Abstract
The primary aim of the INTERGROWTH-21st Project is to construct new, prescriptive standards describing optimal fetal and preterm postnatal growth. The anthropometric measurements include the head circumference, recumbent length and weight of the infants, and the stature and weight of the parents. In such a large, international, multicentre project, it is critical that all study sites follow standardised protocols to ensure maximal validity of the growth and nutrition indicators used. This paper describes, in detail, the selection of anthropometric personnel, equipment, and measurement and calibration protocols used to construct the new standards. Implementing these protocols at each study site ensures that the anthropometric data are of the highest quality to construct the international standards.
Keywords: Anthropometry, fetal growth, INTERGROWTH-21st, newborn measurements, standards
Introduction
The International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) is a large-scale, population-based, multicentre project involving health institutions from eight geographically diverse countries, which aims to assess fetal, newborn and preterm growth under optimal conditions, in a manner similar to that adopted by the World Health Organization (WHO) Multicentre Growth Reference Study (MGRS).1 The INTERGROWTH-21st Project has three major components, which were designed to create: (1) longitudinally derived, prescriptive, international, fetal growth standards using both clinical and ultrasound measures; (2) preterm, postnatal growth standards for those infants born at ≥ 26+0 but < 37+0 weeks of gestation in the longitudinal cohort; and (3) birthweight-for-gestational-age standards derived from all newborns delivering at the study sites over an approximately 12 month period.2
To achieve uniformity in such a large multicentre study, it was important for all study sites to follow standardised protocols to ensure maximal validity of the growth and nutrition indicators. In addition, to promote continuity with MGRS, several members of the MGRS Anthropometry Group helped to adapt the anthropometric protocols for INTERGROWTH-21st; this included matching measurement techniques, and accuracy and precision limitations. This paper describes the anthropometric measurement protocols that are being used to construct the new standards.
Selection of anthropometrists
The local principal investigator at each study site recruited a team of anthropometrists. The size of each team varied because of differences in the number of health institutions involved, and whether full or part-time personnel were needed. The number of personnel employed was sufficient to allow for sickness, holiday cover and fluctuations in workload levels as the project progressed. Only personnel who were highly motivated, educated to secondary school level or higher, capable of working in a clinical environment and interacting easily with the infants’ parents, had legible handwriting, and were able to speak the local language and dialects, were employed. There were no more than 12 anthropometrists per health institution to ensure adherence to the protocols and quality control measures. A lead anthropometrist was selected at each study site, on the basis of experience, leadership abilities and availability for the duration of the project. That person attended the central training and standardisation sessions organised by the INTERGROWTH-21st Anthropometry Group.3
Training of anthropometry personnel
It is critical, because of the small size of the babies, that the anthropometric measurements being used to construct the standards are influenced by as little variation as possible. Therefore, standardised procedures were employed to maximise the validity of the indicators of growth and nutrition.
It was also vitally important that the research staff were able to develop, refine and maintain techniques to take accurate and precise measurements yielding repeatable or reproducible values. Before data collection, rigorous training and standardisation sessions were conducted at each study site, organised by the local lead anthropometrist and supervised by an anthropometry expert from the INTERGROWTH- 21st Anthropometry Group. Training materials were prepared by the INTERGROWTH-21st Project Coordinating Unit, based on the published MGRS protocols.1 During training sessions, the anthropometrists familiarised themselves with the equipment and measurement techniques. They watched the MGRS anthropometry training video (www.who.int/childgrowth/training/en/), which highlights key measurement techniques and calibration procedures. This was followed by a formal standardisation session with detailed instructions (see description elsewhere in this supplement).3
Measurement procedures
Fetal Growth Longitudinal Study
The infant measurements taken within 12 hours of birth in the longitudinal component of the INTERGROWTH-21st Project—the Fetal Growth Longitudinal Study (FGLS)—are weight, length and head circumference (HC). The mother’s weight is recorded when the fetal ultrasound scans are performed, i.e. at ~ 5-weekly (± 1 week) intervals throughout pregnancy. The heights of both parents are taken once during the study.
