Abstract
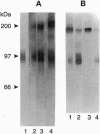
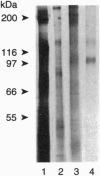
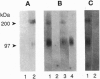
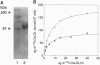
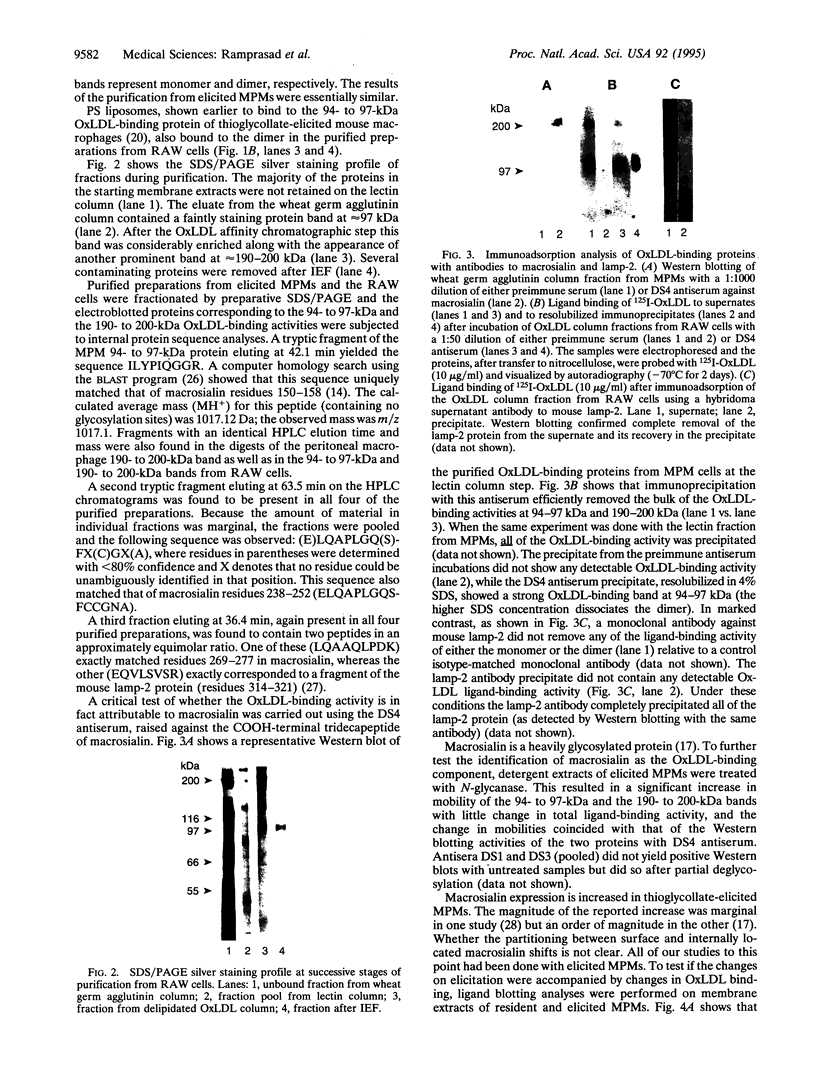
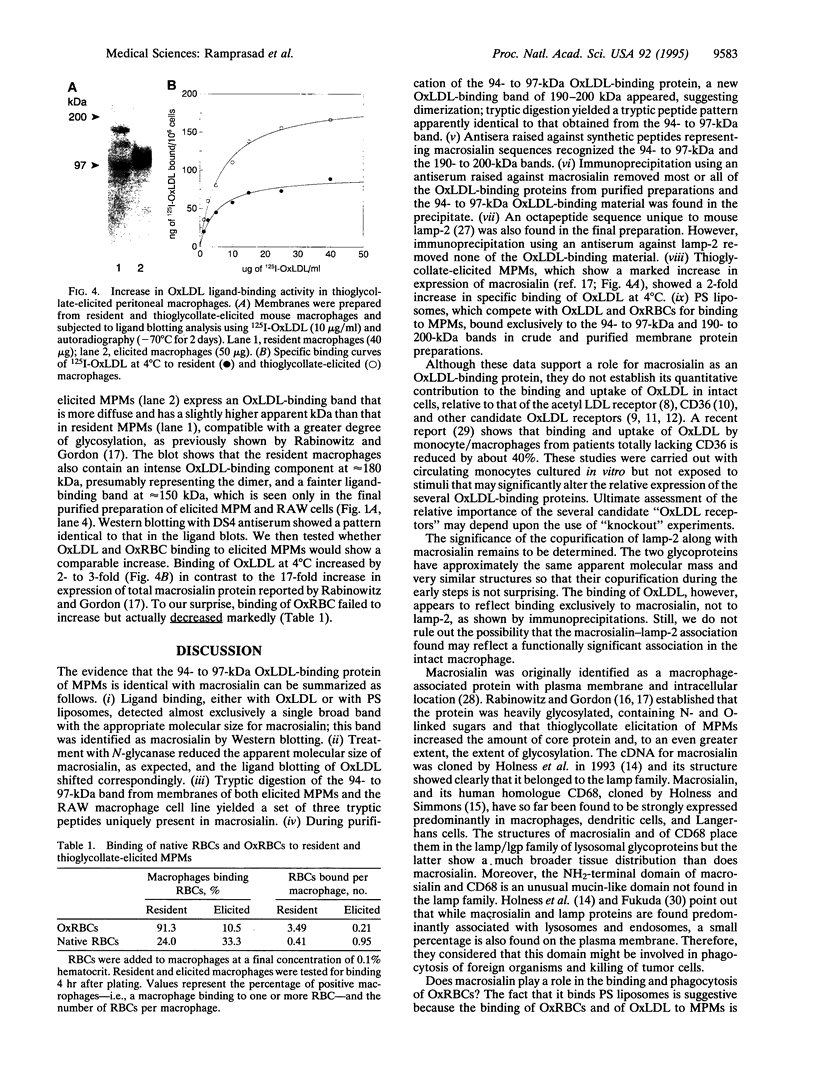
We have previously reported the partial purification of a 94- to 97-kDa plasma membrane protein from mouse peritoneal macrophages that binds oxidatively modified low density lipoprotein (OxLDL) and phosphatidylserine-rich liposomes. We have now identified that protein as macrosialin, a previously cloned macrophage-restricted membrane protein in the lysosomal-associated membrane protein family (mouse homologue of human CD68). Early in the course of purification of the 94- to 97-kDa protein, a new OxLDL-binding band at 190-200 kDa appeared and copurified with the 94- to 97-kDa protein. The HPLC pattern of tryptic peptides from this higher molecular mass ligand-binding band closely matched that derived from the 94- to 97-kDa band. Specifically, the same three macrosialin-derived tryptic peptides (9, 9, and 15 residues) were present in the purified 94- to 97-kDa band and in the 190- to 200-kDa band and antisera raised against peptide sequences in macrosialin recognized both bands. An antiserum against macrosialin precipitated most of the 94- to 97-kDa OxLDL-binding material. We conclude that the binding of OxLDL to mouse macrophage membranes is in part attributable to macrosialin. Our previous studies show that OxLDL competes with oxidized red blood cells and with apoptotic thymocytes for binding to mouse peritoneal macrophages. Whether macrosialin plays a role in recognition of OxLDL and oxidatively damaged cells by intact macrophages remains uncertain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton S. L., Scherer P. E., Lodish H. F., Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994 Aug 19;269(33):21003–21009. [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arai H., Kita T., Yokode M., Narumiya S., Kawai C. Multiple receptors for modified low density lipoproteins in mouse peritoneal macrophages: different uptake mechanisms for acetylated and oxidized low density lipoproteins. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1375–1382. doi: 10.1016/0006-291x(89)92262-6. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y., Holland S. M., August J. T. The cDNA sequence of mouse LAMP-2. Evidence for two classes of lysosomal membrane glycoproteins. J Biol Chem. 1990 Mar 25;265(9):5008–5013. [PubMed] [Google Scholar]
- Endemann G., Stanton L. W., Madden K. S., Bryant C. M., White R. T., Protter A. A. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993 Jun 5;268(16):11811–11816. [PubMed] [Google Scholar]
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992 Apr 1;148(7):2207–2216. [PubMed] [Google Scholar]
- Freeman M., Ekkel Y., Rohrer L., Penman M., Freedman N. J., Chisolm G. M., Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991 Nov 15;266(32):21327–21330. [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983 Mar-Apr;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Holness C. L., Simmons D. L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993 Mar 15;81(6):1607–1613. [PubMed] [Google Scholar]
