Abstract
Population level data support that consumption of fructose and fructose-based sweeteners has dramatically increased and suggest that high dietary intake of fructose is an important factor in the development of the cardiorenal metabolic syndrome (CRS). The CRS is a constellation of cardiac, kidney and metabolic disorders including insulin resistance, obesity, metabolic dyslipidemia, high blood pressure, and evidence of early cardiac and kidney disease. The consequences of fructose metabolism may result in intracellular ATP depletion, increased uric acid production, oxidative stress, inflammation, and increased lipogenesis, which are associated with endothelial dysfunction. Endothelial dysfunction is an early manifestation of vascular disease and a driver for the development of CRS. A better understanding of fructose overconsumption in the development of CRS may provide new insights into pathogenesis and future therapeutic strategies.
Keywords: Cardiorenal metabolic syndrome, Nitric oxide, Lipogenesis, Insulin resistance, Hypertension, Oxidative stress, Inflammation, Estrogen
Introduction
Fructose is a monosaccharide that is widely available in natural food sources such as fruits and honey. Fructose is widely consumed as sucrose and high fructose corn syrup, which represents up to 10 % of total energy intake in the US and several European countries [1]. Population level data support that intake of fructose has significantly increased over the past three decades. In the US, yearly per capita caloric sweetener consumption has risen approximately 20 % since 1970 [2]. The largest source of added sugar in the western diet is sweetened beverages, which account for more than 10 % of energy intake [3]. Increased total fructose consumption has been implicated in the obesity epidemic [4] and contributes to the rising frequency of cardiorenal metabolic syndrome (CRS)-associated insulin resistance, type 2 diabetes mellitus, and hypertension (Fig. 1) [5, 6]. Recent data from the third National Health and Nutrition Examination Survey (NHANES) indicate that consumption of sugar-sweetened beverages is significantly associated with plasma uric acid (UA) concentrations [7]. UA is associated with endothelial dysfunction, which is aggravated by increased oxidative stress and inflammation [6]. Endothelial dysfunction is a manifestation of CRS, and is a maladapted endothelial phenotype characterized by reduced nitric oxide (NO) bioavailability, increased oxidative stress, elevated expression of pro-inflammatory and pro-thrombotic factors, and reduced endothelial-derived vasodilation [8]. However, the molecular mechanisms of fructose and UA on endothelial cell (EC) dysfunction and subsequently CRS are not completely understood. The objectives of this review are to provide a better understanding of the interaction between fructose overconsumption, hyperuricemia, and endothelial dysfunction in the development of CRS.
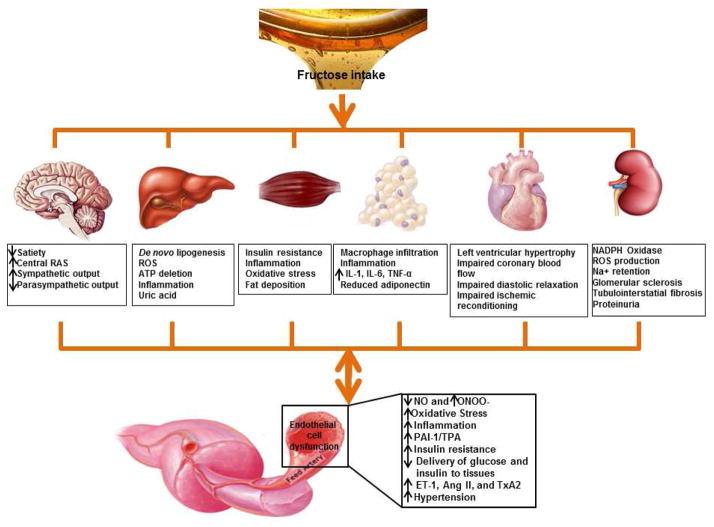
Fig. 1.