Preterm Postnatal Follow-up Study
All preterm infants (≥ 26+0 but < 37+0 weeks) born to mothers in the FGLS cohort enter the Preterm Postnatal Follow-up Study (PPFS) and are followed for 8 months after birth. Measurements are taken every 2 weeks for the first 8 weeks of life and then every month until 8 postnatal months of age (Table 1). The protocol allows measurements to be delayed by up to 10% of the child’s age4 (e.g. 2 days at 2 weeks, and 9 days at 3 months), but considerable effort is made to collect anthropometric data on the scheduled visit dates, particularly at the youngest ages. This is possible because of the relatively small number of preterm infants in PPFS at each study site.
Table 1.
Measurement schedule for infants in PPFS, with maximum allowable delays in parentheses.
| Visit | Age | Visit | Age |
|---|---|---|---|
| 0 | Within 12 hours of birth | 6 | 3 months (9 days) |
| 1 | 48–72 hours after birth | 7 | 4 months (12 days) |
| 2 | 2 weeks (2 days) | 8 | 5 months (15 days) |
| 3 | 4 weeks (3 days) | 9 | 6 months (18 days) |
| 4 | 6 weeks (4 days) | 10 | 7 months (21 days) |
| 5 | 8 weeks (5 days) | 11 | 8 months (24 days) |
Newborn Cross-Sectional Study
In the Newborn Cross-Sectional Study (NCSS), which aims to create birth-weight-for-gestational-age standards, the weight, length and HC of all newborns at each site during the study period are measured within 12 hours of birth. The heights of the mothers are measured before they are discharged from hospital.
Equipment selection
All study sites use the same measuring equipment, which was selected for accuracy, precision and robustness as demonstrated in previous, large, multicentre growth studies. Wherever possible, the same equipment is used as in MGRS.
Weight
Infant weight is measured using a portable, electronic weighing scale (Seca model 376; Seca, Hangzhou, China). It has a tare facility and weighs in kilograms to the nearest 5 g up to 7.5 kg, and to the nearest 10 g up to 20 kg. These scales are easy to use, and the tare function allows the baby to be covered in a blanket in cold climates and in cultures where it is unacceptable to undress the baby.
Recumbent length
Infant length is measured using the Harpenden Infantometer (range 300–1100 mm; Chasmors Ltd, London, UK), which has a fixed headboard and moveable footboard with a digital counter to 0.1 cm.
Head circumference
HC is measured using a self-retracting, flat metal tape measure (CMS ref. 3105; Chasmors Ltd), 0.7 cm wide, range 0–2 m, precise to 1 mm with an 8 cm blank lead-in. This tape was chosen because it is durable and stays in a single plane around the head.
Both the Harpenden Infantometer and the HC measuring tapes are identical to the equipment used in MGRS. Spare tape measures, batteries and digit counters are available at each study site in case replacements are needed. The SECA scale is classified as Class III and is approved for medical use. It was chosen for its high precision, which is essential when weighing newborns, especially small preterm infants. The scales are calibrated according to the gravitational force at each site, which varies by as much as 0.5% across the surface of the Earth.5
The Seca 877 Scale (Seca, Germany) was chosen to measure maternal weight because of its portability, stability and accuracy to the nearest 100 g. Scales were ordered with footmarks pasted on to show women where to stand. To measure maternal and paternal height, the Seca Stadiometer 242 (range 62–210 cm) (Seca, Germany) with an accuracy of ± 0.2 cm was selected. All adult height measurements are taken in clinic settings where the stadiometers are wall-mounted. The stadiometer head plate contains an opto-sensor, which transmits wirelessly to a digital display thereby minimising the possibility of introducing human error into the reading.
Calibration
All the equipment is calibrated twice weekly. Identical aluminium rods are used to calibrate the infantometer (40 and 75 cm) and stadiometer (150 and 200 cm) across all study sites (Chasmors Ltd). Certified calibration weights to cover the potential weight ranges for both infants (0.5–8 kg) and adults (40–80 kg) were purchased locally and are used by each study site to calibrate the scales.
