Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transduced, irradiated tumor vaccines induce potent, T-cell-mediated antitumor immune responses in preclinical models. We report the initial results of a Phase I trial evaluating this strategy for safety and the induction of immune responses in patients with metastatic renal cell carcinoma (RCC). Patients were treated in a randomized, double-blind dose-escalation study with equivalent doses of autologous, irradiated RCC vaccine cells with or without ex vivo human GM-CSF gene transfer. The replication-defective retroviral vector MFG was used for GM-CSF gene transfer. No dose-limiting toxicities were encountered in 16 fully evaluable patients. GM-CSF gene-transduced vaccines were equivalent in toxicity to nontransduced vaccines up to the feasible limits of autologous tumor vaccine yield. No evidence of autoimmune disease was observed. Biopsies of intradermal sites of injection with GM-CSF gene-transduced vaccines contained distinctive macrophage, dendritic cell, eosinophil, neutrophil, and T-cell infiltrates similar to those observed in preclinical models of efficacy. Histological analysis of delayed-type hypersensitivity responses in patients vaccinated with GM-CSF-transduced vaccines demonstrated an intense eosinophil infiltrate that was not observed in patients who received nontransduced vaccines. An objective partial response was observed in a patient treated with GM-CSF gene-transduced vaccine who displayed the largest delayed-type hypersensitivity conversion. No replication-competent retrovirus was detected in vaccinated patients. This Phase I study demonstrated the feasibility, safety, and bioactivity of an autologous GM-CSF gene-transduced tumor vaccine for RCC patients.
INTRODUCTION
Cancer cell vaccines modified to secrete cytokines by ex vivo gene transfer generate antitumor immunity in preclinical models (1–13). A comparison involving multiple cytokine genes found that GM-CSF gene-transduced vaccines were the most potent inducers of long-lasting, specific tumor immunity even in poorly immunogenic tumor models (7). The efficacy of GM-CSF3-transduced vaccines has been shown in preclinical models of melanoma, colon cancer, renal cancer, lung cancer, lymphoma, and prostate cancer (8–15). The therapeutic activity of GM-CSF involves the paracrine (local) action of the cytokine at the vaccine site in activating APCs. These GM-CSF-activated APCs include dendritic cells (the most potent APCs for T cells known) and macrophages (8–15). Systemic antitumor immunity is mediated subsequently by APC priming of CD4+ and CD8+ T cells, which recognize tumor-associated antigens at metastatic sites (8–17). The word “vaccine” is used in this context as the genetically engineered antigen source (cancer cell) for activation of immune responses against established metastatic cancer, as opposed to prophylactic immunization.
Clinical translation of this approach involves tumor resection, culture of the cancer cells, and ex vivo GM-CSF gene transfer, followed by patient vaccination with the genetically modified, lethally irradiated autologous cancer cells (18, 19). RCC was chosen to evaluate this strategy of ex vivo gene therapy, given large yields of tumor cells at surgery and the potential responsiveness of RCC to immunotherapies (18). The retroviral vector MFG permitted high efficiency GM-CSF gene transfer in primary RCC cultures without drug selection (18–20). With this vector, successful establishment of a permanent tumor cell line is not required for each patient, and primary culture potentially maintains a greater diversity of RCC antigens (18, 19).
Patients were randomized to receive escalating vaccine cell doses of lethally irradiated, autologous RCC cells expanded in short-term culture and transduced with the human GM-CSF gene or equivalent doses of expanded, irradiated, nontransduced RCC cells. Nontransduced, cultured RCC secretes low levels of GM-CSF; therefore, randomization was performed to distinguish toxicities related to vaccine cell expansion from toxicities due to higher levels of GM-CSF secretion from human GM-CSF gene transfer. Randomization was double-blind until the conclusion of both toxicity and immunological evaluation to objectively measure biological effects in patients without bias toward the gene therapy arm. The primary objectives were: (a) to evaluate the safety and distinguish toxicities of injections of cultured, lethally irradiated, autologous RCC cells from similarly prepared RCC cells transduced with the human GM-CSF gene, secreting the cytokine at greater than 40 ng/106 vaccine cells/24 h; and (b) to assay both in vitro and in vivo the additional contribution, if any, of GM-CSF gene transduction to induction of tumor immunity induced by irradiated, autologous RCC vaccines.
PATIENTS AND METHODS
Selection of Patients
The protocol has been published previously (19). Patients with stage III T4b (inferior vena cava tumor thrombus above the level of the diaphragm), or stage IV (metastatic) RCC (histologically confirmed after surgery) were eligible. Systemic IL-2-based regimens or other investigational agents were also offered as treatment options for these patients. Eligibility criteria included primary RCC in place; Eastern Cooperative Oncology Group performance status of 0 or 1; appropriate surgical candidate (21) and estimated life expectancy of at least 6 months; no major surgery, radiotherapy, chemotherapy, immunotherapy, or immunosuppressive medications within 1 month prior to enrollment; age ≥18 years; absence of active infection, WBC count ≥ 4,000/µ1, platelets ≥ 100,000/µl, total bilirubin ≤ 1.5 mg/dl, and creatinine ≤2.0 mg/dl; HIV seronegative; no history of autoimmune disease. The study was reviewed and approved by the Johns Hopkins Joint Committee on Clinical Investigation, Institutional Biosafety Committee, Food and Drug Administration, and the NIH Recombinant DNA Advisory Committee.
Study Design
Patients were enrolled from November 1993 to May 1995. After nephrectomy and after informed consent was obtained, patients were randomized to receive autologous vaccine prepared either with or without GM-CSF gene transfer (Table 1; Ref. 19). Rules for dose escalation have been described previously (19). Three fully evaluable patients were vaccinated on each arm of the trial prior to escalation of dose level. Patients had history, physical examination, and laboratory tests 1 week prior to vaccination. Radiography and physical examinations were performed before surgery, before each vaccination, and monthly after each vaccination. Patients received vaccine at the assigned dose and were evaluated for 28 days. Vaccine dose was not escalated between treatments in individual patients. In the event of stable disease, clinical improvement, low toxicities, and sufficient vaccine cells remaining, additional vaccinations every 28 days were allowed to assess cumulative toxicities.
Table 1. Vaccination cell dose and route of administration.
Autologous irradiated RCC vaccines with or without human GM-CSF gene transfer were administered at the assigned dose level; 50% of total dose was given intradermally and 50% s.c. to compare patient tolerability of the two routes of administration.
| Dose level | Autologous cell dose |
Intradermal injections |
Cell dose and volume | s.c. injections |
Cell dose and volume |
|---|---|---|---|---|---|
| 1 | 4 × 106 cells | 2 | 1 × 106 cells/0.5 cm3 | 2 | 1 × 106 cells/0.5 cm3 |
| 2 | 4 × 107 cells | 2 | 1 × 107 cells/0.5 cm3 | 2 | 1 × 107 cells/0.5 cm3 |
| 3 | 4 × 108 cells | 16 | 1.2 × 107 cells/0.6 cm3 | 5 | 4 × 107cells/2cm3 |
Peripheral blood was obtained (as per NIH Recombinant DNA Advisory Committee and Food and Drug Administration guidelines) for detecting RCR before treatment, after each vaccination, monthly for 3 months, every 3 months for the next 9 months, and then yearly. Long-term follow-up included a 6-month evaluation for autoimmune disease. The plasma pharmacokinetics of systemic absorption of GM-CSF were monitored as described (11, 19). Toxicity studies, including autoimmune serologies, were graded using the National Cancer Institute Common Toxicity Criteria.