Proposed roles of fructose and uric acid in the development of endothelial cell dysfunction and CRS. Fructose intake increases uric acid production, oxidative stress, inflammation, and de novo lipogenesis, subsequently results in endothelial cell dysfunction, whereas endothelial dysfunction further aggravates fructose-induced and uric acid-induced CRS. Abbreviations: RAS renin-angiotensin-aldosterone system; ROS reactive oxygen species; IL interleukin; TNF tumor necrosis factor; NO nitric oxide; ONOO−, peroxynitrite; PAI plasminogen activator inhibitor; TPA tissue plasminogen activator; ET-1 endothelin-1; Ang II angiotensin II; TxA2 thromboxane A2
Fructose and Uric Acid Metabolism
Our western diet includes added fructose that leads to typical daily consumption of 85–100 grams of fructose per day [6]. Fructose is readily absorbed and rapidly metabolized by the human liver through the glucose transporters (GLUT) 2 and 5 [9]. Most fructose is metabolized by fructokinase, which phosphorylates fructose to fructose 1-phosphate. Fructose 1-phosphate is subsequently cleaved by aldolase B to yield dihydroxyacetone phosphate (DHAP) and glyceraldehyde. DHAP can be isomerized into glyceraldehyde 3-phosphate and subsequently proceeds to the glycolytic pathway into acetyl-coenzyme A, which is either oxidized in the tricarboxylic acid cycle or committed towards fatty acid synthesis [10]. The trioses can also contribute to lipid synthesis by forming glycerol 3-phosphate, which serves as the backbone for triglyceride [11]. Unlike glucose, whose phosphorylation is tightly regulated so that adenosine triphosphate (ATP) levels are never depleted, the phosphorylation of fructose results in a decrease in intracellular phosphate and ATP depletion, resulting in transient inhibition of protein synthesis [12]. As a result, continued fructose metabolism results in intracellular phosphate depletion, activation of adenosine monophosphate (AMP) deaminase, increasing inosine monophosphate (IMP) that is further degraded to xanthine and hypoxanthine by xanthine oxidase and ultimately generates UA [13].
Fructose in the Development of CRS
The increase in fructose consumption has paralleled the rise in obesity from 13 % to 34 % since 1960, and the rise in diagnosed type 2 diabetes from 5 % to 8 % since 1988 [14]. In children, intake of artificially sweetened beverages was found to be positively associated with adiposity [15]. A prospective cohort analysis of non-diabetic women in the Nurses’ Health Study II concluded that a higher consumption of sugar-sweetened beverages is associated with a greater magnitude of weight gain and an increased risk for the development of type 2 diabetes [15]. In the Framingham Heart Study, the relationship between soft drink consumption and cardiovascular risk was evaluated in 6,039 participants; consumption of more than one can of soft drink per day was significantly associated with the prevalence of the metabolic syndrome [7]. Thus, fructose has been implicated in promoting obesity and the CRS by altering appetite, inducing leptin resistance, and resulting in increased food intake [16••].
The CRS consists of a constellation of cardiac, kidney, and metabolic disorders including elevated serum triglycerides (TG), small low-density lipoprotein (LDL) particles, low high-density lipoprotein (HDL) cholesterol, central obesity, insulin resistance, glucose intolerance, and elevated blood pressure, and is associated with an increased risk of type 2 diabetes and coronary heart disease (Fig. 1) [17•]. In this context, fructose cannot be directly absorbed into the bloodstream like glucose. A large fraction of ingested fructose is converted to glucose and then released into the blood through the liver [18]. When ingested, excess fructose enhances glycogen storage either directly from glucose or through gluconeogenesis. First, fructose is converted to DHAP and glyceraldehyde by fructokinase and aldolase B. The resultant glyceraldehyde then undergoes phosphorylation to glyceraldehyde-3-phosphate. The increased concentrations of DHAP and glyceraldehyde-3-phosphate in the liver drive the gluconeogenic pathway toward glucose-6-phosphate, glucose-1-phosphate and glycogen formation [19]. It appears that fructose is a better substrate for glycogen synthesis than glucose and that glycogen replenishment takes precedence over triglyceride formation.