Measurement protocols
Infant measurements
Several factors are taken into consideration when measuring newborns and infants. First, the measurer’s professional confidence and composure can do much to reassure the parents. Measurements can sometimes provoke anxiety and crying in infants, which can be distressing to the parents if they have not been reassured that these measurements are harmless. Second, older infants (such as the 6- to 8-month-old preterms followed up in PPFS) can resist, particularly during the length measurement. The help of the mother in reassuring and calming the older infant is useful in obtaining a precise measurement with minimum resistance from the infant. During all measurements, the indoor temperature should be comfortable for the infant and there should be enough light in the room.
The general operational procedures for obtaining the measurements are described below. For infants, all measurements are taken and recorded independently (blinded) by two anthropometrists measuring the baby once (one after the other). All measuring takes place either in the labour ward, nursery, neonatal intensive care unit or preterm follow-up clinic. Informed consent is obtained at enrolment.
The anthropometrists first introduce themselves to the parent(s) and explain the procedures. They answer any questions that the parents have and, if necessary, demonstrate the procedures to the parents. For some infants at certain study sites, it was necessary to remove hair clips or ornaments before measuring HC. This is measured first, followed by length and weight. If infants becomes agitated at any stage in the process, the anthropometrists wait for them to calm down before continuing. This is important for the parents, the babies and the measurement values.
Head circumference
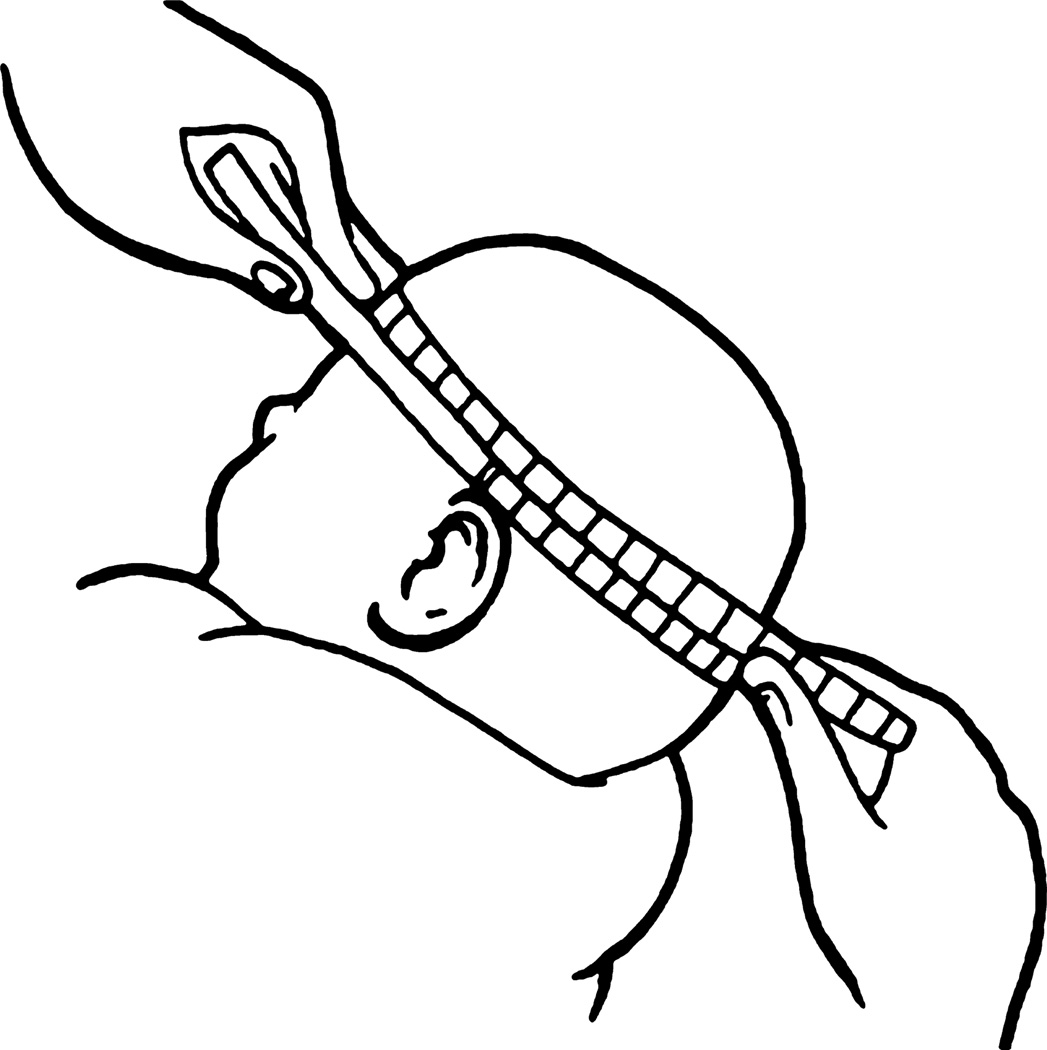