- Holness C. L., da Silva R. P., Fawcett J., Gordon S., Simmons D. L. Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem. 1993 May 5;268(13):9661–9666. [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Arai H., Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages. J Biol Chem. 1990 Mar 25;265(9):5226–5231. [PubMed] [Google Scholar]
- Ottnad E., Parthasarathy S., Sambrano G. R., Ramprasad M. P., Quehenberger O., Kondratenko N., Green S., Steinberg D. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low density lipoprotein: partial purification and role in recognition of oxidatively damaged cells. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1391–1395. doi: 10.1073/pnas.92.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Ylä-Herttuala S., Rosenfeld M. E., Butler S. W., Socher S. A., Parthasarathy S., Curtiss L. K., Witztum J. L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990 May-Jun;10(3):325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. S., Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991 Oct 1;174(4):827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S., Gordon S. Differential expression of membrane sialoglycoproteins in exudate and resident mouse peritoneal macrophages. J Cell Sci. 1989 Aug;93(Pt 4):623–630. doi: 10.1242/jcs.93.4.623. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S., Horstmann H., Gordon S., Griffiths G. Immunocytochemical characterization of the endocytic and phagolysosomal compartments in peritoneal macrophages. J Cell Biol. 1992 Jan;116(1):95–112. doi: 10.1083/jcb.116.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasad M. P., Li R., Gianturco S. H., Bradley W. A. Purification of the human THP-1 monocyte-macrophage triglyceride-rich lipoprotein receptor. Biochem Biophys Res Commun. 1995 May 16;210(2):491–497. doi: 10.1006/bbrc.1995.1687. [DOI] [PubMed] [Google Scholar]
- Ren Y., Silverstein R. L., Allen J., Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995 May 1;181(5):1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti A., Acton S. L., Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995 Jul 7;270(27):16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- Sambrano G. R., Steinberg D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992 Oct;90(4):1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci. 1987 Feb;87(Pt 1):113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J Biol Chem. 1989 Feb 15;264(5):2599–2604. [PubMed] [Google Scholar]
- Stanton L. W., White R. T., Bryant C. M., Protter A. A., Endemann G. A macrophage Fc receptor for IgG is also a receptor for oxidized low density lipoprotein. J Biol Chem. 1992 Nov 5;267(31):22446–22451. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Witztum J. L. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990 Dec 19;264(23):3047–3052. [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijke Y. B., van Berkel T. J. Rat liver Kupffer and endothelial cells express different binding proteins for modified low density lipoproteins. Kupffer cells express a 95-kDa membrane protein as a specific binding site for oxidized low density lipoproteins. J Biol Chem. 1994 Jan 14;269(2):824–827. [PubMed] [Google Scholar]