It has been recognized that a diet high in fructose increases plasma TG in healthy volunteers and in patients with insulin resistance or type 2 diabetes [7]. De novo lipogenesis (DNL), an important feature of the state of high energy charge and high carbohydrate, is higher in subjects consuming fructose compared to those consuming glucose, suggesting that both increased very low density lipoprotein (VLDL) secretion and decreased TG clearance contributed to the effects of fructose to increase postprandial triglycerides [20]. Increased hepatic lipids are associated with decreased apoB degradation and increased VLDL synthesis and secretion, specifically TG-rich VLDL1. Increased secretion of VLDL1, reduced post-heparin lipoprotein lipase (LPL) activation by insulin, and competition for LPL-mediated TG hydrolysis by chylomicrons all contribute to a longer VLDL residence time, allowing for augmented lipoprotein remodeling [20]. Thus, lipid synthesis enhanced by excess fructose contributes to dyslipidemia, ectopic lipid deposition in liver and muscle tissue, and promotes hepatic and whole body insulin resistance [21••]. Fructose may alter the activity of pyruvate dehydrogenase (PDH) by inhibiting PDH kinase (PDK), the mechanisms by which fructose promotes lipogenesis [10]. Meanwhile, an increase of the hepatic lipogenesis may also induce insulin resistance, possibly through increased intrahepatic levels of diacylglycerol, which activates protein kinase C (PKC), since activated PKC may decrease insulin sensitivity, increase hepatic glucose production, increase fasting glucose, and impair glucose tolerance [22]. Thus, fructose induced-gluconeogenesis and lipogenesis play important roles in the development of CRS.
UA as Amplifying Fructose Effects on CRS
High fructose consumption is associated with CRS through an effect on UA production. There is increasing evidence that intracellular ATP depletion and UA generation may have important roles in the ability of fructose to induce CRS features such as increases in the body mass index (BMI), waist circumference, and dyslipidemia [23]. Since UA has the ability to prevent peroxynitrite-induced protein nitrosolyation, lipid and protein peroxidation, and inactivation of tetrahydrobiopterin, elevated plasma UA concentration has been considered as a beneficial phenomenon that assumes a compensatory role in response to increased oxidative stress in conditions such as cardiovascular disease [24]. Although UA appears to possess anti-oxidant activity in the extracellular environment [24], it has detrimental effects once it enters cells including ECs, vascular smooth muscle cells (VSMCs), and adipocytes. The injurious impact of UA includes an inhibitory effect on NO production, induction of platelet aggregation, and pro-inflammatory activity [25].
UA may promote insulin resistance by inhibiting endothelial function because insulin depends on NO for stimulation of glucose uptake through NO-induced vasodilatation in skeletal muscle. Consequently, UA reduces glucose uptake in skeletal muscle [26]. Therefore, excess UA reduces NO bioavailability and hyperuricemia plays an important role in the development or worsening of insulin resistance [27]. Asymptomatic hyperuricemia in humans is also associated with endothelial dysfunction; lowering UA with allopurinol improves endothelial function. In this regard, allopurinol improves a number of features of CRS in fructose-fed rats, including hypertension, hypertriglyceridemia, hyperinsulinemia, insulin resistance, and renal microvascular disease [28]. Previous evidence also associates increased serum UA concentrations with elevated visceral adipose tissue mass. One possible explanation for this finding is that increased free fatty acid flux to the liver, as seen in visceral obesity, not only increases triglyceride-rich VLDL production, but also stimulates UA synthesis [29]. Indeed, adipocytes may produce over 50 active substances such as monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein (CRP), and angiotensin II (Ang II), all of which are released into circulation and are involved in the regulation of energy balance, lipid metabolism, insulin sensitivity, immune response, angiogenesis, vascular function, arterial blood pressure, coagulation and acute inflammation [30]. For example, MCP-1 has a key role in the macrophage infiltration in the adipose tissue of obesity and development of insulin resistance. The ability of UA to induce MCP-1 expression was first demonstrated in rat VSMCs, and the effect of UA was mediated by mitogen-activated protein kinases (MAPK) and nuclear factor-κB in a redox-dependent manner [31]. Furthermore, it has been shown that UA may stimulate the innate immune response through CD8+ T lymphocytes. It is well recognized that the immune response plays a role in the development of cardiovascular and kidney disease [32]. Thus, UA promotes inflammation in the development of CRS.