To measure HC, one anthropometrist positions the infant on his/her lap while supporting the head as still as possible. The other anthropometrist loops the tape (with the cm markings on the outside), with the zero end in the inferior position, before slipping it over the infant’s head. The tape is anchored just above the eyebrows, with the zero point on the side of the infant’s head closest to the anthropometrist taking the measurement. The forehead anchor point is important for standardised measurement within and across study sites. At the back of the head, the tape is positioned over the fullest protuberance of the skull. The other anthropometrist assists by positioning the tape correctly, i.e. level, on the other side of the infant’s head. Once the tape is positioned correctly, the anthropometrist pulls the tape tight to compress the hair and skin (Figure 1), being careful not to pull the tape too tight and cause discomfort, especially to newborns.
Figure 1.
Head circumference measurement.
Recumbent length
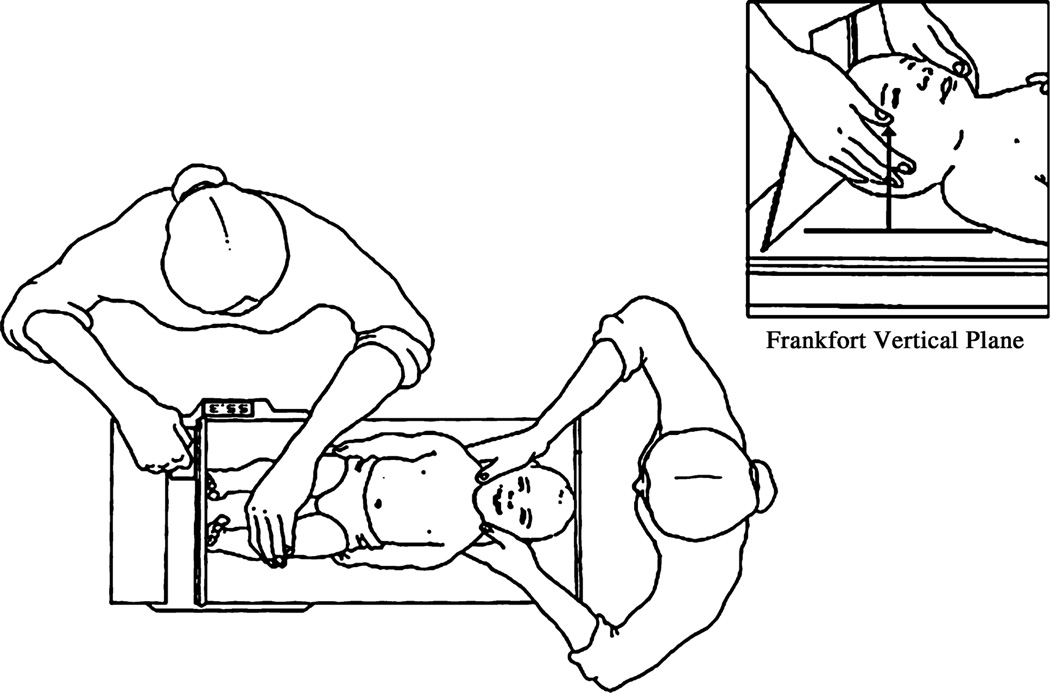
To measure recumbent length, the infantometer is placed on a raised, flat surface that is level and stable. The infant is undressed and positioned on the horizontal board, which is covered with a thin cloth or soft paper. The anthropometrist stands by the side of the infantometer with the digital counter so as to be able to read the measurement. With the left hand, the anthropometrist holds the infant’s legs, leaving the right hand free to manoeuvre the footboard. The second anthropometrist stands at the headboard and positions the infant’s head in the Frankfort Vertical Plane, with a vertical line between the ear canal and the lower border of the orbit of the eye running perpendicular to the horizontal board (Figure 2). The first anthropometrist positions the infant so that the shoulders and hips are aligned at right angles to the long axis of the body. Gentle pressure is applied to the knees to straighten the legs. The knees of newborns, especially preterm infants, are very delicate and therefore minimal pressure is applied so as not to cause any harm.