Vaccine Preparation and Administration
Methods of autologous RCC vaccine preparation and MFG-GM-CSF gene transfer have been described previously (18, 19). Briefly stated, histopathologically confirmed RCC tissue was mechanically disassociated and transported to Somatix Therapy Corporation. Primary cultures were established and transduced at first passage (for vaccines on the gene transfer arm of the study). Following in vitro expansion, vaccine cells were irradiated at 150 Gy to prevent clonogenic survival; tested for GM-CSF production (R&D ELISA), microbial contaminants, and RCR; and stored in liquid nitrogen. On the day of vaccination, the cells were thawed, washed three times with HBSS, checked for viability by trypan blue exclusion, and administered with 1-cc syringes with 25 gauge needles. Table 1 describes the cell doses used. To compare local toxicities from different routes of administration, one-half of the total cell dose was administered intradermally and one-half s.c. in either thigh or abdomen. Viable vaccine cells were measured by trypan blue exclusion and then formulated to meet dose at each dose level. For the study, the range of viable, total vaccine cells following thawing was 54–96% (mean, 76.4%; SD, 10.1%).
Monitoring Patient Immune Function
To evaluate the status of each patient’s cell mediated immunity before and after treatment, DTH testing was performed using seven common recall antigens (Multitest CMI, Connaught Laboratories; Refs. 22–27). Simultaneously, patients were tested for reactivity against autologous, irradiated RCC obtained at nephrectomy. RCC cells for DTH testing were prepared from the surgical specimen by enzymatic digestion with collagenase IV (Sigma Chemical Co.); cells were washed and frozen in 90% fetal bovine serum and 10% DMSO until the day of testing. Cells were irradiated with 150 Gy. RCC cells were washed three times with HBSS and frozen in liquid nitrogen until the day of testing. On the day of DTH testing, unpassaged DTH cells were thawed, washed in 20 volumes of HBSS, counted, and injected at 106 cells/0.2 ml intradermally. Induration was measured at 48 h as described previously (23). DTH was performed 5 days prior to initial treatment to generate a baseline and 1 month after each treatment.
Histological Studies
Six-mm punch biopsies were performed on days 3 and 7 following the first vaccination at both the s.c. and intradermal injection sites. A prevaccination skin biopsy was obtained for comparison. Biopsies were fixed in 10% neutral buffered formalin, paraffin embedded and stained with H&E, Leder stain (granulocytes), HAM56 (macrophages), S100+ (dendritic cells), OKT3 (T cells), MBP (eosinophils), and L26 (B cells; all from DAKO Corp., Carpinteria, CA). The antibody reagents used to delineate dispersed tumor vaccine cells were targeted at cytokeratins (antibodies AE1 and AE3 from Boehringer Mannheim, Indianapolis, IN). A representative tissue section (5 µm) from formaldehyde-fixed, paraffin-embedded tissues was stained with two antibodies specific for granule proteins found within the eosinophil: EG2 (IgGl, antieosinophil cationic protein, Kabi Pharmacia Diagnostics, Uppsala, Sweden) and MBP (polyclonal, anti-MBP, G. Gleich, Mayo Clinic). EG2 staining was performed using the Vectastain ABC-AP kit and the Vector Red Substrate Kit (Vector Laboratories, Burlingame, CA; Ref. 28). An indirect immunoflurorescent assay, described previously, using a polyclonal antibody to eosinophil MBP, was used to semiquantify and localize these granule proteins to intracellular or extracellular locations (29, 30). A species-matched, immunoglobulin subtype-matched protein was used as a negative control for each antibody; nasal polyposis tissue served as positive control tissue.
All EG2-stained specimens were scored by two independent investigators (L. A. B. and S. D.) in a blinded fashion and presented as number of positively staining cells/mm (2). The MBP staining was scored by one blinded investigator (K. M. L.) and one blinded technician; the results were averaged and did not differ by more than 0.5 between the scorers. MBP staining was quantified using a scoring system from 0 to 3+ based on the extent and intensity of the fluorescent stain (31).
Slides were evaluated under code with the pathologist blinded to patient and transduction status. Variables ranked were number of cells stained with the above-mentioned antibodies per high-powered field. Only vaccine sites with tumor cells present (cytokeratin-positive cells) were ranked. For each cell type studied, the slides were ranked from lowest to highest by repeatedly comparing slides until the ranking was achieved.
Statistical Features of the Trial Design
This was a dose-finding trial intended to evaluate the maximum safely tolerated dose of vaccine cells prepared with or without GM-CSF gene transfer. A standard type of dose escalation was used, treating three patients on each treatment arm at each dose level, and escalating to the next higher dose if fewer than two patients experienced dose-limiting toxicity (19). This clinical trial also used an unusual design. To increase objectivity, investigators and patients remained blinded with respect to gene transfer until toxicity and immunological evaluation was completed. Patients were randomly assigned to treatment arms consisting of vaccine cells alone or with MFG-GM-CSF-transduced cells. The structure of this trial, although it was formally a double-blind, randomized trial, was not intended to make efficacy comparisons with high statistical power. Prior to lifting the blind, all clinical, immunohistological, and immunological data acquired were compiled.
RESULTS
Autologous Vaccine Yield and Gene Transfer
In this trial, primary RCC cultures were generated from large, advanced cancers with significant areas of necrosis. The rate of successful vaccine cell expansion at dose level 1 was 70%, (7 of 10), dose level 2 was 88% (8 of 9), and dose level 3 was 20% (2 of 10). If vaccine yield was insufficient to treat at the dose level assigned, patients were treated at the next lower dose level (19). A single transduction with MFG-GM-CSF generated GM-CSF secretion greater than 40 ng/106 cells/24 h in eight of nine cases. Human GM-CSF cDNA gene copy number after a single transduction with MFG in transduced vaccines ranged from 0.1 copy/haploid genome to 1 copy/haploid genome (mean, 0.5 copy/haploid genome) by quantitative Southern blot analysis. The GM-CSF secretion by nontransduced vaccines ranged from 0 to 19 ng/106 cells/24 h, compared to 42–149 ng/106 cells/24 h after human GM-CSF gene transfer (Table 2). In preclinical models, the expression of paracrine GM-CSF greater than 35 ng/106 cells/24 h by vaccine cells induced antitumor immunity (8, 10–15).
Table 2. Autologous vaccine GM-CSF secretion by dose level.
Transduction for patient 2 fell below the specifications of the trial (17 ng/106 cells), and patient 27 was treated on dose level 2 on a compassionate basis, because of failure to achieve dose level 3 yields. Patient 28 did not have enough vaccine for one fully evaluable treatment at dose level 2 and was treated with 2 × 107 cells. These patients were treated as if specifications were met and were evaluated for all safety and toxicity endpoints but did not occupy a full evaluable patient treatment position for the dose escalation rules. Patient 25 did not have confirmed metastatic disease following surgery and was not treated.
| Without GM-CSF gene transfer GM-CSF, ng/106 cells/24 h |
With GM-CSF gene transfer |
||
|---|---|---|---|
| Patient | GM-CSF, ng/106 cells/24 h |
MFG-GM-CSF copies/genome |
|
| Dose level 1 | |||
| 1a | 1 | ||
| 3a | 1 | ||
| 5a | Not tested | ||
| 2a | 17 | 0.6 | |
| 6a | 95 | 1.0 | |
| 8a | 99 | 0.7 | |
| 11a | 51 | 0.8 | |
| Dose level 2 | |||
| 12a | 1 | ||
| 13 | 19 | ||
| 18a | 1 | ||
| 19a | 9 | ||
| 16a | 42 | 0.2 | |
| 23a | 85 | 0.2 | |
| 24a | 149 | 0.5 | |
| 25 | 66 | 0.4 | |
| 27a | 6 | ||
| 28 | 69 | 0.1 | |
| Dose level 3 | |||
| 22a | 7 | ||
| 26a | 6 | ||
Patients fully evaluable for toxicity and immunological effects.
