Significance
The [URE3] prion (infectious protein) is an amyloid (filamentous polymer) of Ure2p. Overproduction of Btn2p or Cur1p cure this prion, and Btn2p colocalizes with Ure2p aggregates in the curing process. We show that most [URE3] variants arising in the absence of Btn2p and Cur1p are cured by restoring the normal levels of these two proteins. The variants cured by normal levels of Btn2p and Cur1p are those with low seed number, a low number of heritable particles, consistent with seed aggregation, and sequestration as the curing mechanism. These Btn2p and Cur1p antiprion proteins are members of the Hook protein family, metazoan members of which have roles in microtubule-dependent movement of organelles and aggregates in the cell.
Keywords: Bmh1, Sis1, prion seed sequestration
Abstract
[URE3] is an amyloid prion of the Saccharomyces cerevisiae Ure2p, a regulator of nitrogen catabolism. Overproduction of Btn2p, involved in late endosome to Golgi protein transport, or its paralog Cur1p, cures [URE3]. Btn2p, in curing, is colocalized with Ure2p in a single locus, suggesting sequestration of Ure2p amyloid filaments. We find that most [URE3] variants generated in a btn2 cur1 double mutant are cured by restoring normal levels of Btn2p and Cur1p, with both proteins needed for efficient curing. The [URE3] variants cured by normal levels of Btn2p and Cur1p all have low seed number, again suggesting a seed sequestration mechanism. Hsp42 overproduction also cures [URE3], and Hsp42p aids Btn2 overproduction curing. Cur1p is needed for Hsp42 overproduction curing of [URE3], but neither Btn2p nor Cur1p is needed for overproduction curing by the other. Although hsp42Δ strains stably propagate [URE3-1], hsp26Δ destabilizes this prion. Thus, Btn2p and Cur1p are antiprion system components at their normal levels, acting with Hsp42. Btn2p is related in sequence to human Hook proteins, involved in aggresome formation and other transport activities.
The yeast prion [URE3] is a self-propagating amyloid of Ure2p (1–4). Ure2p normally functions as a soluble regulator of nitrogen catabolism (5, 6), and its conversion to the aggregated amyloid prion form produces inappropriate derepression of many genes of nitrogen catabolism, resulting in slightly slowed growth (7). Many [URE3] isolates have a severe toxic effect, producing extremely slowed growth (8). A single protein sequence can assume any of many different heritable/infectious forms with different biological properties and, one infers, different protein conformations. The parallel in-register β-sheet architecture of the [URE3] prion amyloid (9) can explain the templating properties of this prion (10) and the ability of the Ure2p amyloid to act as a gene with multiple alleles (4).
[PSI+] is similarly an amyloid prion of Sup35p, a subunit of the translation termination factor [reviewed by Liebman and Chernoff (11)], and [PIN+] is a prion of Rnq1p, a protein of unknown function (12–14).
Eukaryotic cells deal with protein aggregates in several ways, including resolubilization, degradation, sequestration, and combinations of these processes. Several organelles and systems have been recognized to play a role in these processes, including vacuoles (the yeast equivalent of the mammalian lysosomes), the ubiquitin–proteasome systems, the autophagy system, and the various chaperones.
In mammalian cells the aggresome is a centrosomal structure to which aggregates are brought in a microtubule-dependent process (15). A yeast site near the spindle pole body (the yeast centrosome) collects huntingtin/polyQ aggregates in a process that depends on microtubule function, suggesting that this is the yeast aggresome (16). Bmh1p, a 14-3-3 protein, was found associated with huntingtin in yeast aggresomes, and bmh1Δ prevented aggresome accumulation of huntingtin (16).
Btn2p and Cur1p were found to cure the [URE3] prion when overproduced (17). These proteins are paralogs, members of the Hook family, consistent with their similar effects, but Btn2 localized to a site next to the nucleus, whereas Cur1 was localized largely within the nucleus (17). During the curing process, those [URE3] cells having both Ure2p-GFP aggregates and Btn2-RFP dots show striking colocalization (17–19). Partial colocalization of Btn2-RFP with Sup35NM-GFP in a [PSI+] strain or with the huntingtin-like Q103-GFP were also observed (17), but overexpression of Btn2p or Cur1p did not cure [PSI+]. Doubly deleted btn2Δ cur1Δ strains show an elevated seed number of [URE3] and partial resistance to prion curing by dimethyl sulfoxide, overexpression of Ssa1p, overexpression of Hsp104, and other agents (17). These results suggested that Btn2p and Cur1p expressed at their normal levels affect [URE3] propagation. It was suggested that at least Btn2p collects Ure2p amyloid aggregates at the Btn2 site near the nucleus, thereby decreasing the operative seed number and destabilizing the prion (17).
Another study described two morphological sites of accumulation of nonspecific aggregates, one near the nucleus (JUNQ) and the other peripheral to the nucleus (IPOD), neither localized at the spindle pole body (20). Ubiquitinated proteins accumulated at the juxtanuclear site, whereas aggregates of overproduced “amyloidogenic” prion proteins Ure2p and Rnq1p, in strains not carrying prions, were reported to be moved to the peripheral site (20). Prion formation by Ure2p is induced 20- to 200-fold by overproduction of the prion protein (2), but still only a relatively small minority of cells become [URE3], although most cells may have aggregates of Ure2p while it is overproduced. The same is true of induction of [PSI+], a prion of Sup35p (2, 21, 22) and of [PIN+], a prion of Rnq1p (14). Thus, aggregates of overproduced Ure2p or Rnq1p in cells not carrying the corresponding prion will be largely nonprion aggregates, and there is no reason to assume that they are amyloid, even though those proteins are indeed “amyloidogenic.” The formation of both IPOD and JUNQ foci were inhibited by benomyl (20), a drug that depolymerizes microtubules.
Specht et al. (23) found that Hsp42 was necessary for aggregate accumulation at the peripheral site, but not at the juxtanuclear site. They found that Hsp42 did not affect the localization of “amyloidogenic” aggregates of Rnq1p, but again, the cells were not carrying the corresponding prion, [PIN+], so these aggregates were likely nonamyloid. Specht et al. found that although benomyl inhibited formation of both peripheral and juxtanuclear aggregate foci, a benomyl-resistant tubulin2 mutant showed the same effect. In contrast, LatrunculinA, which depolymerizes the actin cytoskeleton, inhibited accumulation of aggregates at both sites, but a mutation in actin-1 that prevents LatrunculinA action prevented this action of the drug (23). Thus, the actin cytoskeleton is invovled in IPOD and JUNQ focus formation, distinguishing both from the aggresome. Overproduction of either Hsp26 or Hsp42 were reported to cure the [PSI+] prion (24), but hsp26Δ did not affect IPOD or JUNQ aggregate focus formation (23). Hsp42p associates with Btn2p in vivo (25).
The prion-curing ability of overproduced Btn2p and Cur1p was confirmed by Malinovska et al. using an artificial prion (25). As in other studies cited above, this group overproduced the RFP-labeled prion domains of Ure2, Sup35, Rnq1, or Nrp1 in a strain carrying none of the corresponding prions. There was no significant colocalization with Btn2-GFP, from which it was inferred that Btn2p was not directly associating with prion amyloid in the curing process (25). As discussed above, only a tiny minority of cells overexpressing these prion domains form prions, although most cells show aggregates of the overproduced protein, so this inference is not justified. Indeed, Btn2p does colocalize with amyloid aggregates of Ure2p in a [URE3] strain early in the curing process (17–19). Btn2p is also partially colocalized with amyloid aggregates of Sup35p in a [PSI+] strain (17). Btn2p also colocalizes with nonamyloid aggregates of optineurin, PrP, Rnq1p, and Q103P (19). Malinovska et al. further showed that Btn2p and Cur1p each interact physically with the Hsp40 family member Sis1p, and that both Btn2p and Cur1p are drawn into the nucleus as a result of this association (25). It was suggested that overproduced Btn2p or Cur1p cure prions by sequestering the Sis1p that is needed for prion propagation (25).
Here we show that normal levels of Btn2p and Cur1p can cure most [URE3] variants despite being 20–400 times less abundant in the cell than Sis1p. [URE3] variants cured by normal levels of Btn2 and Cur1 are much lower in copy number than those not cured under this condition, suggesting that sequestration of seeds is the mechanism of prion curing. Btn2p and Cur1p are thus elements of normal prion-curing systems, suggesting that yeast does not welcome [URE3] but has systems in place to rapidly eliminate it. We find that Hsp42 is important for curing by overproduction of Btn2p or Cur1p and that overproduction of Hsp42 itself cures [URE3] in a process requiring Cur1p, but not Btn2p.
Results
Most [URE3] Variants Are Cured by Normal Levels of Btn2p and Cur1p.