During the last decade, studies have also supported a link between fructose, UA, and hypertension. Fructose uptake has also been associated with elevated blood pressure in both adolescents and adults with no previous history of hypertension [9]. It has been speculated that the increase in blood pressure is secondary to the development of insulin resistance and hyperinsulinemia since both fructose and UA may induce endothelial dysfunction, sympathetic activation, and kidney injury [33]. Hyperuricemia following excess fructose intake may raise systemic blood pressure by increasing inflammation, activating the renin-angiotensin system, and decreasing NO production contributing to renal vasoconstriction, and thus resulting in hypertension [9]. However, the importance of a link among fructose, elevations in UA, and cardiovascular events in the general population still remains to be clarified.
Fructose and UA Initiate EC Injury
The arterial endothelium provides a continuous barrier between the elements of blood and the arterial wall, and is a critical component to vascular homeostasis, actively responding to biochemical and physical stimuli through the release of a diverse set of vasoactive substances [34••]. Dysfunction of the endothelium as in the CRS can be regarded as a pivotal event in the initiation of the EC function process. LDL particles produced from fructose in blood plasma invade the endothelium and become oxidized, creating risk for a subsequent inflammatory response and ultimately cardiovascular disease. Monocytes enter the artery wall from the bloodstream with platelets adhering to the area of insult, differentiate into macrophages, and eventually form foam cells. Foam cells die and further propagate the inflammatory process [35]. Under conditions of fructose intake and fatty acid over-nutrition, nutrient overflow into cells prompts electrons transferring to oxygen without ATP production, and further favors a state of increased reactive oxygen species (ROS), which potentially leads to oxidative damage within mitochondria [36]. Meanwhile, UA is catalyzed from the oxidation of hypoxanthine to xanthine by xanthine oxidase (XO), which produces ROS [32]. Eventually, the excessive ROS generation is known to impair endothelial nitric oxide synthase (eNOS) activity and NO production, affecting endothelium-dependent vasodilation [37•]. Therefore, the presence of ROS and consecutive disorders of the mitochondrial respiratory chain are believed to contribute to endothelial dysfunction and subsequent development of atherosclerosis and cardiovascular disease in CRS (Fig. 1).
ROS production may induce a chronic inflammatory reaction of the endothelium in cardiovascular disease. Indeed, increased inflammatory activation has also been linked to vascular complications in CRS. Data from one cohort study support the association between endothelial biomarkers of soluble endothelial selectin, thrombomodulin, intercellular adhesion molecules 1, von willebrand factor, inflammation CRP, serum amyloid A, IL-6, IL-8, TNF-α, and soluble intercellular adhesion molecule 1 (ICAM-1), all of which have been associated with cardiovascular events. This study elucidated biomarkers of endothelial dysfunction and association of inflammation cytokines with cardiovascular disease [38]. Recently, it has been recognized that the adaptive immune system is important in the genesis of endothelia injury. Specifically, a population of immune cells, vascular T lymphocytes, has been identified in the artery walls. Activated T cells can be sub-typed according to their cytokine profile. T helper (Th)1 cells secrete IL-2, TNF-β, and IFN-γ, whereas Th2 cells typically produce IL-4, -5, -6, and -10 [39]. Increased Th secretion of cytokines, chemokines, growth factors, and hydrolytic enzymes leads to an inflammatory process. However, CD4+CD25+Foxp3+ regulatory T cells (Tregs) can protect the pro-inflammatory activation of vascular cells. Several mechanisms of Treg-mediated inflammatory suppression have been proposed, including secretion by the Tregs of immunosuppressive cytokines, cell contact–dependent suppression, and functional modification or killing of activated protein C [40].