Safety of Administration and Systemic Toxicities
Patient characteristics are described in Table 3. The cohort was not heavily pretreated. Of the 33 patients enrolled, 18 ultimately received vaccine therapy. Of 28 patients with confirmed RCC at surgery, one patient did not have metastatic RCC, seven patients’ primary culture failed to meet vaccine cell yield specifications even for dose level 1, and two patients had progressive disease requiring radiotherapy and steroids for palliation prior to treatment, making them ineligible. No surgical complications were encountered precluding subsequent vaccination. Vaccine yield and clinical status permitting, multiple vaccinations were allowed for the study of cumulative side effects. Three patients received one vaccination, six received two vaccinations, seven received three vaccinations, and two received four vaccinations. Sixteen patients received 39 fully evaluable, 28-day treatment cycles through a dose escalation of 100-fold for vaccine cell dose. A fully evaluable patient was vaccinated at the assigned dose level with a 28-day period of toxicity and immunological assessment (19). However, progression of disease limited study of multiple vaccinations. In vitro expansion of vaccine cells would have permitted five of seven fully evaluable patients at dose level 1 to receive three vaccinations, six of seven patients at dose level 2, and two of two patients at dose level 3. The study was stopped before full dose level 3 accrual was completed, because dose level 3 vaccine yields were not regularly attainable.
Table 3.
Characteristics of treated patients
| Characteristic | No. |
|---|---|
| No. treated (fully evaluable) | 18(16) |
| Median age | 59 |
| Range | 44–79 |
| Male/female | 10/8 |
| Performance status (ECOGa) = 0 | 18 |
| Prior therapy | |
| IL-2 | 1/18 |
| Radiotherapy | 1/18 |
Eastern Cooperative Oncology Group.
No dose limiting systemic or cutaneous toxicities were observed in either arm of the trial (Table 4). The two most concerning potential toxicities, vaccine site-specific ulceration and development of acute autoimmune disease (specifically, nephritis in uninephric patients), were not observed. In addition, this was the first human clinical trial using the MFG retroviral vector. No RCR was detected in patients receiving MFG-GM-CSF gene-transduced vaccines.
Table 4. Adverse clinical effects associated with RCC vaccine.
Episodes of toxicity. Toxicities, where applicable, are graded by National Cancer Institute Common Toxicity Criteria.
| Dose level 1 |
Dose level 2 |
Dose level 3 |
|||
|---|---|---|---|---|---|
|
−GM-CSF gene |
+GM-CSF gene |
−GM-CSF gene |
+GM-CSF gene |
−GM-CSF gene |
|
| No. of fully evaluable patients | 3 | 4 | 4 | 3 | 2 |
| No. of fully evaluable cycles | 7 | 8 | 13 | 7 | 4 |
| Systemic side effects | |||||
| Fever | 1a | ||||
| Nausea/vomiting | 1b | ||||
| Constipation | 3c | ||||
| Pruritus (non-vaccine site) | 2 | 2 | |||
| Urticaria without bronchospasm | 2d | ||||
| Myalgia | 2 | ||||
| Other deep vein thrombosis | 1e | ||||
| Clinical evidence of autoimmune disease | |||||
| Renal function change: creatinine/proteinuria | |||||
| Thyroid | |||||
| Rheumatoid arthritis | |||||
| Lupus | |||||
| Acute vaccine site effects | |||||
| Injection site erythema ID/SQ | 7/0 | 6/0 | 13/3 | 7/4 | 4/4 |
| Site tenderness/swelling | 6ae | 7e | 2e | ||
| Pruritus | 2 | 3f | 1 | ||
Grade 2.
Grade 1.
Grade 2, 2 episodes; Grade 1, 1 episode; same patient.
1 episode at DTH site, 1 episode at vaccine site; same patient.
Occurred on nonvaccinated limb in a patient on bed rest for non-cancer-related back injury.
Intermittent pruritus in patient 24.
The apparent lack of acute, systemic toxicities in this trial was also paralleled by the lack of plasma elevations of GM-CSF in pharmacokinetic studies following treatment at each dose level, independent of gene transfer (data not shown). Long-term toxicity and immunological follow-up was limited by short survival; 13 of 18 treated patients died of RCC progression within 12 months of their initial vaccination, despite further therapy with IL-2, other investigational agents, or the best supportive care.
Cutaneous Reactions at Vaccination Sites
Cutaneous reactions at vaccination sites were clearly cell dose dependent. At dose level 1 (1 × 106 cells at four vaccination sites), little erythema, pruritus, or induration was noted at either the s.c. or intradermal injection sites, irrespective of GM-CSF gene transfer. Moderate to beefy red erythema occurred at dose levels 2 and 3 at both the untransduced and transduced intradermal vaccine sites (1.0 × 107 to 1.2 × 107 cells per vaccination site), with the maximum measure of erythema and induration noted between 24 and 48 h. At dose levels 2 and 3, vaccine site edema resolved in 2 days without medical intervention. One patient (patient 24), who experienced a partial regression of metastases (see below), experienced unique symptoms at the vaccine sites after treatment with transduced vaccine. In addition to edema encompassing the anterior thigh, draining inguinal lymph nodes were tender to palpation for 7 days after each vaccine cycle. Within minutes of receiving the third cycle of GM-CSF gene-transduced vaccine cells, this patient had intense vaccine site pruritus and generalized pruritus on the scalp, neck, and abdomen, but not generalized urticaria, bronchospasm, hypotension, or eosinophilia. Resolution occurred within 20 min without medical management. Up to 6 months after completion of vaccination, this patient experienced episodic pruritus at former vaccine sites.
Histopathology of the Vaccine Site
The histopathology of the vaccine sites was evaluated blind to treatment assignment. Cytokeratin staining of biopsies for RCC vaccine cells at 3 and 7 days following first vaccination, compared to the pretreatment control biopsy, showed that intradermal vaccine sites were more informative than s.c. sites. Thirty-two of 33 (98%) intradermal site biopsies had tumor vaccine cells present, whereas only 7 of 33 (21%) s.c. site biopsies had identifiable tumor vaccine cells; these cells were more dispersed than those of the intradermal site biopsies. Individual tumor cells, however, were clearly identifiable with cytokeratin staining as single-spindled to ovoid cells with enlarged nuclei. At all dose levels, regardless of GM-CSF gene transfer, reactive fibroblasts in a loose basophilic matrix regularly surrounded the tumor cells. At dose level 1, independent of gene transfer, and with untransduced tumor vaccines at dose levels 2 and 3, the fibroblastic response predominated over inflammatory cell infiltration compared to GM-CSF-transduced vaccine biopsies.