Formation of the [URE3] prion inactivates the nitrogen regulation function of Ure2p, resulting in the derepression of DAL5 (among many genes). We used strains having the Ure2p-regulated DAL5 promoter linked to the ADE2 gene (4, 26), so that [URE3] cells become Ade+, but cells lacking the prion are Ade−. To determine whether normal levels of Btn2p or Cur1p can cure [URE3] variants, we induced prion formation in a btn2Δ cur1Δ strain by overproduction of the Ure2p prion domain (27), selecting Ade+ clones. As a simple screening method, we restored Btn2p and Cur1p by mating isolates with the BTN2 CUR1 [ure-o] strain 4592. We found that 38 of 48 Ade+ isolates produced only Ade− diploids. All 48 haploid candidates became uniformly Ade− when grown to single colonies on 4 mM guanidine hydrochloride, indicating that they carried a [URE3] prion.
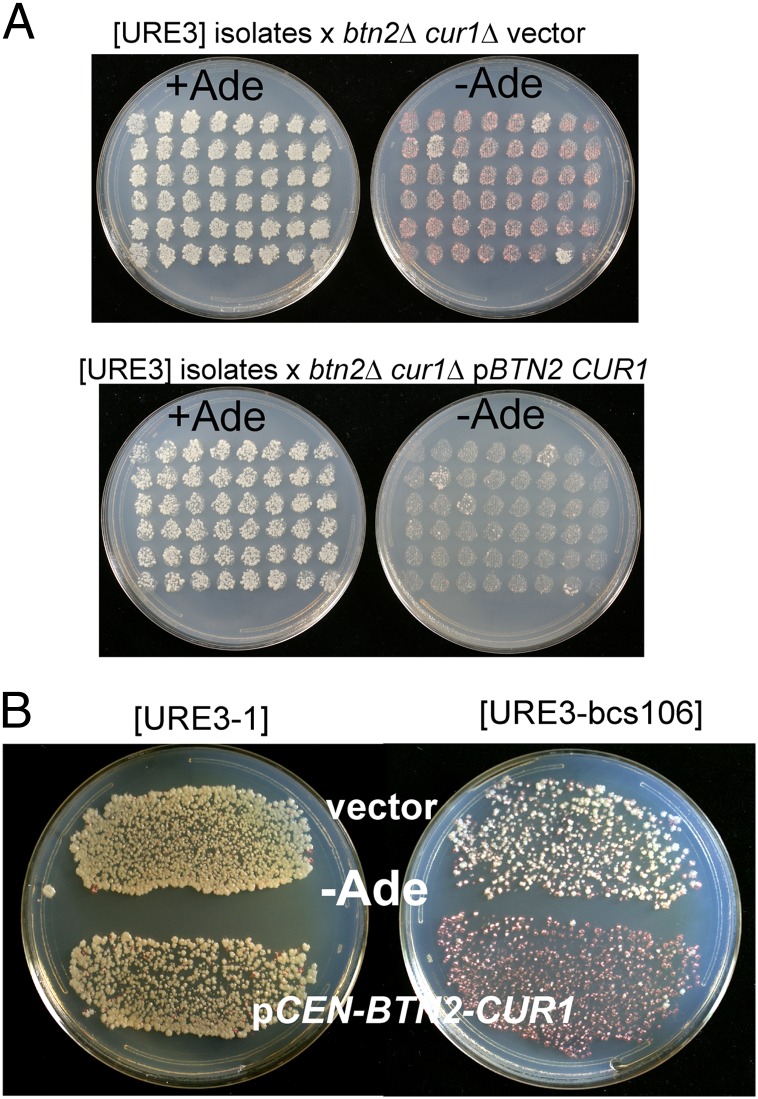
To determine whether the loss of the Ade+ phenotype in the btn2Δ/+ cur1Δ/+ diploids was due to restoration of Btn2 and Cur1, we mated another series of Ade+ haploid isolates with the btn2Δ cur1Δ [ure-o] strain 5197 carrying either the vector or p1482, a plasmid that expresses Btn2p and Cur1p at their normal levels (Fig. 1A). Diploids with Btn2p and Cur1p restored were cured of [URE3] in 44 of 48 cases, but not in the diploids with the empty vector that remained btn2Δ cur1Δ (Fig. 1A). In addition, those haploid isolates (six tested) that became Ade− on mating with a wild-type strain each also became Ade− on transformation with p1482 (BTN2 and CUR1 expressed at normal levels) (Fig. 1B). This plasmid (p1482) destabilized neither the [URE3] of an isolate that produced Ade+ diploids nor [URE3-1] in the same background. We denote these frequently arising [URE3] variants as [URE3-bcs], for Btn2-Cur1-hypersensitive.
Fig. 1.
Normal expression of Btn2p and Cur1p cures Btn2-Cur1 hypersensitive [URE3]s ([URE3-bcs]). (A) Strain 5190 (btn2Δ cur1Δ pGAL:URE2 prion domain) was grown for 2 d in 2% (wt/vol) raffinose, 2% (wt/vol) galactose minimal medium and plated for Ade+ ([URE3]) cells. Candidates were picked to an array on –Ade medium, grown for 1 d, replica-plated to YPAD, and mated for 1 d with strain 5197 (also btn2Δ cur1Δ) carrying either the vector (pRS316) or pBTN2-CUR1 (p1482) and replica-plated to minimal medium with and without adenine. Diploids formed with 5197 carrying the vector are still btn2Δ cur1Δ, grow on –Ade, and are pink, owing to a weak phenotype and frequent loss of the prion. Diploids formed with 5197 carrying pBTN2-CUR1 are Ade− owing to curing of the [URE3-bcs] prion. Four isolates are not efficiently cured by mating with 5197 carrying pBTN2-CUR1. (B) Strain dk133 (btn2Δ cur1Δ) carrying either [URE3-1] (28) or [URE3-bcs106] was transformed with the single-copy vector pRS316 or the same plasmid carrying BTN2 and CUR1 controlled by their own promoters. Transformants were selected in the presence of adenine and replica plated to plates without adenine. Growth of Btn2-Cur1 insensitive papillae derived from [URE3-bcs106] can be seen.
To distinguish the roles of Btn2 and Cur1 in eliminating [URE3-bcs], we transformed several such isolates with pRS316 (CEN vector), p1478 (pRS316-CUR1), p1479 (pRS316-BTN2), or p1482 (pRS316-BTN2-CUR1), single-copy plasmids with the genes controlled by their native promoters. Each gene increased the loss of each of several [URE3-bcs] variants examined, but both were needed for maximal curing (Table 1). As is evident from Table 1 for the four [URE3-bcs] isolates transformed with the vector, all of the more than 50 [URE3-bcs] variants examined were quite unstable, compared with either [URE3-1] (28) or non-bcs [URE3] variants isolated in the same experiment. The inverse correlation of seed number and instability well known from previous work (29–31) suggests that these Btn2/Cur1 hypersensitive [URE3] variants are characterized by low seed number. This question is further addressed below.
Table 1.
Btn2p and Cur1p both needed (at normal levels) for maximal elimination of [URE3-bcs]
| [URE3] variant | Transformant clones |
Phenotype of subclones from Ade− clones that lost pBTN2-CUR1 |
||||||||||||
| vector |
pBTN2 |
pCUR1 |
pBTN2-CUR1 |
|||||||||||
| % Ade+ / sectored / Ade− |
Ade+ | Ade− | ||||||||||||
| [URE3-1] | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | N.A. | |
| [URE3-bcs106] | 92 | 8 | 0 | 27 | 48 | 25 | 0 | 65 | 35 | 0 | 3 | 97 | 0 | 63 |
| [URE3-bcs60] | 80 | 20 | 0 | 58 | 33 | 9 | 48 | 40 | 12 | 40 | 42 | 18 | 0 | 39 |
| [URE3-bcs66] | 25 | 75 | 0 | 12 | 73 | 15 | 13 | 68 | 19 | 13 | 15 | 72 | 0 | 96 |
| [URE3-bcs121] | 43 | 52 | 5 | 34 | 60 | 16 | 26 | 52 | 22 | 0 | 68 | 32 | ||
Strain dk133 (btn2Δ cur1Δ) carrying the indicated [URE3-bcs] or [URE3-1] (28) was transformed with the CEN vector pRS316, or the vector carrying BTN2 and/or CUR1 under their native promoters (p1478, p1479, or p1482). Transformants were selected in the presence of adenine and replica plated to a plate lacking adenine. More than 100 transformant clones were examined in each case. Results are expressed as percent Ade+/sectored/ Ade−. From each of four Ade− transformants with p1482 (pBTN2-CUR1) of cells carrying [URE3-bcs106], [URE3-bcs60], and [URE3-bcs66], Ura- subclones that had lost p1482 were tested for adenine auxotrophy by replica-plating. The results, summed and shown in the rightmost column, show that [URE3-bcs] had been cured by pBTN2-CUR1. N.A., not applicable.
Does restoring Btn2p and Cur1p cure [URE3-bcs] or simply suppress the Ade− phenotype? The presence of frequent sectoring on introduction of BTN2, CUR1 or both (Table 1) suggests that curing is occurring. Loss of pBTN2-CUR1 (p1482) from Ade− transformants of several [URE3-bcs] isolates did not restore the Ade+ phenotype, showing that curing had occurred (Table 1). Further, sporulation of Ade− diploids formed by mass mating each of three [URE3-bcs] isolates with wild-type strain 4592 produced exclusively 4 Ade−: 0 segregation. In particular, all of the btn2Δ cur1Δ segregants (10 or more in each cross) were Ade−, implying that the [URE3] prion was gone and not simply phenotypically suppressed by Btn2p and Cur1p.
