto MV. Consistency and variation in phenotypic selection exerted by a community of seed predators. Evolution. 2013;67(1):157–169. doi: 10.1111/j.1558-5646.2012.01736.x. [DOI] [PubMed] [Google Scholar]
- 26.Talluto MV, Benkman CW. Landscape-scale eco-evolutionary dynamics: Selection by seed predators and fire determine a major reproductive strategy. Ecology. 2013;94(6):1307–1316. doi: 10.1890/12-2058.1. [DOI] [PubMed] [Google Scholar]
- 27.Elliott PF. Foraging behavior of a central-place forager: Field tests of theoretical predictions. Am Nat. 1988;131(2):159–174. [Google Scholar]
- 28.Larsen KW, Boutin S. Movements, survival, and settlement of red squirrel (Tamiasciurus hudsonicus) offspring. Ecology. 1994;75(1):214–223. [Google Scholar]
- 29.Schoennagel T, Turner MG, Romme WH. The influence of fire interval and serotiny on postfire lodgepole pine density in Yellowstone National Park. Ecology. 2003;84(11):2967–2978. [Google Scholar]
- 30.Rusch DA, Reeder WG. Population ecology of Alberta red squirrels. Ecology. 1978;59(2):400–420. [Google Scholar]
- 31.Gurnell J. Home range, territoriality, caching behaviour and food supply of the red squirrel (Tamiasciurus hudsonicus fremonti) in a subalpine lodgepole pine forest. Anim Behav. 1984;32(4):1119–1131. [Google Scholar]
- 32.Wheatley M, Larsen KW, Boutin S. Does density reflect habitat quality for North American red squirrels during a spruce-cone failure? J Mammal. 2002;83(3):716–727. [Google Scholar]
- 33.Peters DPC, Bestelmeyer BT, Turner MG. Cross-scale interactions and changing pattern-process relationships: Consequences for system dynamics. Ecosystems. 2007;10(5):790–796. [Google Scholar]
- 34.Chave J. The problem of pattern and scale in ecology: What have we learned in 20 years? Ecol Lett. 2013;16(Suppl 1):4–16. doi: 10.1111/ele.12048. [DOI] [PubMed] [Google Scholar]
- 35.Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21(3):465–477. [Google Scholar]
- 36.Post DM, Palkovacs EP. Eco-evolutionary feedbacks in community and ecosystem ecology: Interactions between the ecological theatre and the evolutionary play. Philos Trans R Soc Lond B Biol Sci. 2009;364(1523):1629–1640. doi: 10.1098/rstb.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tack AJM, Johnson MTJ, Rosiln T. Sizing up community genetics: It’s a matter of scale. Oikos. 2012;121(4):481–488. [Google Scholar]
- 38.Hahm WJ, Riebe CS, Lukens CE, Araki S. Bedrock composition regulates mountain ecosystems and landscape evolution. Proc Natl Acad Sci USA. 2014;111(9):3338–3343. doi: 10.1073/pnas.1315667111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benkman CW, Siepielski AM. A keystone selective agent? Pine squirrels and the frequency of serotiny in lodgepole pine. Ecology. 2004;85(8):2082–2087. [Google Scholar]
- 40.Perry DA. An estimate of the effective range of pollen dispersal in lodgepole pine (Pinus contorta Dougl.) Ann Bot (Lond) 1978;42(4):1001–1002. [Google Scholar]
- 41.May RM, Endler JA, McMurtrie RE. Gene frequency clines in the presence of selection opposed by gene flow. Am Nat. 1975;109(970):659–676. doi: 10.1086/283036. [DOI] [PubMed] [Google Scholar]
- 42.Linhart YB, Grant MC. Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst. 1996;27:237–277. [Google Scholar]
- 43.Hendry AP, Day T, Taylor EB. Population mixing and the adaptive divergence of quantitative traits in discrete populations: A theoretical framework for empirical tests. Evolution. 2001;55(3):459–466. doi: 10.1554/0014-3820(2001)055[0459:pmatad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17(4):183–189. [Google Scholar]
- 45.Savolainen O, Pyhäjärvi T, Knürr T. Gene flow and local adaptation in trees. Annu Rev Ecol Evol Syst. 2007;38:595–619. [Google Scholar]
- 46.Richardson JL, Urban MC, Bolnick DI, Skelly DK. Microgeographic adaptation and the spatial scale of evolution. Trends Ecol Evol. 2014;29(3):165–176. doi: 10.1016/j.tree.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Muir PS, Lotan JE. Disturbance history and serotiny of Pinus contorta in western Montana. Ecology. 1985;66(5):1658–1668. [Google Scholar]
- 48.Petit RJ, Hampe A. Some evolutionary consequences of being a tree. Annu Rev Ecol Evol Syst. 2006;37:187–214. [Google Scholar]
- 49.Whitlock C. Postglacial vegetation and climate of grand teton and southern Yellowstone national parks. Ecol Monogr. 1993;63(2):173–198. [Google Scholar]
- 50.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 51.Barnosky AD, et al. Approaching a state shift in Earth’s biosphere. Nature. 2012;486(7401):52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- 52.Flannigan MD, Krawchuk MA, de Groot WJ, Wotton BM, Gowman LM. Implications of changing climate for global wildland fire. Int J Wildland Fire. 2009;18(5):483–507. [Google Scholar]
- 53.Brown CD, Johnstone JF. Once burned, twice shy: Repeat fires reduce seed availability and alter substrate constraints on Picea mariana regeneration. For Ecol Manage. 2012;266:34–41. [Google Scholar]
- 54.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 55.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. 2nd Ed. New York: Springer; 2002. [Google Scholar]
- 56.Ricker WE. Stock and recruitment. J Fish Res Board Can. 1954;11(5):559–623. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.