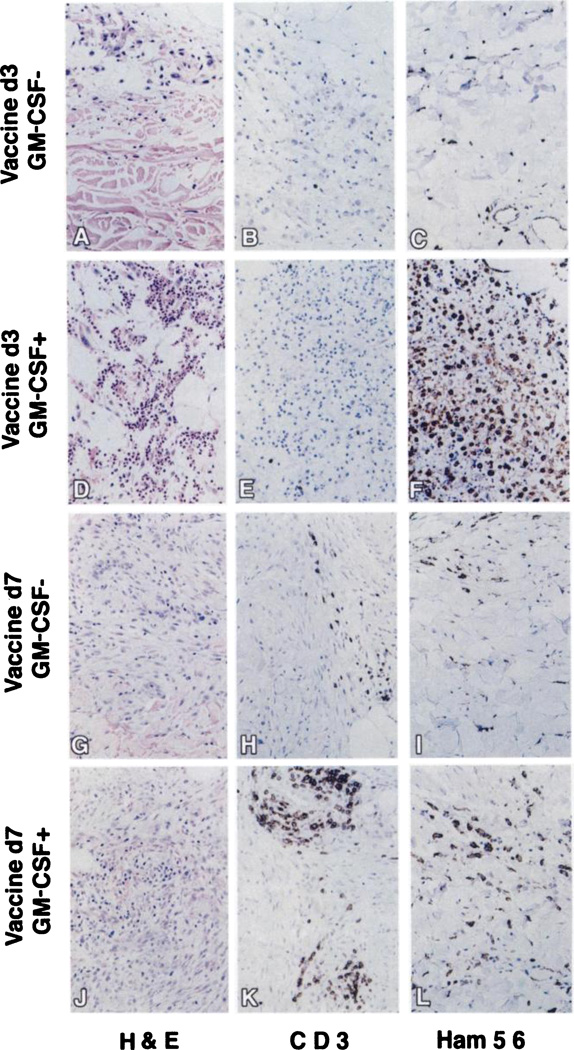
The intensity of cellular infiltration at the intradermal vaccine sites correlated with increasing total vaccine dose, and the phenotypes of the infiltrating cells were affected by GM-CSF transduction and time of biopsy. Compared to the minimal inflammatory cell infiltrates at dose level 1, dose level 2 biopsies were highly informative. At dose level 2, 3 days postvaccination, the GM-CSF-transduced tumor vaccine sites had more abundant macrophages (Ham56+) and granulocytes (H&E and Leder staining) compared to untransduced sites (Fig. 1, C versus F and A versus D). Most granulocytes were neutrophils by H&E and Leder staining (Fig. 1D), but eosinophils were evident by H&E and MBP staining. Few CD3+ T lymphocytes were present on day 3 (Fig. 1, B and E). Peritumoral S100+ cells, consistent with dendritic cells, were rare. B lymphocytes were not detected in either transduced or nontransduced vaccine sites (data not shown). By 7 days after vaccination, granulocyte and macrophage infiltration lessened (Fig. 1, I and L), and the predominant infiltrating cell type was CD3+ T lymphocytes. GM-CSF-transduced vaccine sites had distinctly more CD3+ T infiltrating cells than nontransduced sites (Fig. 1, H versus K). Peritumoral dendritic cells increased at day 7 following vaccination in the GM-CSF-transduced vaccines relative to untransduced biopsies (data not shown).
Fig. 1.
Photomicrographs of day 3 and day 7 vaccine sites at dose level 2. Representative intradermal biopsies at day 3 and day 7 in patients treated with or without MFG-GM-CSF-transduced RCC vaccines. H&E staining shows vaccine cells and inflammatory cellular infiltrates (A, D, G, and J). T-cell-specific staining using the CD-3 marker are represented (B, E, H, and K). Macrophage-specific Ham-56 staining is represented (C, F, I, and L). In every patient, a prevaccine site negative control was run for background staining. Cytokeratin-positive vaccine cells were evident by immunohistochemistry in each representative tissue section (data not shown). ×200.
Induction of Systemic Immune Responses
As in previous tumor vaccine studies, DTH tests in this trial served as a qualitative measurement of T-cell response (22–27). DTH was measured as bidimensional induration at 48 h at the site of test antigen administration (23). Patients were tested 48 h prevaccination for T-cell anergy to 7 common recall antigens using the Multitest CMI (Connaught Laboratories). With the exception of two anergic patients enrolled at dose level 1, all subjects had CMI DTH scores within the range of normal volunteers or patients with localized cancer (24).
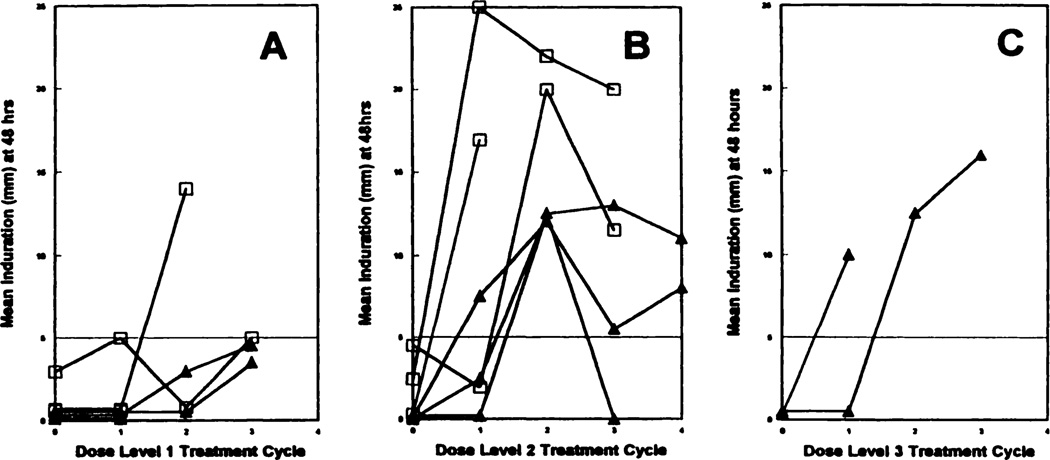
With the exception of two patients, significant DTH reactivity (≥5 mm) to unpassaged, irradiated autologous tumor cells (RCC) was not observed in patients prior to treatment (Fig. 2). Significant DTH reactivity was not noted at dose level 1. However, at dose level 2, DTH conversions to RCC were observed in patients receiving both transduced and nontransduced vaccine cells (Fig. 2). A trend toward increased DTH reactions was observed in GM-CSF-transduced vaccines at dose level 2, but the study was too small to estimate a statistical difference. In addition to induration, cutaneous reactions surrounding the indurated areas of DTH sites were noted in some patients. Twenty-eight days after first treatment with GM-CSF-transduced vaccine, patient 24 had an 80 × 80-mm area of patchy erythema surrounding the largest indurated RCC cell DTH conversions recorded in the trial. Concomitantly, this patient experienced regression of multiple pulmonary metastases on CT scan (see below). After three vaccinations, edematous, macular erythematous skin reactions surrounding RCC autologous cell DTH sites were noted in patients 23, 24 (GM-CSF-transduced arm, dose level 2), and 26 (nontransduced arm, dose level 3). DTH responses were also seen against autologous normal kidney cells, and to a much lesser extent against peripheral blood lymphocytes; however, the possibility that some of the reaction is against residual contaminating collagenase or fetal bovine serum precludes drawing conclusions about antigen specificity.