Endothelial Dysfunction Aggravates Fructose-Induced and UA-Induced CRS
Insulin resistance and resulting hyperinsulinemia are frequently present in type 2 diabetes, obesity, hypertension, and dyslipidemias [41]. Mitochondria dysfunction, accumulation of lipid metabolite, and increased ROS induced by fructose and UA in ECs cause activation of serine/threonine kinases, including IκB kinase (IKKβ), c-Jun N-terminal kinase (JNK), and PKCs, all of which increase serine phosphorylation of IRS proteins and contribute to insulin resistance. Increased serine phosphorylation of insulin receptor substrate (IRS) protein leads to decreased activity of insulin downstream signaling pathways, including phosphatidylinositide 3-kinases (PI3K), protein kinase B (Akt), and PKC, and culminates in decreased glucose uptake, increased glucose production, and reduced vasodilation and insulin secretion [42]. Our recent studies have explored the signaling pathways by which enhanced tissue renin angiotensin system contributes to insulin resistance in ECs. To this point, Ang II increased serine phosphorylation of IRS-1 and inhibited the insulin-stimulated phosphorylation of eNOS through activation of S6 kinase (S6K) signaling pathway. An inhibitor of rapamycin (mTOR) attenuated the Ang II-stimulated phosphorylation of p70S6K and IRS-1, and blocked the ability of Ang II to impair insulin-stimulated phosphorylation of eNOS and NO dependent -arteriole vasodilation. From these results, we conclude that activation of mTOR/p70S6K by Ang II in vascular endothelium may contribute to the impairment of insulin-stimulated vasodilation through phosphorylation of IRS-1 [43•]. These observations suggest that improving endothelial function might be a beneficial therapeutic in fructose-induced and UA-induced CRS.
Insulin metabolic signaling is an important contributor to normal vascular function and homeostasis. In this regard, insulin stimulates production of the vasodilator NO via activation of IRS-1/PI3K signaling in ECs. In contrast, insulin stimulates production of the vasoconstrictor endothelin-1 (ET-1) via a MAPK–dependent signaling pathway [44]. Typically, endothelial insulin resistance is accompanied by reduced PI3K-NO pathway and heightened MAPK-ET-1 pathway. Indeed, vascular homeostasis is tightly controlled by EC secreting the vasodilatory substances, such as NO, endothelium-derived hyperpolarizing factor (EDHF), and prostacyclin (PGI2), and vasoconstrictory substances, such as ET-1, Ang II, and thromboxane A2 (TxA2) [45]. These EC secreting vasoactivity substances have been proposed to mediate the link between insulin resistance or hyperinsulinemia and hypertension in the fructose-fed animal models, including continued activation of the sympathetic nervous system, increased production and activity of vasoconstrictors, such as ET-1, Ang II and TxA2, and impaired endothelium-dependent relaxation [46•]. Thus, endothelial dysfunction has been suggested as a common underlying mechanism that links insulin resistance, hyperinsulinemia, and hypertension.
Recently, our investigative group reported that there is a gender difference in mitochondrial function and that this difference contributes to development of the CRS in mice fed a western diet high in fructose [47•]. Indeed, sex differences influence the progression of insulin resistance and hypertension [46•]. A study has shown that female rats are protected against fructose-induced changes in metabolism and blood pressure until removal of estrogen by ovariectomy [48]. In contrast, gonadectomized male rats fed a high fructose diet did not develop hypertension, suggesting that testosterone may act as a causal link between insulin resistance/hyperinsulinemia and hypertension, and it is the absence of testosterone that prevented the development of fructose-induced hypertension [49]. In general, physiological concentrations of estrogen have beneficial effects on lipoproteins, insulin, and glucose metabolism [50]. Estrogen can improve insulin action in the liver, muscle, and adipose tissue by increasing glycogen deposition, glucose uptake, and lipogenesis [51]. Estrogen plays an important role in the regulation of vascular tone and pathophysiology of cardiovascular disease through estrogen receptor α and β, which are both expressed in ECs [52]. Estrogen may promote endothelium-dependent vasodilation by increasing the releases of NO, PGI2, and EDHF. In comparison, estrogen decreases the release of ET-1 and Ang II, which are potent vasoconstrictors and procoagulants [53]. Furthermore, estrogen can decrease sympathetic activity, circulating levels of norepinephrine and blood pressure [54]. The mechanisms in estrogen-induced eNOS activation and NO expression on ECs, that estrogen activates PI3K/Akt pathways, leads to phosphorylation and activation of eNOS and increases NO production [55]. Estrogen also increases eNOS activity by causing rapid estrogen receptor-dependent activation of MAPK [56]. Thus, estrogen plays important roles in insulin and glucose metabolism of CRS through maintaining EC physiological function.