Btn2/Cur1-Hypersensitive [URE3] Variants Have Low Seed Number.
The increase in [URE3] seed number in btn2Δ cur1Δ strains (17), and the colocalization of Btn2p and Ure2p in [URE3] strains undergoing curing by overproduced Btn2p (17–19), along with our observation above that [URE3-bcs] variants are all unstable, suggest that [URE3] variants with particularly low seed number might be those that are hypersensitive to Btn2p and Cur1p at their normal levels. Guanidine is a specific inhibitor of Hsp104 (32–34) in its filament-cleaving activity (35), and Cox et al. (29) have used these facts to develop methods of measuring prion seeds. As a prion-containing cell grows on medium containing guanidine, new seeds are not generated, but seeds already present segregate until there is only one seed per cell. The number of prion-containing cells in the colony is presumed to equal the number of seeds originally present in the founder cell of the clone. Plating the entire colony on media selective for the prion (-Ade plates) without guanidine gives a measure of the seed number (29). Using this method, we found that each [URE3-bcs] isolate had seed numbers significantly lower than that of [URE3-1] (Table 2). Different experiments gave significantly different seed numbers for [URE3-1], a variability similar to that seen by Cox et al. for [PSI+] (29), but perhaps owing to the slightly different guanidine concentrations used. Data within a single experiment show clear differences, with all bcs variants having consistently lower seed numbers.
Table 2.
Seed number of [URE3] variants
| Prion variant | Seed number |
| Experiment 1 | |
| [URE3-1] | 708 ± 338 |
| [URE3-bcs6] | 323 ± 93 |
| [URE3-bcs2] | 260 ± 147 |
| [URE3-bcs3] | 306 ± 66 |
| [URE3-bcs4] | 70 ± 23 |
| Experiment 2 | |
| [URE3-1] | 274 ± 138 |
| [URE3-bcs101] | 49 ± 9 |
| [URE3-bcs102] | 97 ± 87 |
| [URE3-bcs103] | 101 ± 24 |
| [URE3-bcs104] | 136 ± 96 |
| [URE3-bcs105] | 63 ± 12 |
| [URE3-bcs106] | 82 ± 14 |
| [URE3-bcs107] | 48 ± 8 |
| [URE3-bcs108] | 40 ± 7 |
| [URE3-bcs109] | 60 ± 20 |
| [URE3-bcs110] | 92 ± 37 |
Strains were streaked for single colonies on YPAD containing 4 mM guanidine HCl (experiment 1) or 5 mM guanidine HCl (experiment 2). Single colonies were suspended in water and plated on –Ade plates. Ade+ colonies are a relative measure of the number of prion seeds in the cell founding the colony (29). Seed number ± SD is shown.
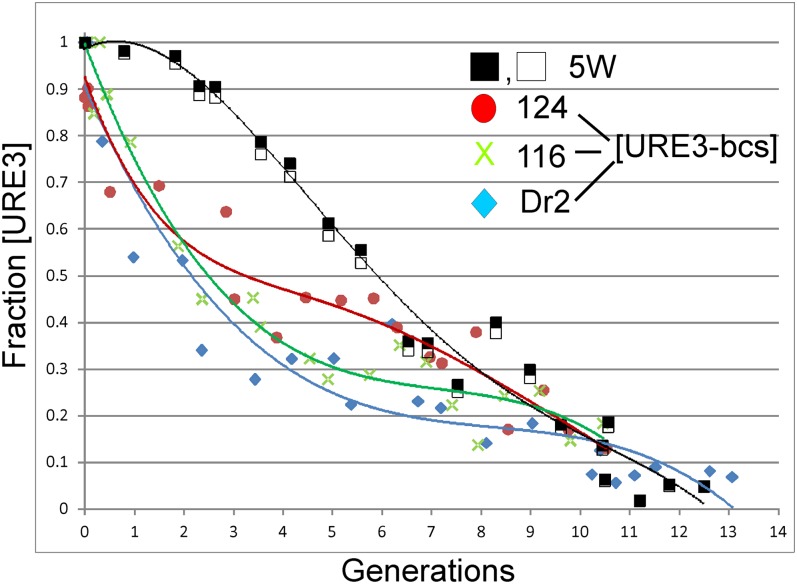
A second method for measuring seed number is to grow a culture in the presence of guanidine, measure the kinetics of loss of the prion, and use those data to calculate seed number (29). Again, this method showed that [URE3-bcs] isolates had significantly lower seed numbers than a [URE3] that is maintained in the presence of normal levels of Btn2 and Cur1 (Fig. 2).
Fig. 2.
Btn2-Cur1 hypersensitive [URE3] variants have significantly fewer seeds than an insensitive [URE3] variant arising in the same btn2Δ cur1Δ background. Btn2-Cur1 insensitive [URE3] strain 5W (black and white squares) and Btn2-Cur1 hypersensitive [URE3] variants 124, 116, and Dr2 were selected from –Ade plates and added to either YPD or YPD containing 3.3 mM guanidine hydrochloride at 1 × 104 cells per mL initial density. Aliquots were removed at time 0 and every hour for 24 h, plating 100 cells per 1/2 YPD plate. After 6 d growth at 30 °C, plates were photographed and colonies were counted using OpenCFU Version 3.8.11, selecting cured red colonies with color filter: hue 16 plus and minus 4 units. Total colonies on the plates and volumes added each hour to the 1/2 YPD plates from the master stock were used to calculate generation number. The values shown are corrected for spontaneous prion loss at each time point by the formula: Fraction [URE3] shown = Fraction [URE3] in guanidine/Fraction [URE3] without guanidine. Uncorrected data are shown in Fig. S1. The shift to the left shows that the [URE3-bcs] isolates have lower seed numbers than the variant 5W not cured by restoring normal levels of Btn2p and Cur1p.
Mutation and Segregation of [URE3-bcs] Isolates.
Subclones of [URE3-bcs] isolates were analyzed by mating with wild-type strain (4592) or by mating with strain 5197 carrying p1482 (CEN URA3 BTN2 CUR1). Although many subclones retained the sensitivity to normal levels of Btn2p and Cur1p, others became completely insensitive to curing by these proteins, and a few produced no diploids (with or without adenine present) (Table S1). Further subcloning of each type of variant showed that each could give rise to the other types at some frequency. Subclones that became insensitive to curing often remained insensitive. Thus, in addition to frequent loss of [URE3-bcs], the prion can change (mutate) to different variants, and these can segregate. We suggest that continued selection for prion maintenance selects for a more stable variant arising in the population, such variants are stable because they have higher seed number, and higher seed number makes them insensitive to curing by normal levels of Btn2p and Cur1p. Specifically variant 5W, whose seed number is shown in Fig. 2 to be higher than several bcs variants, was a Btn2/Cur1-insensitive variant derived from a hypersensitive variant (Table S1).
Spontaneous Prion Generation Elevated in btn2Δ cur1Δ Strains.
If Btn2p and Cur1p are eliminating many [URE3] variants as they arise, the frequency of spontaneous [URE3] should be higher in a doubly deleted strain than in an isogenic wild type. Indeed we found that strain dk133 (btn2Δ cur1Δ) carrying the vector pRS316 produced 106 ± 15 [URE3] clones per 106 cells plated, whereas the same strain carrying p1482 (a CEN plasmid expressing both BTN2 and CUR1, each from their native promoter) produced 19 ± 6 [URE3] clones per 106 cells.
Curing by Overproduction of Btn2p (Cur1p) Does Not Require Cur1p (Btn2p).
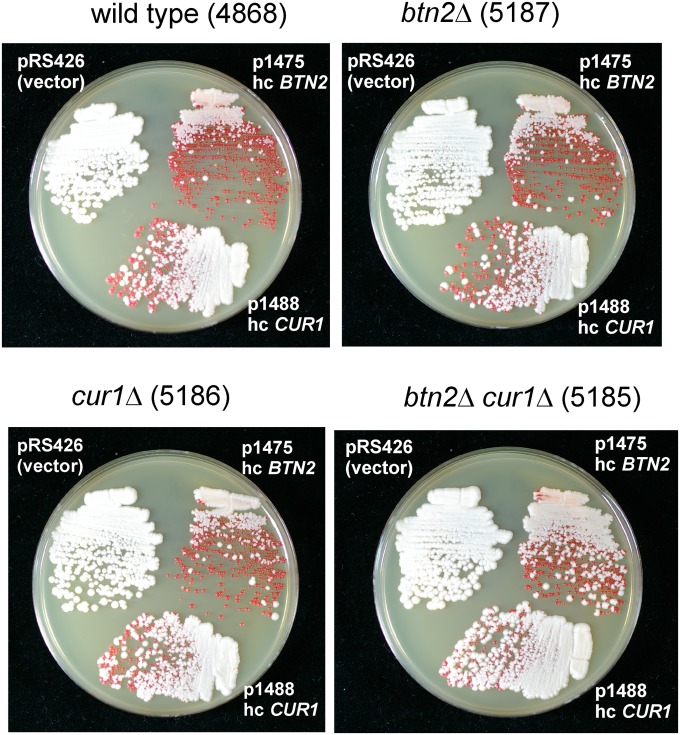
Overexpression of Btn2p in a cur1Δ strain gave similar curing efficiency as in a wild-type host. Similarly, overexpression of Cur1p in a btn2Δ strain cured [URE3-1] as well as in the wild type (Fig. 3). This indicates that neither protein depends on the other for curing. It is possible that Btn2p and Cur1p participate in completely different systems, but their sequence similarity suggests that they may be alternative components of the same antiprion apparatus.