Fig. 2.
DTH reactivity at 48 h to autologous irradiated RCC was measured as described previously. The mean of the tridimensional measure of induration at ≥5 mm is considered a positive conversion from vaccination (23). The horizontal line at 5 mm defines reactivity □. GM-CSF-transduced vaccines; ▲, untransduced vaccines. A, dose level 1; B, dose level 2; C, dose level 3. The time course of DTH measurement is plotted for every evaluable patient treated at his or her respective dose level.
Histological Evaluation of DTH Sites
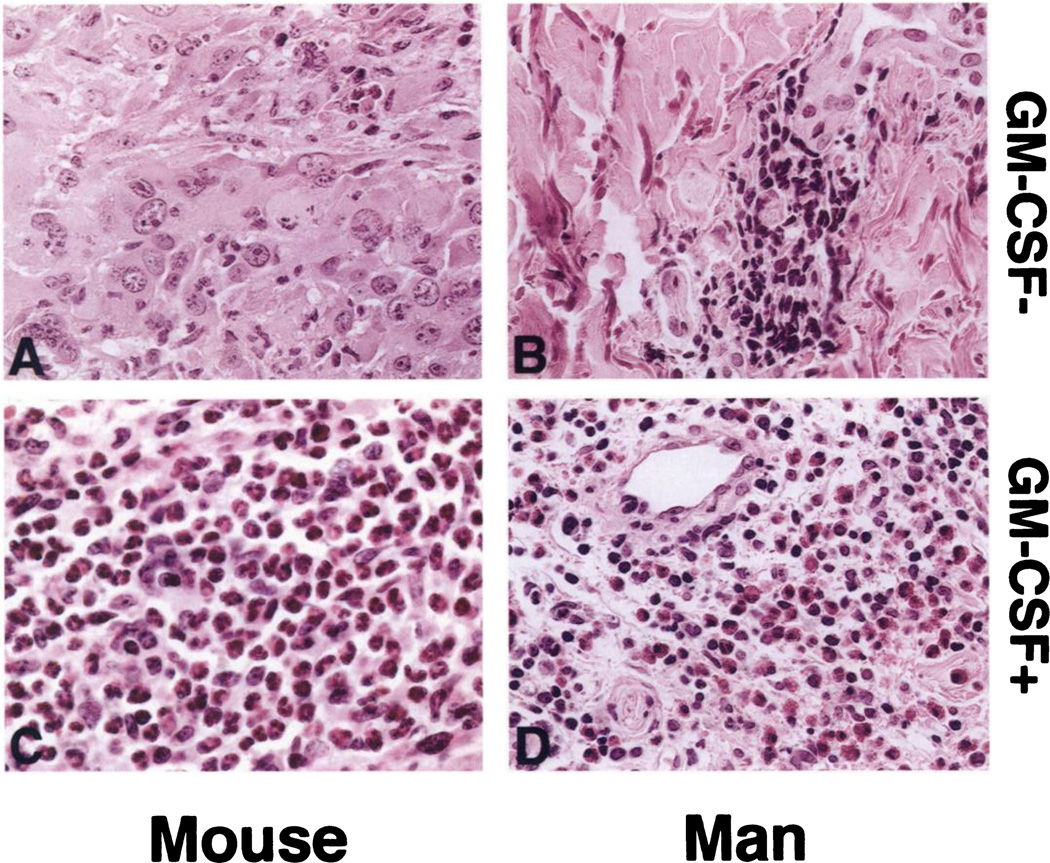
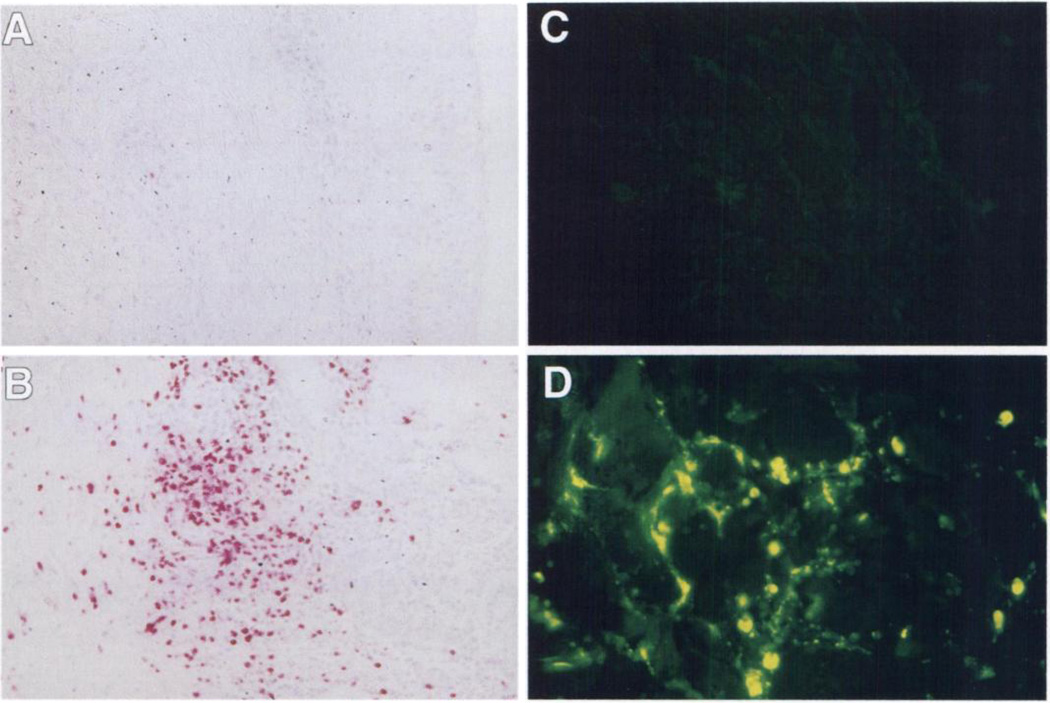
DTH biopsies manifested a cellular infiltration pattern similar to that generated by autologous irradiated tumor cell DTH testing in mice treated with GM-CSF-transduced vaccines (Fig. 3). In all patients, the characteristic DTH response consisted of mononuclear cell infiltration and perivascular cuffing by lymphocytes. Notably, an intense eosinophil infiltration was present at the reactive DTH sites of patients treated with GM-CSF-transduced vaccines at dose level 2, which was not observed in DTH biopsies of patients receiving nontransduced vaccines (Figs. 3, B and D, and 4). This eosinophil infiltration closely mirrors a difference observed between the DTH response of GM-CSF-transduced versus nontransduced tumor vaccines in murine tumor models (Fig. 3, A and C; Refs. 8 and 9). Staining for EG2 and MBP, a major secretory protein specific to eosinophil granules, was intense in the DTH biopsies from patients vaccinated with GM-CSF-transduced vaccines. Furthermore, much of the MBP had been released into the interstitial tissues, indicating significant degranulation characteristic of activated eosinophils (Fig. 4).
Fig. 3.