Figure 2.
Infant length measurement.
The anthropometrist slides the footboard towards the infant’s feet with the soles flat on the footboard and the toes pointing upwards. To straighten the feet upwards, the infant’s soles can be scratched slightly to initiate the Babinski reflex. The second anthropometrist checks that the infant’s spine is not arched when the reading is taken. The footboard is pressed against the feet to compress the soles slightly before the measurement is taken. Both length and HC are recorded in cm to the last completed (not the nearest) mm. For example, if the length value lay between 50.2 cm and 50.3 cm, the value is recorded as 50.2 cm.
Weight
Infant weight is measured last because of the need to remove all the infant’s clothes. If a blanket or cloth is required to wrap the infant, the scale is first tared with the item to give a zero reading. The infant is then placed carefully on the scale and once he/she stops moving the anthropometrist presses the ‘Hold’ button on the scale to obtain a stable weight and freeze the reading on the display. The value is then recoded on the data collection form.
In the case of an extremely sick infant in a neonatal intensive care unit, the incubator’s electronic scale (if available) is used, having been calibrated in the same way as the baby scales. For most babies in an incubator, HC is measured as described above. For those reliant on nasal continuous positive airflow pressure, the HC measurement is delayed until the nasal tubes are removed. Length is measured within the first 7 postnatal days, where possible, once the baby has been removed from the incubator. The date and time of all measurements, as well as the infant’s date and time of birth, are recorded on the data collection forms.
Each anthropometrist performs these three measurements as described above in sequence, after which they compare their values. The maximum allowable differences (0.7 cm for length, 0.5 cm for HC) are the same as those used in MGRS.1 Any pair of measurements outside those maximum allowed differences is repeated by both anthropometrists. If the repeat values exceed the limits for that measurement, the anthropometrists repeat the measurement for a third and final time. After a third failed repeat measurement, the likelihood of obtaining an accurate measurement is very small, as the infant is likely to be distressed and uncooperative by this point. All measurements are entered on the form, which is checked for completeness before ending the session.
Adult measurements
For FGLS, the mother’s height and weight are taken at study entry twice by the same anthropometrist. Thereafter, weight alone is taken at each study visit. The father’s height is obtained once during the study. For NCSS, maternal height is recorded once before hospital discharge. Prepregnancy weight is obtained from the medical records.
To measure height, women remove their shoes and any headwear, and stand up straight with their back to the measuring rod with feet slightly apart, the trunk balanced over the waist, knees straight, arms and shoulders relaxed. The subject’s head is positioned in a Frankfort horizontal plane, with a horizontal line between the lower border of the orbit of the eye and the opening of the ear canal, running perpendicular to the vertical rod of the stadiometer. The headboard is moved down until it touches the top of the head, and the subject is asked to take in a deep breath, which straightens the spine. The measurement value is read from the digital display and recorded in centimetres to one decimal place.