Fig. 3.
Btn2p and Cur1p work independently to cure [URE3-1]. High-copy plasmids expressing Btn2p (p1475), Cur1p (p1488), or the vector (pRS426) were transformed into the wild-type [URE3-1] strain 4648 or the isogenic btn2Δ, cur1Δ, or btn2Δ cur1Δ strains. After growth to single colonies on media selective for the presence of the plasmids but containing adenine, a mixture of colonies was streaked on 1/2 YPD.
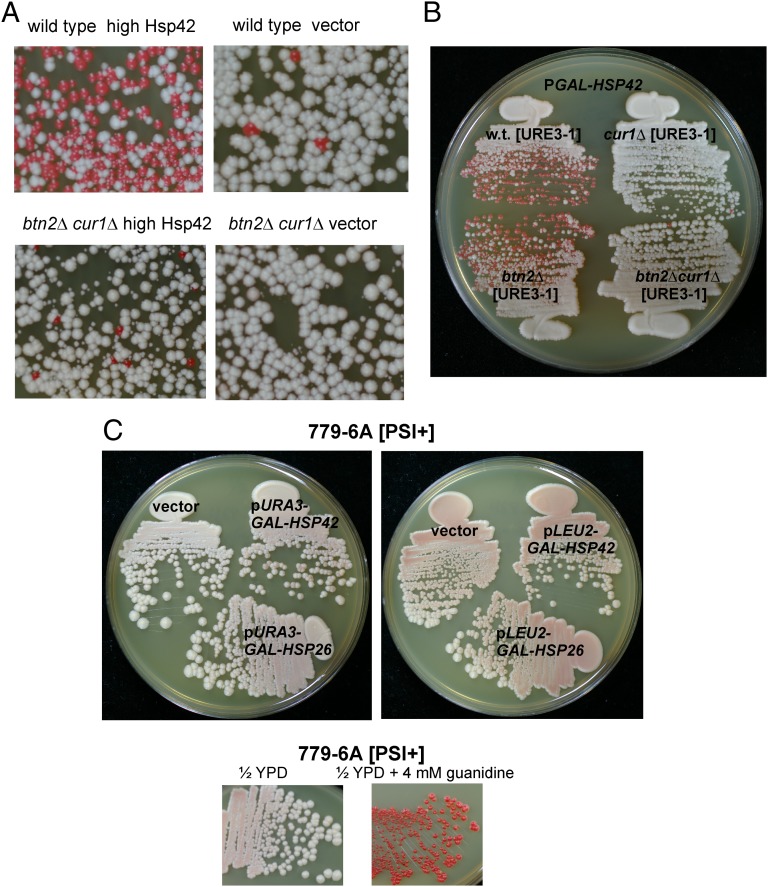
Hsp42 Overproduction Cures [URE3-1], Requires Cur1p.
Hsp42 is known to associate with some aggregates (23), to interact with Btn2p (25), and is reported to cure [PSI+] when overproduced (24). We found that overexpression of Hsp42 cures [URE3-1] and that this curing is essentially eliminated in a btn2Δ cur1Δ strain (Fig. 4A). Comparing Hsp42 overproduction curing in single btn2Δ and cur1Δ mutants showed that Btn2p is dispensable for Hsp42 overproduction curing but that Cur1p is necessary (Fig. 4B).
Fig. 4.
Hsp42 overexpression curing of [URE3]. (A) Wild-type strain 4648 or the isogenic strain dk133 (btn2Δ cur1Δ), each bearing [URE3-1], were transformed with vector p1062 or p1062-HSP42 (p1505, CEN URA3 GAL1:HSP42), grown in galactose medium for 3 d, and plated on 1/2 YPD. (B) Cur1p, but not Btn2p, is needed for curing of [URE3-1] by overexpression of Hsp42. (C) Hsp42 and Hsp26 overproduction do not cure [PSI+] of strain 779-6A. 779-6A ([PSI+]) transformed with vectors p1062 and p896, p1505 and p1508 (GAL1:HSP42), and p1511 and p1510 (GAL1:HSP26) were grown 100-fold twice in Gal-Raf medium selecting for the plasmids. From the second growth (4 d later) aliquots were streaked for single colonies on 1/2YPD and grown for 3 d at 30 °C.
The same Hsp42 overproduction plasmids that efficiently cured [URE3-1] did not detectably cure [PSI+] from strain 779-6A (Fig. 4C). We also found that similar overproduction of Hsp26 did not cure either [URE3-1] (not shown) or [PSI+] (Fig. 4C). It is possible that our results differ from those of Duennwald et al. (24) because of prion or host strain differences.
Although hsp42Δ did not prevent [URE3-1] propagation, hsp26Δ destabilized this prion. Two hsp26Δ isolates from strain BY241 were generated, and [URE3-1] was introduced by cytoduction from strain 1735 into the parent and both hsp26Δ isolates. Wild-type cytoductants were Ade+ and white on 1/2 YPD, whereas all hsp26Δ cytoductants were pink and weakly Ade+. Wild-type and hsp26Δ cytoductants were then used as donors to the [ure-o] strain 4591. All cytoductants from the wild type carried [URE3-1], but most of those from the hsp26Δ cytoductants did not (Table 3). Thus, hsp26Δ destabilizes [URE3-1].
Table 3.
hsp26Δ destabilizes [URE3-1]
| Donor | Recipient | Cytoductants |
|
| Ade+ | Ade− | ||
| BY241 w.t. 1 | 4591 ρo | 37 | 0 |
| 5 | 31 | 0 | |
| 8 | 31 | 0 | |
| BY241 hsp26Δ1-1 | 16 | 40 | |
| 1-1 | 16 | 66 | |
| 1-5 | 0 | 55 | |
| 1-6 | 3 | 112 | |
| BY241 hsp26Δ2–4 | 22 | 130 | |
| 2-4 | 5 | 42 | |
| 2-5 | 12 | 102 | |
| 2-6 | 19 | 83 | |
| 2-6 | 11 | 74 | |
[URE3-1] was transferred by cytoduction (cytoplasmic mixing) from strain 1735 into the parent and each of two hsp26Δ isolates. Several cytoductants of each were used as cytoduction donors to strain 4591 ([ure-o] ρo), and cytoductants scored for [URE3] by their Ade+/− phenotype.
Sis1p Overproduction Prevents Overproduction Curing by Btn2, Cur1, or Hsp42.
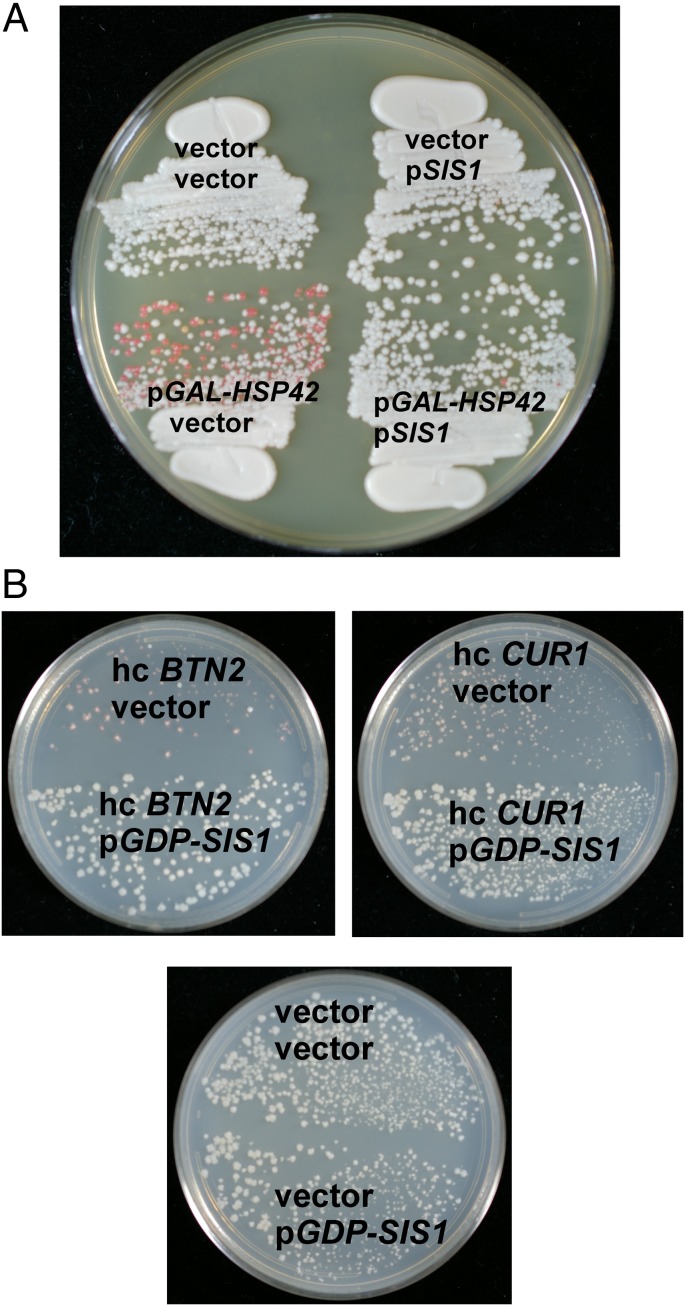
Sis1p is an essential Hsp40 whose depletion results in rapid loss of [PIN+] and [URE3] and slower loss of [PSI+] (36). Malinovska et al. (25) found that Sis1p overproduction largely prevented Btn2 or Cur1 overproduction curing of their Nrp1-Sup35C–based prion. We found that constitutive overproduction of Sis1p blocked curing of [URE3-1] by overproduced Btn2p, Cur1p, or Hsp42 (Fig. 5). Malinovska et al. showed that overproduction of Sis1p does not substantially affect total levels of Btn2p or Cur1p (25).