Eosinophils predominate in the DTH reaction following vaccination with MFG-GM-CSF-transduced vaccines. Representative H&E-stained sections of posttreatment DTH biopsies of mice vaccinated with untransduced irradiated B16 melanoma vaccine (A) are compared with those transfected with MFG-GM-CSF vector secreting GM-CSF at 360 ng/106 cells/24 h (C), autologous irradiated human RCC (B), and irradiated RCC transduced with MFG-GM-CSF (D). For both murine and human DTH tests, the cell preparations, including freezing in fetal bovine serum, irradiation, and cell innocula, were equivalent. Eosinophils were quantitated as described in “Patients and Methods” for intradermal biopsies. Three eosinophils/200× hpf were seen in the DTH site of patient vaccinated with nontransduced RCC and 588 eosinophils/200× hpf in the DTH site of patient vaccinated with GM-CSF-transduced RCC. ×400.
Fig. 4.
EG2 and MBP staining of DTH reactions following vaccination with GM-CSF-transduced and untransduced RCC vaccines. Post-dose level 2 treatment DTH biopsies from the same samples presented in Fig. 3 [untransduced (A and C) and GM-CSF-transduced (B and D)] were stained with anti EG2 (A and B) and MBP (C and D) as described in “Patients and Methods.” Mean number of eosinophils in the biopsies (quantitated as described in “Patients and Methods”) was 3 EG2 positive cells/mm2 for A (untransduced) and 588 EG2-positive cells/mm2 for B (GM-CSF-transduced).
Objective Antitumor Responses
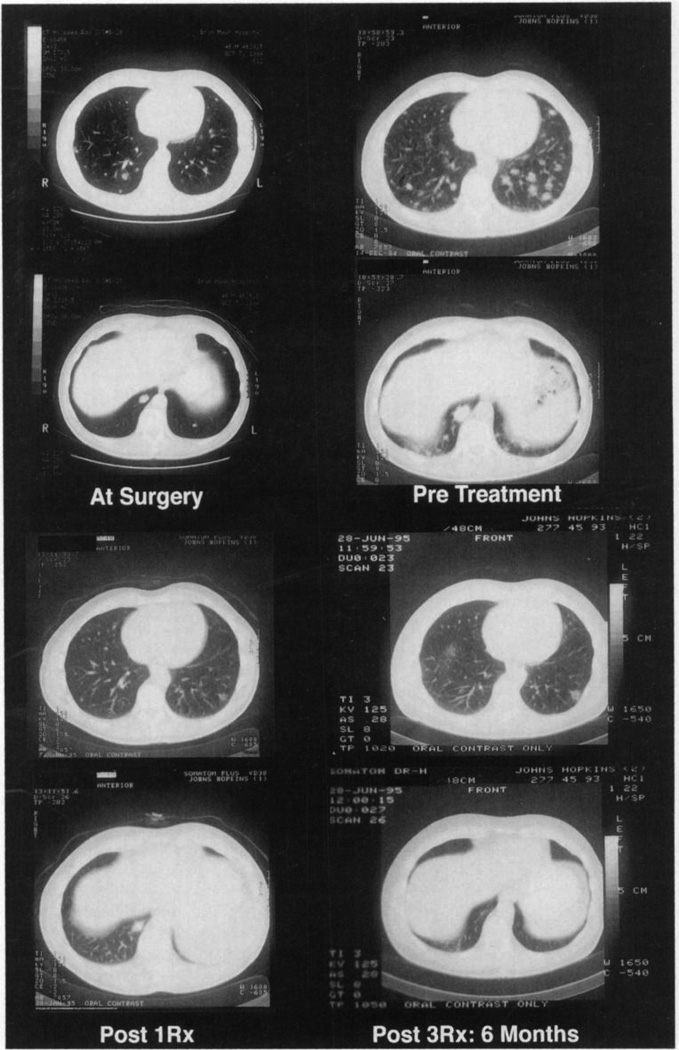
Assessment of efficacy was not a primary objective of this Phase I study. One patient (patient 24) had regression of multiple pulmonary metastases following treatment at dose level 2 (Fig. 5). Of note, this patient had progression of multiple pulmonary metastases during the 2 months between nephrectomy and first vaccination. This patient received three vaccinations with MFG-GM-CSF gene-transduced vaccines secreting 149 ng/106 cells/24 h. The patient received no prior systemic therapy, and the objective partial remission endured for 7 months. No other patient treated in the trial had objective evidence of treatment related tumor responses.
Fig. 5.
Regression of metastases in patient 24. This patient was treated with 4 × 107 autologous MFG-GM-CSF-transduced RCC cells secreting GM-CSF at 149 ng/106 cells/24 h for three doses, consuming all vaccine. The patient’s CT scan is presented at four time points: at surgery, before treatment (2 months postnephrectomy), 28 days after the first vaccination, and after 3 treatments (6 months postvaccination).
DISCUSSION
This trial was conducted to evaluate and compare the safety of escalating doses of irradiated autologous RCC vaccines with and without ex vivo GM-CSF gene transfer. Only minor toxicities were encountered from the effects of primary culture alone or the addition of human GM-CSF gene transfer (Table 4). The continued clinical evaluation of MFG-GM-CSF gene-transduced tumor cell vaccines in outpatients thus appears justified.
A major limitation to the vaccine cell dose administered was not adverse reactions, but rather the vaccine yields from in vitro cell expansion. Achievable tumor vaccine cell yields from stage II and III RCC patients are apparently higher than those observed in this trial. (18, 19) Possibly, the more necrotic tissue encountered in larger primary RCC tumors of stage IV patients do not grow in vitro as well under the culture conditions used for vaccine cell propagation. Technical improvements in primary tumor culture conditions may permit improved yields in the future. Nevertheless, multiple vaccinations with 4 × 107 RCC vaccine cells appear technically achievable for stage IV patients.
In contrast to technical limits in autologous vaccine cell expansion, transfer of the GM-CSF gene and increased secretion of GM-CSF above 40 ng/106 cells/24 h was readily accomplished with a single transduction in nearly all cases (eight of nine cases). Variability in GM-CSF secretion was observed from tumor to tumor (Table 3). Heterogeneity in biological properties of tumor cells was anticipated in a vaccine strategy exploiting potential autologous tumor antigens. Preclinical studies indicate that heterogeneity in GM-CSF secretion does not interfere with vaccination efficacy, because equivalent antitumor immunity was produced over a wide range of GM-CSF secretion rates above a threshold of 35 ng/106 cells/24 h (8, 10–15). Nevertheless, it is unclear from this study whether or not the threshold of GM-CSF secretion necessary for optimum induction of systemic antitumor immunity in humans was achieved. For many RCC patients, autologous vaccine cells secreting bioactive levels of GM-CSF can be generated using retroviral gene transfer. Efficacy studies using a vaccine preparation of 4 × 107 autologous RCC cells secreting GM-CSF at >40 ng/106 cells/24 h, an immunologically active dose, appear safe and technically feasible.
In the context of sufficient tumor vaccine cell dose, however, paracrine GM-CSF following gene transfer produced specific immunological effects. Blind analysis of intradermal vaccine biopsy specimens at dose level 2 identified a stark contrast between the intensity of infiltration of APCs at GM-CSF-transduced vaccine sites compared to nontransduced vaccine sites. Dense infiltrates of APCs predominate on day 3 at the intradermal vaccine site of GM-CSF gene-transduced vaccines. By day 7, the macrophage infiltration appears to be largely replaced by T-lymphocytes. In mouse models, this particular histological feature is one of the most distinct indicators of immunological priming by GM-CSF-transduced tumor vaccines at the vaccine site (8). Although the kinetics of cellular infiltrates was similar at the intradermal biopsy sites of nontransduced vaccines, fewer infiltrating APCs (day 3) and lymphocytes (day 7) were observed at untransduced vaccine sites, even biopsies as dose level 3 compared to GM-CSF-transduced vaccine site biopsies at dose level 2.