To measure weight, women are asked to wear minimal, light clothing to their appointments. If it is not possible for a woman to remove heavier clothing, the total weight of her clothing is calculated (based on the list of weights of typical items prepared by each study site), and subtracted from her weight. Women remove their shoes and stand on the scale placing their feet on the footmarks. They remain still until a stable weight value appears. The scale is always placed on a hard flat surface with no obstructions.
A detailed manual with instructions for carrying out all the infant and adult measurement techniques, as well as the procedures for the calibration and maintenance of equipment, was prepared by the INTERGROWTH-21st Anthropometry Group.4 The manual was translated where necessary and copies were sent to all study sites. This printed reference material and the MGRS training video are used to train any new anthropometrists recruited during the course of the project.
Discussion
The rigorous anthropometric protocols described above were implemented at each study site to ensure that the anthropometric data were of the highest quality to construct international standards for fetal growth, postnatal growth of preterm infants, and birthweight for gestational age. The earlier MGRS protocols were used as templates; the procedures used in MGRS were robust and accurate in a multicentre research setting and were therefore adapted for the INTERGROWTH-21st Project. Wherever possible, the MGRS protocol was adhered to, with only minor adaptations made to suit the needs of the INTERGROWTH-21st Project: for example, the measurement of preterm infants. This conformity allows for continuity between these two large, multicentre studies. Similar continuity has occurred for other large, national, multicentre studies.1
Anthropometric measurements are the result of a complex process of interactions in the setting in which the measurements are taken, including the techniques of the measurers, the behaviour of the subject, and the accuracy and precision of the instruments. All these factors were considered in the design of the anthropometry component of the INTERGROWTH-21st Project. Key aspects included the selection and calibration of the equipment; the use of standardised measurement procedures by all personnel; highquality training conducted by experts, and frequent standardisation sessions.3 Together, these factors serve to control variability both within and between study sites so that the data can be combined into a single data set for the purposes of constructing the new international growth standards.
Interestingly, the overall quality of infant measurements in the health institutions participating in the INTERGROWTH-21st Project appears to have improved with the introduction of these anthropometry protocols. Many of the health institutions have adopted the additional measurements as part of their routine clinical care, thereby providing an important practical contribution to the effective monitoring of infant growth in these centres around the world.
Acknowledgements
A full list of Members of the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH- 21st) and its Committees appears in the preliminary pages of this supplement.
Funding
This Project was supported by the INTERGROWTH-21st Grant ID# 49038 from the Bill & Melinda Gates Foundation to the University of Oxford, for which we are very grateful.
Footnotes
Disclosure of interests
None.
Contribution to authorship
L Cheikh Ismail and W Chumlea wrote the manuscript and all the authors read and approved the final version. All line drawings were prepared by Stuart Chromik.
Details of ethics approval
The INTERGROWTH-21st Project was approved by the Oxfordshire Research Ethics Committee ‘C’ (reference:08/ H0606/139) and the research ethics committees of the individual participating institutions and corresponding health authorities where the Project was implemented.
References
- 1.de Onis M, Onyango AW, Van den Broeck J, Chumlea WMC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(Suppl 1):S27–S36. doi: 10.1177/15648265040251S104. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Altman DG, Purwar M, Noble JA, Knight HE, Ruyan P, et al. for the International Fetal and Newborn Growth Consortium (INTERGROWTH-21st) The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG. 2013 doi: 10.1111/1471-0528.12047. [DOI] [PubMed] [Google Scholar]
- 3.Cheikh Ismail L, Knight HE, Ohuma EO, Hoch L, Chumlea WC for the International Fetal and Newborn Growth Consortium (INTERGROWTH-21st) Anthropometric standardisation and quality control protocols for the construction of new international fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG. 2013 doi: 10.1111/1471-0528.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.INTERGROWTH-21st Anthropometry Group. Anthropometry Manual for the INTERGROWTH-21st Project. [Accessed 21 January 2011]; Available at [ www.intergrowth21.org.uk]. [Google Scholar]
- 5.Cornell University. [Accessed 7 October 2010]; [ http://curious.astro.cornell.edu/question.php?number=310]. [Google Scholar]