Fig. 5.
Overproduction of Sis1p prevents curing of [URE3-1] by overproduction of Hsp42, Btn2p, or Cur1p. (A) Wild-type [URE3-1] strain 4648 carrying pGDP1-SIS1 or the vector pRS315 and pGAL-HSP42 or the vector p1062, was grown in raffinose-galactose medium for 3 d, and cells were streaked on 1/2 YPD. (B) [URE3-1] strain 4648 carrying pGDP1-SIS1 or the vector was transformed with high copy BTN2, CUR1 or the plasmid vector, transformants were again grown on medium selecting for the plasmids and streaked on 1/2 YPD.
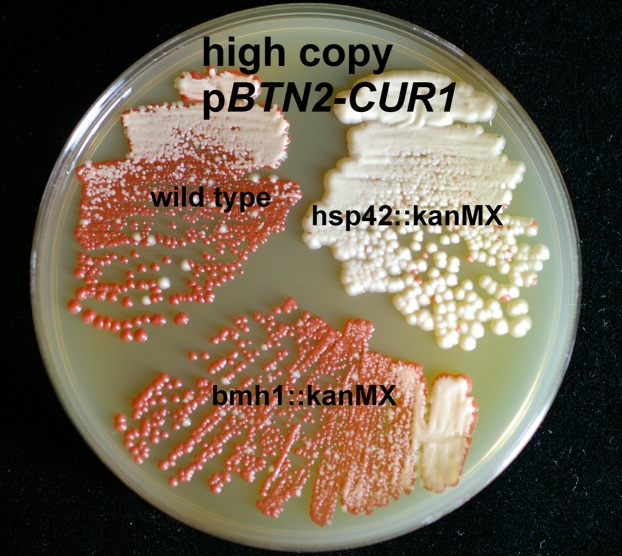
Curing of [URE3-1] by Overproduction of Btn2p and Cur1p Is Promoted by Hsp42, but Not Bmh1p.
Isogenic wild-type, hsp42Δ, and bmh1Δ strains carrying [URE3-1] were used as hosts for curing. Overproduction of Btn2p and Cur1p efficiently cured [URE3-1] from the wild-type host, but only slightly from the hsp42Δ cells (Fig. 6). When Btn2p or Cur1p were overexpressed individually, curing by Cur1p was still effective in hsp42Δ cells, but curing by Btn2p was largely blocked (Fig. S2). In contrast, the bmh1Δ mutation did not affect curing by either Btn2p or Cur1p (Fig. 6 and Fig. S3), suggesting that Btn2p and Cur1p curing does not involve the yeast aggresome as defined by Wang et al. (16).
Fig. 6.
Hsp42, but not Bmh1p, is important for curing of [URE3-1] by overproduced Btn2p and Cur1p. The [URE3-1] strain 4648 or isogenic hsp42::kanMX or bmh1::kanMX derivatives were transformed with the high-copy vector pRS426 or pRS426-BTN2 (p1475) or pRS426-CUR1 (p1488), grown on media selecting for the plasmids for 4 d, and streaked for single colonies on 1/2 YPD. Btn2p levels are not substantially altered by hsp42Δ (25).
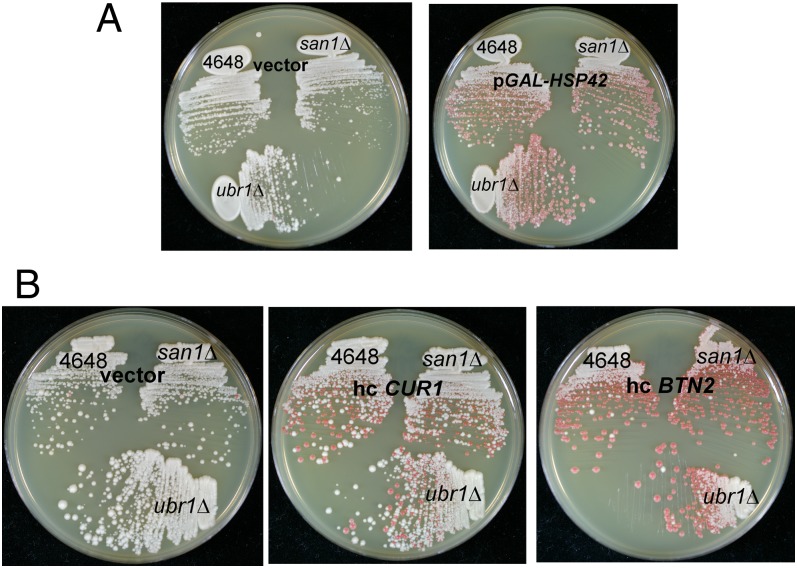
Overproduction Curing by Btn2p or Cur1p Is Independent of the E3 Ubiquitin Ligases San1p and Urb1p.
Two ubiquitin E3 ligases, San1p and Ubr1p, have been identified as responsible for marking misfolded cytoplasmic proteins for degradation in nuclear proteasomes (37, 38). We find that neither Btn2 nor Cur1 overproduction curing depends on San1p or Urb1p (Fig. 7). Curing also proceeds in san1Δ ubr1Δ double mutants. This suggests that the mechanism of curing does not involve this part of the ubiquitin–proteasome system.
Fig. 7.
Curing of [URE3-1] by overexpression of Hsp42, Cur1p, or Btn2p is not affected by san1Δ or urb1Δ. (A) Wild-type [URE3-1] strain 4648 or isogenic san1Δ or urb1Δ strains were transformed with a plasmid carrying GAL-HSP42 (p1505) or the empty vector. Transformants were grown for 3 d on raffinose-galactose medium and then streaked for single colonies on 1/2 YPD. (B) Wild-type [URE3-1] strain 4648 or isogenic san1Δ or urb1Δ strains were transformed with high-copy (hc) plasmids overexpressing Btn2p (p1475) or Cur1p (p1488) or the empty vector. A mixture of transformant clones was regrown on medium selecting for plasmid-carrying cells, and then streaked on 1/2 YPD.
[PIN+] Is Not Cured by Overproduced Btn2p or Cur1p.
The [PIN+] strain 4970 was transformed with pRS425 (2micron DNA LEU2 vector), p1486 (pRS425 CUR1), or p1476 (pRS425 BTN2). Strain 4970 cured of the prion by growth in the presence of guanidine was used as a [pin-] control. Transformants were grown selecting for the plasmid for 5 d on solid medium, mated with the [pin-] strain 5075 carrying a plasmid expressing an RNQ1-GFP fusion protein. Diploids were selected and examined by fluorescence microscopy. [PIN+] cells have a single bright dot of Rnq1-GFP fluorescence, whereas [pin-] cells (such as the diploids formed with the [pin-] control here) show even, bright fluorescence of the cytoplasm. Diploids formed with 4970 carrying the vector, or overexpressing Btn2p or Cur1p each showed >95% of cells with a single bright dot of Rnq1-GFP, indicating that no detectable curing of [PIN+] was occurring. The same plasmids produce >90% curing of [URE3] under similar conditions.