Measurement of DTH responses using dissociated autologous RCC showed a trend toward greater responses at dose level 2 in patients treated with GM-CSF-transduced vaccines. The small number of patients in each group precludes statistical analysis. Of note, because the DTH cells were prepared by collagenase digestion and stored in fetal bovine serum, it is impossible to distinguish responses against these contaminating foreign proteins versus true RCC antigens. Ongoing analysis using T-cell clones from vaccinated patients will ultimately define the antigen specificity of induced immune responses (32–43). Nonetheless, because all DTH specimens were prepared identically, quantitative differences between patients vaccinated with GM-CSF-transduced versus nontransduced cells suggests that the paracrine production of GM-CSF at the vaccine site contributes to systemic immune activation.
The most clear-cut difference between patients vaccinated with GM-CSF-transduced versus untransduced vaccines was reflected in the histological analysis of the DTH sites. The identification of eosinophils at the reactive RCC DTH biopsy sites in patients treated with GM-CSF-transduced vaccines is provocative (Figs. 3 and 4). In preclinical studies, eosinophils were observed at vaccine sites of IL-4 and GM-CSF-transduced tumor vaccines (4, 8–9). Although GM-CSF-stimulated eosinophils are weak APCs in vitro, they are more conventionally understood to act as effector cells (44–48). Eosinophils are involved in both allergic reactions and cytocidal responses to parasite infections (47–50). The predominance of eosinophils at the reactive RCC DTH challenge sites appears to be a hallmark of treatment with GM-CSF gene-transduced tumor vaccines. Eosinophils may act as effector cells following vaccination. Indeed, the finding of large amounts of interstitial MBP from degranulated eosinophils is indicative of eosinophil activation. Of note, urticaria and pruritus at DTH sites followed treatment of patient 24 with the GM-CSF-transduced vaccine. These allergic symptoms were experienced after an objective tumor response, which was measurable by CT scan.
Our recent studies in murine models of GM-CSF gene-transduced vaccines have shed some light on the basis and relevance of the eosinophil infiltrate.4 Analyses of systemic immunity induced by treatment with B16-GM-CSF vaccines in IL-4 and IL-5 knockout mice show that both of these cytokines are necessary for the eosinophil infiltrate and antitumor activity. Thus, the eosinophil infiltrate appears to result from the Th2 component of a dual Thl/Th2 response induced by paracrine GM-CSF tumor vaccines.
Finally, reduction in pulmonary metastases followed treatment of patient 24 with MFG-GM-CSF gene-transduced vaccines secreting GM-CSF at 149 ng/106 cells/24 h. A spontaneous regression cannot be formally excluded in this patient. However, over 20 new pulmonary metastases appeared on CT scan after nephrectomy and before vaccination (Fig. 5). If a spontaneous regression occurred, it took place around the start of vaccine treatment. A nonreactive DTH to RCC cells measured prior to vaccination was followed 28 days later by the largest postvaccination DTH conversions measured in the trial. Observation of the potential efficacy of GM-CSF-transduced cancer vaccines in this toxicity study was unexpected for two reasons. First, the sample size was small. Second, the patients treated had established tumor burdens greater than 1010 cells, in excess of the established tumor burdens in which GM-CSF-transduced tumor vaccines have efficacy in animal models (8–15). Prospective validation is required in trials statistically powered to estimate efficacy. Potentially important parameters for further evaluation include absolute amount of GM-CSF secreted/vaccine cells, optimum cell dose, frequency of vaccination, and duration of treatment. The low toxicity and bioactivity of GM-CSF gene-transduced tumor vaccines make efficacy evaluation compelling in patients with minimal residual cancer following surgery.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the significant contributions of Drs. Suzanne Topalian, Anton Berns, Gordon Parry, Donald S. Coffey, Hayden Braine, Thomas R. Hendrix, Michael Amey, David Blake, Alan W. Partin, Joel B. Nelson, and James Zabora and of Sujatha Ayyagari, Barbara Starklauf, Devon Young, Jo Foster, and Kimberly Cordwell.
Footnotes
This work was supported by the NIH GCRC at Johns Hopkins, the Kirby Award of the Cancer Research Institute, the Longrifles Endowment, the Burroughs Wellcome Experimental Therapeutics Award, and the CaPCURE Foundation. Partial funding for this study was provided by Somatix Corporation. Under an agreement between Somatix and the Johns Hopkins University, D. P. is entitled to a share of sales royalty received by the University from Somatix. The terms of this arrangement are being managed by the University in accordance with its conflict of interest policies.
The abbreviations used are: GM-CSF, granulocyte-macrophage colony-stimulating factor, APC, antigen-presenting cell; RCC, renal cell carcinoma; IL, interleukin; RCR, replication-competent retrovirus; DTH, delayed-type hypersensitivity; MBP, major basic protein; CT, computed tomography.
K. Hung, R. Hayashi, C. Lowenstein, H. Levitsky, and D. Pardoll. A dual Thl/Th2 response is required for maximal sytemic antitumor immunity, submitted for publication.