Conclusions
Based on the present knowledge of fructose and its detrimental metabolic effects, it is clear that fructose is responsible for dyslipidemia, elevated blood pressure, high plasma glucose, prothrombotic disorder, pro-inflammatory state, and associated cardiac and renal disease (Fig. 1). However, many of these alterations in humans are observed when fructose is experimentally administered in amounts largely exceeding the usual fructose intake with hypercaloric conditions. Future studies should explore the dose threshold of fructose on the CRS. Indeed, currently there are no common opinions to support that low or moderate intake of fructose is unsafe, since fructose consumption in everyday life cannot be blamed as the only culprit for all metabolic disorders. Clearly, there is a need for clinical trials with variable amounts of fructose intake to determine the effects on metabolic outcomes, rather than depending on meta-analyses of existing studies of mixed design and duration. A number of therapeutic agents that decrease the effect of high fructose diets on diabetes and insulin resistance include statins, metformin, and angiotensin-converting-enzyme inhibitor attenuated diabetes and insulin resistance. Recently, estrogens are suggested to protect against development of the CRS and the prevalence of obesity, insulin resistance, and type 2 diabetes increases in post-menopausal woman. Overall, further studies are warranted to help correct unhealthy dietary habits and develop new therapeutic approaches for metabolic disorders and the associated cardiovascular complications.
Acknowledgments
The authors would like to thank Brenda Hunter for her editorial assistance. This research was supported by National Institutes of Health grants HL-73101 and HL-107910 to J.R.S. and AG-040638 to A.W.-C. and the Veterans Affairs Merit System 0018 (J.R.S.) and CDA-2 (A.W.-C.).
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Guanghong Jia, Annayya R. Aroor, Adam T. Whaley-Connell, and James R. Sowers declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Sheludiakova A, Rooney K, Boakes RA. Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur J Nutr. 2012;51:445–54. doi: 10.1007/s00394-011-0228-x. [DOI] [PubMed] [Google Scholar]
- 2.Lecoultre V, Egli L, Theytaz F, et al. Fructose-induced hyperuricemia is associated with a decreased renal uric acid excretion in humans. Diabetes Care. 2013;36:e149–50. doi: 10.2337/dc13-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakhan SE, Kirchgessner A. The emerging role of dietary fructose in obesity and cognitive decline. Nutr J. 2013;8;12:114. doi: 10.1186/1475-2891-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cozma AI, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012;35:1611–20. doi: 10.2337/dc12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kretowicz M, Johnson RJ, Ishimoto T, et al. The impact of fructose on renal function and blood pressure. Int J Nephrol. 2011;2011:315879. doi: 10.4061/2011/315879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindal A, Garcia-Touza M, Jindal N, et al. Diabetic kidney disease and the cardiorenal syndrome: old disease, new perspectives. Endocrinol Metab Clin North Am. 2013;42:789–808. doi: 10.1016/j.ecl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 8.Rajwani A, Cubbon RM, Wheatcroft SB. Cell-specific insulin resistance: implications for atherosclerosis. Diabetes Metab Res Rev. 2012;28:627–34. doi: 10.1002/dmrr.2336. [DOI] [PubMed] [Google Scholar]
- 9.Collino M. High dietary fructose intake: Sweet or bitter life? World J Diabetes. 2011;2:77–81. doi: 10.4239/wjd.v2.i6.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011;22:60–5. doi: 10.1016/j.tem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Fushinobu S, Nishimasu H, Hattori D, et al. Structural basis for the bifunctionality of fructose -1,6-bisphosphate aldolase/phosphatase. Nature. 2011;478:538–41. doi: 10.1038/nature10457. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Sanchez-Lozada LG, Nakagawa T. The effect of fructose on renal biology and disease. J Am Soc Nephrol. 2010;21:2036–9. doi: 10.1681/ASN.2010050506. [DOI] [PubMed] [Google Scholar]