Discussion
We generated and isolated a large series of [URE3] variants in a btn2Δ cur1Δ strain and restored normal levels of both Btn2p and Cur1p either by mating with a wild-type strain or by transformation with a CEN plasmid carrying both genes with their native promoters. We found that the majority of [URE3] variants (called Btn2-Cur1 hypersensitive, bcs) are cured by restoration of normal levels of Btn2p and Cur1p, levels that do not cure [URE3-1], the original isolate of Lacroute (28). This indicates that Btn2p and Cur1p, without being overproduced, are antiprion components. Each shows some [URE3-bcs]-curing activity without the other, but they are most effective when both are expressed. Likewise, overexpressed Btn2p and Cur1p can each cure [URE3-1] in the absence of the other. Thus, Btn2p and Cur1p do not depend on each other, but their sequence similarity suggests that they may, in some sense, do the same thing.
The fact that the cell has a prion-curing system suggests that at least the cured prions are not generally advantageous to the cell. Indeed, population studies indicate that even the mildest forms of the [URE3], [PSI+], and [PIN+] prions confer a substantial detriment on their host cells (39, 40). Furthermore, most variants of [PSI+] are actually lethal or nearly so, and many [URE3] variants dramatically slow growth (8).
Like the toxic or lethal variants of [PSI+] and [URE3] (8), and the variants of [PSI+] and [URE3] affecting passage through an intraspecies or interspecies barrier (41–43), the [URE3-bcs] variants expand the range of known possibilities of yeast prion characteristics. The [URE3-bcs] variants that we describe here would not be detected in a wild-type host, because they are rapidly eliminated by the combined action of Btn2p and Cur1p. Because infectious prion formation occurs readily in vitro with purified Ure2p, and a range of variants are so generated (4), it is likely that [URE3-bcs] variants arise in wild-type cells but are rapidly eliminated. Even in a btn2Δ cur1Δ strain, [URE3-bcs] variants are unstable, being lost frequently and also giving rise to white (strong) variants with higher seed number that are insensitive to normal levels of Btn2p and Cur1p. Even without the selection for stability evident in the case of [URE3-bcs], the [PSI+] prion exists as a “prion cloud,” with distinct variants arising spontaneously and segregating during mitotic growth (44). The generation of stable, relatively Btn2-Cur1–insensitive variants from [URE3-bcs] must reflect some mutability of the prion.
How do Btn2p and Cur1p cure [URE3]? All of the many [URE3-bcs] isolates examined are unstable, unlike [URE3-1] and other non-bcs variants. Prion instability suggests low seed number (29–31), and we confirm that [URE3-bcs] variants indeed do have lower seed numbers than variants such as [URE3-1] that are not cured at normal Btn2p and Cur1p levels. In a btn2Δ cur1Δ strain, the copy number of [URE3-1] was higher than in an isogenic BTN2 CUR1 strain (17), again suggesting that these proteins lower prion copy number. The finding that Btn2p colocalizes with Ure2p amyloid aggregates in [URE3] strains undergoing curing (17–19) suggests a direct mechanism involving sequestration of prion seeds as previously proposed (17). As expected for this model, normal levels of Btn2p/Cur1p are enough to sequester a small number of seeds; higher seed [URE3] variants are only cured by overproduction. Just as curing of [URE3] by Btn2p overproduction draws together nearly all of the Ure2p amyloid aggregates into a single locus coincident with Btn2p (17), overproduction of Btn2p reduces the number of optineurin aggregates, and the collected aggregates are coincident with Btn2p (19).
Malinovska et al. (25) confirmed the prion-curing ability of overproduced Btn2p or Cur1p using an artificial prion consisting of a segment of Nrp1p and the nonprion part of Sup35p. These authors noted that overproduction of Sis1p, an essential Hsp40, prevented this curing activity. They found that Sis1p interacts with Btn2p, and overproduction of Sis1p draws both Btn2p and Cur1p into the nucleus (25), potentially explaining the prevention of curing of the cytoplasmic prion. We find that overproduction of Sis1p prevents overproduced Btn2p, Cur1p, or Hsp42 from curing [URE3-1]. An alternate explanation is that overproduced Btn2p or Cur1p ties up a large fraction of Sis1p, impairing its ability to help prion propagation (25). However, this cannot explain the [URE3]-curing activity of Btn2p and Cur1p at their normal levels that we report here, because there is 20-fold less Btn2p (103 molecules per cell) and 400-fold less Cur1p (<50 molecules per cell) than there is Sis1p (YNL007C, 2 × 104 molecules per cell) as estimated by Ghaemmaghami et al. (45). Even the doubly hemizygous btn2Δ/+ cur1Δ/+ diploids, which likely have lower than normal levels of Btn2p and Cur1p, lost [URE3-bcs] variants (bcs candidates mated with strain 4592). Nor can this hypothesis explain the striking colocalization of Btn2p with Ure2p in cells with [URE3] undergoing curing, observed by two different groups (17, 18).
Sis1p is necessary for [URE3] propagation (36) and may bind to the amyloid. Perhaps Btn2p does not directly bind to amyloid but does bind to Sis1p bound to amyloid, and then the Btn2p (and thus the attached amyloid) is moved to the sequestration site. This model [amyloid-Sis1p-Btn2p (possibly with Hsp42)-transporter], first suggested by Kryndushkin et al. (19), could explain all of the results without invoking Sis1p depletion. Excess Sis1p could saturate the Btn2 binding sites, competing with the amyloid-bound Sis1p for transport to the sequestration site.
Despite their sequence similarity and common prion-curing ability, Btn2p and Cur1p are different in several ways. Btn2p is localized to a site near but not in the nucleus, whereas Cur1p is located throughout the nucleus (17). Colocalization of Btn2p with Ure2p aggregates is observed during curing of [URE3] by Btn2p overproduction (17, 18), but the same phenomenon was not seen with Cur1p (17). We find that [URE3] curing by overproduction of Hsp42 requires Btn2p, but not Cur1p. Moreover, curing by overproduction of Btn2p requires Hsp42, but curing by overproduction of Cur1p does not require Hsp42.
BTN2 was first identified as a gene whose transcription was elevated in cells mutant for the BTN1 gene, the latter a homolog of the human CLN3 involved in Batten’s disease, a juvenile lysosomal disease (46). Later surveys found Btn2p is also induced by heat shock (47). Btn2p has been reported to be localized in the cytoplasm (48) and to late endosomes (49). We found that Btn2p was consistently concentrated in one or two dots close to the nucleus (17). Btn2p mediates late endosome–Golgi protein movements (49) and affects several proteins processed by this system (49–51). Nonetheless, the relation of the different activities of Btn2p remains unclear.
We have begun to assess the roles of the various aggregate accumulation sites and pathways in the control of prion infection. Induction of autophagy by starvation does not cure [URE3], and curing of [URE3] by overproducing Btn2p is not reduced in an atg1Δ strain, defective in autophagy (17). The failure of bmh1Δ to detectably alter the efficiency of prion curing by overproduced Btn2p, Cur1p, or Hsp42p suggests that these proteins are not acting through the yeast aggresome defined by Wang et al. (16). Ubr1p and San1p are E3 ubiquitin ligases involved in targeting misfolded cytoplasmic proteins to proteasomes in the nucleus (37, 38). The [URE3] prion is certainly a misfolded form of Ure2p that is in the cytoplasm, but it seems that Btn2p and Cur1p are not working through these ubiquitin ligases.
Two sites of accumulation of nonamyloid aggregates were identified, with one being perinuclear (JUNQ) and the other peripheral (IPOD) to the nucleus (20). The IPOD site is reported to accumulate nonprion aggregates of Ure2p (20), and such nonprion aggregates were found to not coincide with Btn2p (25). However, prion aggregates of Ure2p do associate with Btn2p (17–19). Thus, Btn2p (or associated proteins) recognizes the prion form of Ure2p as distinct from nonprion aggregates of the same protein. Only accumulation of aggregates at the peripheral site requires Hsp42p, and Hsp42p is found to localize at that site (23). We find that Hsp42 is needed for Btn2p overproduction curing, raising the possibility that Btn2p may bring prion aggregates to the peripheral site. Moreover, Btn2p is not restricted to binding prion aggregates: it has been shown by others to bind to a variety of nonamyloid aggregates as well (19, 25).
Btn2p is a member of the “Hook” family (49), whose founding member is a Drosophila gene involved in endocytosis (52). Cur1p does not show homology to metazoan Hook proteins but is similar to Btn2p (17). The Caenorhabditis elegans Hook homolog, zyg-12, links centrosomes to the nucleus through the microtubules (53). The human Hook2 protein has a role in aggresome formation (54), very much like what we have suggested for Btn2p and Cur1p. Btn2p (410 residues) is much closer to Cur1p (252 residues) (32% identity in a 171-residue region of similarity, e = 10−23) than it is to its closest human homolog, Hook1 (32% identity in an 88-residue region, e = 10−3) (Fig. S4), a protein believed to tether cargo proteins to microtubules in the endocytosis process (e.g., refs. 55 and 56). The role shown for Btn2p in endosomal sorting (49) suggests that their functions may be similar in this respect. However, although these sequence similarities are statistically significant and indicate an evolutionary relationship, they are not close enough to ensure functional similarity.
The Btn2/Cur1 pathway(s) that eliminates prions is not the first antiprion system found in yeast. Chernoff described the antiprion effects of Ssb proteins, a group of ribosome-associated Hsp70 chaperones. In ssb1Δ ssb2Δ strains, the frequency of [PSI+] arising was increased more than 10-fold, indicating that the normal levels of these proteins were blocking prion formation by Sup35p (57). Chernoff made the apt analogy of the Ssb proteins to DNA repair systems that have an antimutator effect. Overproduction of Ssb proteins can cure some [PSI+] variants (58) and increased the curing efficiency of overproduced Hsp104 (57). [PSI+] variants arising in ssb deletion strains are not cured by replacement of the normal genes (57).
The existence of cellular antiprion systems active without artificial gene overexpression adds to the growing lines of evidence that at least the well-studied [URE3] and [PSI+] prions, and probably [PIN+], are pathogens (reviewed in ref. 59). Although the deletion or overexpression of any of several genes cures yeast prions, the Btn2/Cur1 system may be unique in curing prions at their normal levels.
Methods
Strains and Media.
Strains are listed in Table 4. Knockout mutants were produced by PCR-amplifying the knocked-out gene from the collection strain (60), transformation of log phase BY241 cells selecting for resistance to G418, and confirmation by PCR that the normal gene was missing and the putative mutant had been formed. Media were as described by Sherman (61). Gal-Raf medium contained 2% (wt/vol) galactose, 2% (wt/vol) raffinose, 0.7% Yeast Nitrogen Base without amino acids (Difco), and required amino acids or bases.
Table 4.
Strains of S. cerevisiae
| Strain | Genotype | Reference |
| BY241 | MATa leu2 trp1 ura3 PDAL5:ADE2 PDAL5:CAN1 kar1 [ure-o] | (4) |
| 4648 | BY241 [URE3-1] | (4) |
| 4592 | MATα leu2 his3 ura3 PDAL5:ADE2 PDAL5:CAN1 kar1 [ure-o] | (4) |
| 1735 | MATα ura2-60 his- [URE3-1] = MA116-8A | (1) |
| 4591 | MATα his3 leu2 trp1 PDAL5:ADE2 PDAL5:CAN1 kar1 [ure-o] | (4) |
| dk133 | BY241 btn2::TRP1 cur1::kanMX [ure-o] | (17) |
| 5190 | dk133 p1330 | This work |
| dk122 | BY241 btn2::kanMX | (17) |
| dk124 | pBY241 cur1::kanMX | (17) |
| 5197 | MATα leu2 ura3 trp1 his3 btn2::TRP1 cur1::kanMX PDAL5:ADE2 kar1 | Segregants of dk133 x 4592 |
| 5200 | BY241 bmh1::kanMX [URE3-1] | This work |
| 5194 | BY241 hsp42::kanMX [URE3-1] | This work |
| 5225 | BY241 ubr1::kanMX [URE3-1] | This work |
| 5223 | BY241 san1::kanMX [URE3-1] | This work |
| 4970 | MATa kar1 leu2 ura3 his4 ade1-14 ho::kanMX [PIN+] | This work |
| 5075 | MATα lys2 leu2 ura3 trp1 rnq1::kanMX p1332 | (40) |
Plasmids.
Plasmids used are listed in Table 5. BTN2, with 500 bp upstream of the ORF, was amplified using primers 667 and 668 (Table S2), cut with XhoI and BamHI, and inserted into pRS316 (CEN URA3) cut with the same enzymes forming p1479. CUR1, with 500 bp upstream of its ORF, was amplified using primers 669 and 670 (Table S2), with XhoI and BamHI and inserted into pRS316 cut with the same enzymes forming p1478. Primers 682 and 683 (Table S2) were used to amplify CUR1, with 500 bp upstream of its ORF, cut with BamHI and SacII and ligated to p1479 cut with the same enzymes, forming p1482. Similar methods were used for constructing p1488. For both BTN2 and CUR1, the 500 bp upstream of the ORF includes all of the intergenic region, suggesting that the native promoter of each gene is included.
Table 5.
Plasmids
| Name | Genes | Source |
| pRS316 | CEN URA3 | (62) |
| pRS426 | 2 μm DNA URA3 | (63) |
| p1479 | pRS316 PBTN2:BTN2 | This work |
| p1478 | pRS316 PCUR1:CUR1 | This work |
| p1482 | pRS316 PCUR1:CUR1 PBTN2:BTN2 | This work |
| p1475 | pRS426 PADH1:BTN2 | (17) |
| p1488 | pRS426 PCUR1:CUR1 | This work |
| pH382 = p1330 | CEN LEU2 PGAL1:URE2(amino acids1–65) | H. K. Edskes, NIDDK, NIH, Bethesda, MD |
| p1332 | CEN URA3 RNQ1-GFP | (39) |
| p1062 = pH392 | pRS316 PGAL1 | H. K. Edskes |
| p1062-HSP42 = p1505 | CEN URA3 PGAL1:HSP42 | This work |
| p1062-HSP26 | CEN URA3 PGAL1:HSP26 | This work |
| p896 = pH316 | CEN LEU2 PGAL1 | H. K. Edskes |
| p896-HSP42 | CEN URA3 PGAL1:HSP42 | This work |
| p896-HSP26 | CEN URA3 PGAL1:HSP26 | This work |
| pMR294 | pRS315 PGDP1:SIS1 CEN LEU2 | Michael Reidy, NIDDK, NIH, Bethesda, MD |
The HSP42 ORF was amplified with oligos 694 and 695 (Table S2), digested with BamHI and HindIII, and inserted into the GAL1 promoter vectors p896 and p1062 digested with the same enzymes forming p1508 and p1505, respectively. Such constructs are denoted GAL1:HSP42, for example. The HSP26 ORF was similarly inserted under control of the GAL1 promoter of these vectors forming p1510 and p1511, respectively.
Measuring Prion Seed Number.
Following Cox et al. (29), we used two methods to assess prion seed number. Freshly isolated [URE3] cells growing on –Ade plates were streaked for single colonies on 1/2 YPD with 4 mM or 5 mM guanidine HCl, which is presumed to stop prion seed generation. Single colonies were picked up in their entirety, suspended in water and plated on a –Ade plate. After 5 d the number of Ade+ colonies appearing was counted and assumed to represent the seed number of the cell founding that colony. A sample of Ade+ colonies arising from the replated cells were checked for guanidine curability. Five to ten clones from each prion variant were assayed in this manner. The details of the population test are given in the legend of Fig. 2.
Supplementary Material
Acknowledgments
We thank Daniel Masison, Kevin O’Connell, and Michael Reidy [all of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)] and Frank Shewmaker and Dmitry Kryndushkin [Uniformed Services University of the Health Sciences (USUHS)] for reading the manuscript; and Herman Edskes (NIDDK), Michael Reidy (NIDDK), and Dmitry Kryndushkin (USUHS) for strains and plasmids. This work was supported by the Intramural Program of the NIDDK of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409582111/-/DCSupplemental.