- 13.Debosch BJ, Chen Z, Finck BN, et al. Glucose Transporter-8 (GLUT8) Mediates Glucose Intolerance and Dyslipidemia in High-Fructose Diet-Fed Male Mice. Mol Endocrinol. 2013;27:1887–96. doi: 10.1210/me.2013-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masterjohn C, Park Y, Lee J, et al. Dietary fructose feeding increases adipose methylglyoxal accumulation in rats in association with low expression and activity of glyoxalase-2. Nutrients. 2013;5:3311–28. doi: 10.3390/nu5083311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khitan Z, Kim DH. Fructose: a key factor in the development of metabolic syndrome and hypertension. J Nutr Metab. 2013;2013:682673. doi: 10.1155/2013/682673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••16.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–15. doi: 10.2337/db12-1814. This study showed the major discovery that fructose-mediated generation of uric acid may have a causal role in diabetes and obesity, and provides new insights into pathogenesis and therapies for this important disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •17.Sowers JR, Whaley-Connell A, Hayden MR. The Role of Overweight and Obesity in the Cardiorenal Syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. This study revealed the potential mechanisms by which obesity and other metabolic abnormalities interact to promote heart and progressive kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhar I, Dhar A, Wu L, Desai KM. Increased methylglyoxal formation with upregulation of Renin Angiotensin system in fructose fed sprague dawley rats. PLoS One. 2013;8:e74212. doi: 10.1371/journal.pone.0074212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinman RD, Fine EJ. Fructose in perspective. Nutr Metab (Lond) 2013;10:45. doi: 10.1186/1743-7075-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanhope KL, Havel PJ. Fructose consumption: recent results and their potential implications. Ann N Y Acad Sci. 2010;1190:15–24. doi: 10.1111/j.1749-6632.2009.05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••21.Chaudhary K, Malhotra K, Sowers J, Aroor A. Uric Acid - Key Ingredient in the Recipe for Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2013;3:208–220. doi: 10.1159/000355405. This study also found that elevated serum levels of uric acid appear to contribute to impaired nitric oxide production/endothelial dysfunction, increased vascular stiffness, inappropriate activation of the renin-angiotensin-aldosterone system, enhanced oxidative stress, and maladaptive immune and inflammatory responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–7. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aroor AR, McKarns S, Demarco VG, et al. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–52. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120:1791–9. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15:175–81. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindal A, Brietzke S, Sowers JR. Obesity and the Cardiorenal Metabolic Syndrome: Therapeutic Modalities and Their Efficacy in Improving Cardiovascular and Renal Risk Factors. Cardiorenal Med. 2012;2:314–327. doi: 10.1159/000343803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sluijs I, Beulens JW, van der ADL, et al. Plasma uric acid is associated with increased risk of type 2 diabetes independent of diet and metabolic risk factors. J Nutr. 2013;143:80–5. doi: 10.3945/jn.112.167221. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos RD. Elevated uric acid, the metabolic syndrome and cardiovascular disease: cause, consequence, or just a not so innocent bystander? Endocrine. 2012;41:350–2. doi: 10.1007/s12020-012-9657-4. [DOI] [PubMed] [Google Scholar]
- 30.Zapolski T, Waciński P, Kondracki B, et al. Uric acid as a link between renal dysfunction and both pro-inflammatory and prothrombotic state in patients with metabolic syndrome and coronary artery disease. Kardiol Pol. 2011;69:319–26. [PubMed] [Google Scholar]
- 31.Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–69. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riegersperger M, Covic A, Goldsmith D. Allopurinol, uric acid, and oxidative stress in cardiorenal disease. Int Urol Nephrol. 2011;43:441–9. doi: 10.1007/s11255-011-9929-6. [DOI] [PubMed] [Google Scholar]
- 33.Gul R, Demarco VG, Sowers JR, et al. Regulation of Overnutrition-Induced Cardiac Inflammatory Mechanisms. Cardiorenal Med. 2012;2:225–233. doi: 10.1159/000339565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••34.Muniyappa R, Sowers JR. Endothelial insulin and IGF-1 receptors: when yes means NO. Diabetes. 2012;61:2225–7. doi: 10.2337/db12-0654. This report also revealed that interventions aimed at downregulating IGF-1R expression may augment endothelial insulin sensitivity in the pathological states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Sowers JR, Ren J. Pathophysiological insights into cardiovascular health in metabolic syndrome. Exp Diabetes Res. 2012;2012:320534. doi: 10.1155/2012/320534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Shen W, Zhao B, et al. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: Hope from natural mitochondrial nutrients. Adv Drug Deliv Rev. 2009;61:1343–52. doi: 10.1016/j.addr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- •37.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5–12. doi: 10.1007/s11154-012-9229-1. This study revealed the cellular mechanisms in the endothelium underlying vascular actions of insulin, the role of insulin resistance in mediating endothelial dysfunction, and the effect of insulin sensitizers in restoring the balance in pro- atherogenic and anti-atherogenic actions of insulin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Bussel BC, Schouten F, Henry RM, et al. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011;58:588–95. doi: 10.1161/HYPERTENSIONAHA.111.174557. [DOI] [PubMed] [Google Scholar]
- 39.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 40.He S, Li M, Ma X, et al. CD4+CD25+Foxp3+ regulatory T cells protect the proinflammatory activation of human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:2621–30. doi: 10.1161/ATVBAHA.110.210492. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Vargas L, Addison SS, Nistala R, et al. Gestational Diabetes and the Offspring: Implications in the Development of the Cardiorenal Metabolic Syndrome in Offspring. Cardiorenal Med. 2012;2:134–142. doi: 10.1159/000337734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–14. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •43.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302:E201–8. doi: 10.1152/ajpendo.00497.2011. This report showed that Ang II-mediated impairment of vascular actions of insulin may help explain the role of Ang II as a link between insulin resistance and hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–9. doi: 10.2337/db12-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–31. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •46.Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–59. doi: 10.1007/s11010-009-0184-4. This study addressed the role of sympathetic nervous system overactivation, increased production of vasoconstrictors, such as endothelin-1 and angiotensin II, and prostanoids in the development of hypertension in fructose-fed rats. [DOI] [PubMed] [Google Scholar]
- •47.Manrique C, DeMarco VG, Aroor AR, et al. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632–42. doi: 10.1210/en.2013-1256. This report also revealed that higher aldosterone levels, in concert with insulin resistance, may promote myocardial stiffness and diastolic dysfunction in response to overnutrition in females. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. 2002;283:H2478–84. doi: 10.1152/ajpheart.00243.2002. [DOI] [PubMed] [Google Scholar]
- 49.Song D, Arikawa E, Galipeau D, et al. Androgens are necessary for the development of fructose-induced hypertension. Hypertension. 2004;43:667–72. doi: 10.1161/01.HYP.0000118018.77344.4e. [DOI] [PubMed] [Google Scholar]
- 50.Martins-Maciel ER, Campos LB, Salgueiro-Pagadigorria CL, et al. Raloxifene affects fatty acid oxidation in livers from ovariectomized rats by acting as a pro-oxidant agent. Toxicol Lett. 2013;217:82–9. doi: 10.1016/j.toxlet.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Manrique C, Lastra G, Habibi J, et al. Loss of Estrogen Receptor α Signaling Leads to Insulin Resistance and Obesity in Young and Adult Female Mice. Cardiorenal Med. 2012;2:200–210. doi: 10.1159/000339563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Lone R, Knorr K, Jaffe IZ, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–96. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 53.Masood DE, Roach EC, Beauregard KG, Khalil RA. Impact of sex hormone metabolism on the vascular effects of menopausal hormone therapy in cardiovascular disease. Curr Drug Metab. 2010;11:693–714. doi: 10.2174/138920010794233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–41. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SA, Kim EY, Jeon WK, et al. The inhibitory effect of raloxifene on lipopolysaccharide-induced nitric oxide production in RAW264.7 cells is mediated through a ROS/p38 MAPK/CREB pathway to the up-regulation of heme oxygenase-1 independent of estrogen receptor. Biochimie. 2011;93:168–74. doi: 10.1016/j.biochi.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Jaubert AM, Mehebik-Mojaat N, Lacasa D, et al. Nongenomic estrogen effects on nitric oxide synthase activity in rat adipocytes. Endocrinology. 2007;148:2444–52. doi: 10.1210/en.2006-1329. [DOI] [PubMed] [Google Scholar]