References
- 1.Aigle M, Lacroute F. Genetical aspects of [URE3], a non-mitochondrial, cytoplasmically inherited mutation in yeast. Mol Gen Genet. 1975;136(4):327–335. doi: 10.1007/BF00341717. [DOI] [PubMed] [Google Scholar]
- 2.Wickner RB. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264(5158):566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 3.Taylor KL, Cheng N, Williams RW, Steven AC, Wickner RB. Prion domain initiation of amyloid formation in vitro from native Ure2p. Science. 1999;283(5406):1339–1343. doi: 10.1126/science.283.5406.1339. [DOI] [PubMed] [Google Scholar]
- 4.Brachmann A, Baxa U, Wickner RB. Prion generation in vitro: Amyloid of Ure2p is infectious. EMBO J. 2005;24(17):3082–3092. doi: 10.1038/sj.emboj.7600772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper TG. Transmitting the signal of excess nitrogen in Saccharomyces cerevisiae from the Tor proteins to the GATA factors: Connecting the dots. FEMS Microbiol Rev. 2002;26(3):223–238. doi: 10.1111/j.1574-6976.2002.tb00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magasanik B, Kaiser CA. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290(1-2):1–18. doi: 10.1016/s0378-1119(02)00558-9. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer C, Masison DC. Antagonistic interactions between yeast [PSI(+)] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol Cell Biol. 2002;22(11):3590–3598. doi: 10.1128/MCB.22.11.3590-3598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGlinchey RP, Kryndushkin D, Wickner RB. Suicidal [PSI+] is a lethal yeast prion. Proc Natl Acad Sci USA. 2011;108(13):5337–5341. doi: 10.1073/pnas.1102762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxa U, et al. Characterization of β-sheet structure in Ure2p1-89 yeast prion fibrils by solid-state nuclear magnetic resonance. Biochemistry. 2007;46(45):13149–13162. doi: 10.1021/bi700826b. [DOI] [PubMed] [Google Scholar]
- 10.Wickner RB, Shewmaker F, Kryndushkin D, Edskes HK. Protein inheritance (prions) based on parallel in-register β-sheet amyloid structures. BioEssays. 2008;30(10):955–964. doi: 10.1002/bies.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191(4):1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics. 1997;147(2):507–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sondheimer N, Lindquist S. Rnq1: An epigenetic modifier of protein function in yeast. Mol Cell. 2000;5(1):163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- 14.Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: The story of [PIN(+)] Cell. 2001;106(2):171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 15.Johnston JA, Ward CL, Kopito RR. Aggresomes: A cellular response to misfolded proteins. J Cell Biol. 1998;143(7):1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, et al. Abnormal proteins can form aggresome in yeast: Aggresome-targeting signals and components of the machinery. FASEB J. 2009;23(2):451–463. doi: 10.1096/fj.08-117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kryndushkin DS, Shewmaker F, Wickner RB. Curing of the [URE3] prion by Btn2p, a Batten disease-related protein. EMBO J. 2008;27(20):2725–2735. doi: 10.1038/emboj.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanneganti V, Kama R, Gerst JE. Btn3 is a negative regulator of Btn2-mediated endosomal protein trafficking and prion curing in yeast. Mol Biol Cell. 2011;22(10):1648–1663. doi: 10.1091/mbc.E10-11-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kryndushkin D, Ihrke G, Piermartiri TC, Shewmaker F. A yeast model of optineurin proteinopathy reveals a unique aggregation pattern associated with cellular toxicity. Mol Microbiol. 2012;86(6):1531–1547. doi: 10.1111/mmi.12075. [DOI] [PubMed] [Google Scholar]
- 20.Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454(7208):1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernoff YO, Derkach IL, Inge-Vechtomov SG. Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr Genet. 1993;24(3):268–270. doi: 10.1007/BF00351802. [DOI] [PubMed] [Google Scholar]
- 22.Kochneva-Pervukhova NV, Poznyakovski AI, Smirnov VN, Ter-Avanesyan MD. C-terminal truncation of the Sup35 protein increases the frequency of de novo generation of a prion-based [PSI+] determinant in Saccharomyces cerevisiae. Curr Genet. 1998;34(2):146–151. doi: 10.1007/s002940050379. [DOI] [PubMed] [Google Scholar]
- 23.Specht S, Miller SBM, Mogk A, Bukau B. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J Cell Biol. 2011;195(4):617–629. doi: 10.1083/jcb.201106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duennwald ML, Echeverria A, Shorter J. Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLoS Biol. 2012;10(6):e1001346. doi: 10.1371/journal.pbio.1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell. 2012;23(16):3041–3056. doi: 10.1091/mbc.E12-03-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlumpberger M, Prusiner SB, Herskowitz I. Induction of distinct [URE3] yeast prion strains. Mol Cell Biol. 2001;21(20):7035–7046. doi: 10.1128/MCB.21.20.7035-7046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masison DC, Wickner RB. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science. 1995;270(5233):93–95. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- 28.Lacroute F. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 1971;106(2):519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox B, Ness F, Tuite M. Analysis of the generation and segregation of propagons: entities that propagate the [PSI+] prion in yeast. Genetics. 2003;165(1):23–33. doi: 10.1093/genetics/165.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall D, Edskes H. Silent prions lying in wait: A two-hit model of prion/amyloid formation and infection. J Mol Biol. 2004;336(3):775–786. doi: 10.1016/j.jmb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442(7102):585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- 32.Jung G, Masison DC. Guanidine hydrochloride inhibits Hsp104 activity in vivo: A possible explanation for its effect in curing yeast prions. Curr Microbiol. 2001;43(1):7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira PC, Ness F, Edwards SR, Cox BS, Tuite MF. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol Microbiol. 2001;40(6):1357–1369. doi: 10.1046/j.1365-2958.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- 34.Jung G, Jones G, Masison DC. Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc Natl Acad Sci USA. 2002;99(15):9936–9941. doi: 10.1073/pnas.152333299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ness F, Ferreira P, Cox BS, Tuite MF. Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol Cell Biol. 2002;22(15):5593–5605. doi: 10.1128/MCB.22.15.5593-5605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higurashi T, Hines JK, Sahi C, Aron R, Craig EA. Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc Natl Acad Sci USA. 2008;105(43):16596–16601. doi: 10.1073/pnas.0808934105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner RG, Nelson ZW, Gottschling DE. Degradation-mediated protein quality control in the nucleus. Cell. 2005;120(6):803–815. doi: 10.1016/j.cell.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Heck JW, Cheung SK, Hampton RY. Cytoplasmic protein quality control degradation mediated by parallel actions of the E3 ubiquitin ligases Ubr1 and San1. Proc Natl Acad Sci USA. 2010;107(3):1106–1111. doi: 10.1073/pnas.0910591107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayashiki T, Kurtzman CP, Edskes HK, Wickner RB. Yeast prions [URE3] and [PSI+] are diseases. Proc Natl Acad Sci USA. 2005;102(30):10575–10580. doi: 10.1073/pnas.0504882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly AC, Shewmaker FP, Kryndushkin D, Wickner RB. Sex, prions, and plasmids in yeast. Proc Natl Acad Sci USA. 2012;109(40):E2683–E2690. doi: 10.1073/pnas.1213449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edskes HK, McCann LM, Hebert AM, Wickner RB. Prion variants and species barriers among Saccharomyces Ure2 proteins. Genetics. 2009;181(3):1159–1167. doi: 10.1534/genetics.108.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen B, et al. Genetic and epigenetic control of the efficiency and fidelity of cross-species prion transmission. Mol Microbiol. 2010;76(6):1483–1499. doi: 10.1111/j.1365-2958.2010.07177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bateman DA, Wickner RB. [PSI+] Prion transmission barriers protect Saccharomyces cerevisiae from infection: Intraspecies ‘species barriers’. Genetics. 2012;190(2):569–579. doi: 10.1534/genetics.111.136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateman DA, Wickner RB. The [PSI+] prion exists as a dynamic cloud of variants. PLoS Genet. 2013;9(1):e1003257. doi: 10.1371/journal.pgen.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 46.Pearce DA, Ferea T, Nosel SA, Das B, Sherman F. Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nat Genet. 1999;22(1):55–58. doi: 10.1038/8861. [DOI] [PubMed] [Google Scholar]
- 47.Gasch AP, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11(12):4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chattopadhyay S, Muzaffar NE, Sherman F, Pearce DA. The yeast model for batten disease: Mutations in BTN1, BTN2, and HSP30 alter pH homeostasis. J Bacteriol. 2000;182(22):6418–6423. doi: 10.1128/jb.182.22.6418-6423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kama R, Robinson M, Gerst JE. Btn2, a Hook1 ortholog and potential Batten disease-related protein, mediates late endosome-Golgi protein sorting in yeast. Mol Cell Biol. 2007;27(2):605–621. doi: 10.1128/MCB.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chattopadhyay S, Pearce DA. Interaction with Btn2p is required for localization of Rsglp: Btn2p-mediated changes in arginine uptake in Saccharomyces cerevisiae. Eukaryot Cell. 2002;1(4):606–612. doi: 10.1128/EC.1.4.606-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chattopadhyay S, Roberts PM, Pearce DA. The yeast model for Batten disease: A role for Btn2p in the trafficking of the Golgi-associated vesicular targeting protein, Yif1p. Biochem Biophys Res Commun. 2003;302(3):534–538. doi: 10.1016/s0006-291x(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 52.Krämer H, Phistry M. Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J Cell Biol. 1996;133(6):1205–1215. doi: 10.1083/jcb.133.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malone CJ, et al. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell. 2003;115(7):825–836. doi: 10.1016/s0092-8674(03)00985-1. [DOI] [PubMed] [Google Scholar]
- 54.Szebenyi G, et al. Hook2 contributes to aggresome formation. BMC Cell Biol. 2007;8:19. doi: 10.1186/1471-2121-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krämer H, Phistry M. Genetic analysis of hook, a gene required for endocytic trafficking in drosophila. Genetics. 1999;151(2):675–684. doi: 10.1093/genetics/151.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maldonado-Báez L, Cole NB, Krämer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J Cell Biol. 2013;201(2):233–247. doi: 10.1083/jcb.201208172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chernoff YO, Newnam GP, Kumar J, Allen K, Zink AD. Evidence for a protein mutator in yeast: Role of the Hsp70-related chaperone ssb in formation, stability, and toxicity of the [PSI] prion. Mol Cell Biol. 1999;19(12):8103–8112. doi: 10.1128/mcb.19.12.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chacinska A, et al. Ssb1 chaperone is a [PSI+] prion-curing factor. Curr Genet. 2001;39(2):62–67. doi: 10.1007/s002940000180. [DOI] [PubMed] [Google Scholar]
- 59.Wickner RB, et al. Amyloids and yeast prion biology. Biochemistry. 2013;52(9):1514–1527. doi: 10.1021/bi301686a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winzeler EA, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285(5429):901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 61.Sherman F. In: Getting Started with Yeast. Guide to Yeast Genetics and Molecular Biology, Methods in Enzymology. Guthrie C, Fink GR, editors. Vol 194. San Diego: Academic; 1991. pp. 3–21. [Google Scholar]
- 62.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.