REFERENCES
- 1.Mulligan RC. The basic science of gene therapy. Science (Washington DC) 1993;260:926. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 2.Jaffee EM, Hurwitz H, Pardoll DM. Gene modifications of tumors. In: De VT, Vita Heilman S, Rosenberg SA, editors. Biologic Therapy of Cancer. Ed. 2. Vol. 774. J. B. Lippincott; 1995. p. 84. [Google Scholar]
- 3.Rosenberg SA. Newer approaches to cancer treatment. In: De VT, Vita Heilman S, Rosenberg SA, editors. Cancer Principles and Practice of Oncology. Ed. 4. Vol. 2598. J. B. Lippincott; 1993. p. 13. [Google Scholar]
- 4.Tepper RI, Pattengale PK, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Pardoll DM, Itaya T, Golumbek P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B, Frost B. Interleukin-2 production by tumor cells bypasses T-helper function in the generation of an antitumor response. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 6.Gansbacher B, Zier K, Daniels B, Cronin K, Bannerji R, Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J. Exp. Med. 1990;172:1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asher AL, Mule JJ, Kasid A, Restifo NP, Salo JC, Reichert CM, Jaffe G, Fendly B, Kriegler M, Rosenberg SA. Murine tumor cells transduced with the gene for tumor necrosis factor-α. J. Immunol. 1990;146:3327–3334. [PMC free article] [PubMed] [Google Scholar]
- 8.Dranoff G, Jaffee EM, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hirofumi H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine GM-CSF stimulates potent, specific and long lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golumbek PT, Lazenby AJ, Levitsky HI, Jaffee E, Karasuyama H, Baker M, Pardoll D. Treatment of established renal cancer by tumor cells engineered to secrete Interleukin-4. Science (Washington DC) 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 10.Sanda MG, Ayyagari SR, Jaffee EM, Epstein JI, Clift SL, Cohen LK, Dranoff G, Pardoll DM, Mulligan RC, Simons JW. Demonstration of a rational strategy for human prostate cancer gene therapy. J. Urol. 1994;151:622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 11.Jaffee EM, Lazenby A, Meurer J, Marshall F, Hauda KM, Counts C, Hurwitz H, Simons JW, Levitsky HI, Pardoll DM. Use of murine models of cytokine-secreting tumor vaccines to study feasibility and toxicity issues critical to designing clinical trials. J. Immunother. 1995;18:1–9. doi: 10.1097/00002371-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Carducci MA, Ayyagari SR, Sanda MG, Simons JW. Gene therapy for human prostate cancer. Cancer (Phila.) 1995;75:2013–2020. [Google Scholar]
- 13.Vieweg J, Rosenthal FM, Bannerji R, Heston WD, Fair WR, Gansbacher B, Gilboa I. Immunotherapy for prostate cancer in the Dunning rat model: use of cytokine gene modified tumor vaccines. Cancer Res. 1994;54:1760–1765. [PubMed] [Google Scholar]
- 14.Jaffee EM, Thomas MC, Huang AY-C, Hauda KM, Levitsky HI, Pardoll DM. Enhanced immune priming with spatial distribution of paracrine cytokine vaccines. J. Immunother. 19:1–8. doi: 10.1097/00002371-199605000-00002. 19%. [DOI] [PubMed] [Google Scholar]
- 15.Levitsky HI, Montgomery J, Ahmadzadeh M. Immunization with GM-CSF-transduced, but not B7-1-transduced lymphoma cells primes idiotype specific T cells and generated potent systemic anti-tumor immunity. J. Immunol. 1997 in press. [PubMed] [Google Scholar]
- 16.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muratmatsu S, and Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;776:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang A, Columbek P, Ahmadzadeh M, Jaffee EM, Pardoll DM, Levitsky HI. Role of bone marrow-derived cells in presenting MHC class I-re-stricted tumor antigens. Science (Washington DC) 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 18.Jaffee EM, Dranoff G, Cohen LK, Hauda KM, Clift S, Marshall FF, Mulligan RC, Pardoll DM. High efficiency gene transfer into primary human tumor explains without cell selection. Cancer Res. 1993;53:2221–2226. [PubMed] [Google Scholar]
- 19.Berns A, Cohen L, Donehower RC, Dranoff G, Hauda KM, Jaffee EM, Lazenby AJ, Levitsky HI, Marshall FF, Mulligan RC, Nelson WG, Owens AH, Pardoll DM, Parry G, Partin AH, Piantadosi S, Zabora JR, Simons JW. Phase Istudy of non-replicating autologous tumor cell injections using cells prepared with or without GM-CSF gene transduction in patients with metastatic renal cell carcinoma. Recombinant DNA Advisory Committee Protocol, active as of 12/93. Hum. Gene Ther. 1995;6:347–368. doi: 10.1089/hum.1995.6.3-347. [DOI] [PubMed] [Google Scholar]
- 20.Danos O, Mulligan RC. Safe and efficient generation of recombinant retrovirus with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci. USA. 1988;85:6460–6465. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walther MM, Alexander RB, Weiss GH, Venzon D, Berman A, Pass HI, Linehan WM, Rosenberg SA. Cytoreductive surgery prior to interleukin-2 based therapy in patients with metastatic renal cell carcinoma. Urology. 1993;42:250–259. doi: 10.1016/0090-4295(93)90612-e. [DOI] [PubMed] [Google Scholar]
- 22.Sokol JE. 1975 measurement of delayed skin test responses. N. Engl. J. Med. 1995;29:501. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 23.McCune CS, O’Donnell RW, Marquis DM, Sahasrabudhe DM. Renal cell Carcinoma treated by vaccines for active specific immunotherapy: correlation of survival with skin testing by autologous tumor cells. Cancer Immunol. Immunother. 1990;32:62–66. doi: 10.1007/BF01741726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oren ME, Herberman RB. Delayed cutaneous hypersensitivity reactions to membrane extracts of human tumor cells. Clin. Exp. Immunol. 1977;9:45–56. [PMC free article] [PubMed] [Google Scholar]
- 25.Hoover HC, Jr, Surdyke M, Dangel RB, Peters LC, Hanna MG., Jr Delayed cutaneous hypersensitivity to autologous tumor cells in colorectal cancer patients immunized with an autologous tumor cell: bacillus Calmette-Guerin vaccine. Cancer Res. 1984;44:671–676. [PubMed] [Google Scholar]
- 26.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–2577. [PubMed] [Google Scholar]
- 27.Berd DJ, Maguire JC, Jr, McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J. Clin. Oncol. 1990;8:1858–1867. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- 28.Beck LA, SteUato L, Beall T, Schall D, Leopold CA, Bickel F, Baroody B, Bocherner BS, Schleimer RP. Detection of chemokine RANTES and endothelial adhesion molecules in nasal polyps. J. Allergy Clin. Immunol. 1996;98:766–774. doi: 10.1016/s0091-6749(96)70126-4. [DOI] [PubMed] [Google Scholar]
- 29.Peters MS, Schroeter AL, Kephart GM, Gleich GJ. Localization of eosinophil granule major basic protein in chronic urticaria. J. Invest. Dermatol. 1983;81:39–45. doi: 10.1111/1523-1747.ep12538380. [DOI] [PubMed] [Google Scholar]
- 30.Leiferman KM, Ackerman SJ, Sampson HS, Haugen PY, Venecie G, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis: comparison with onchocerciasis. N. Engl. J. Med. 1985;313:282–293. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 31.Perez GL, Peters MS, Reda AM, Butterfield JH, Peterson EA, Leiferman KM. Mast cells, neutrophils and eosinophils in prurigo nodularis. Arch. Dermatol. 1993;129:861–870. [PubMed] [Google Scholar]
- 32.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, DePlaen E, Vandeneynde B, Khuth A, Boon T. A gene encoding an antigen recognized cytotoxic T lymphocytes on a human melanoma. Science (Washington DC) 1991;254:1643–1648. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 33.Gaugler B, Van den Eynde B, Van der Bruggen RP, Romero P, Gaforio JJ, DePlaen E, Lethe B, Brasseur F, Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J. Exp. Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brichard V, Van Pel A, Wolfel T, DePlaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, Maltei S, DePlaen E, Lurquin C, Szikora JP. A new gene coding for a differentiation antigen recognized by autologous cytotoxic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakker ABH, Schreurs MWJ, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, Figdor CG. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabonowitz J, Engelhard VH, Hunt DF, Sling-Luff CL. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science (Washington DC) 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 39.Coulie P, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, Buon T. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc. Natl. Acad. Sci. USA. 1995;92:7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, dePlaen E, Hankelm T, Meyer Zum Buschenfelde KH, Beach D. A pl6INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science (Washington DC) 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella F, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 42.Robbins P, El-Gamil M, Kawakami Y, Rosenberg S. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 43.Bernhard H, Karbach J, Wolfel T, Busch P, Storkel S, Stockle M, Wolfel C, Seliger B, Huber C, Meyer H, Zum Buschenfelde KH. Cellular immune response to human renal cell carcinomas: definition of a common antigen recognized by HLA-A2-restricted cytotoxic T lymphocyte (CTL) clones. Int. J. Cancer. 1994;59:837–842. doi: 10.1002/ijc.2910590621. [DOI] [PubMed] [Google Scholar]
- 44.Mawhorter SD, Kazura JE, Boom WH. Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T cell proliferation. Immunology. 1994;81:584–591. [PMC free article] [PubMed] [Google Scholar]
- 45.Del Pozo V, De Andres B, Martin E, Cardaba B, Fernandez JC, Gallardo S, Tramon P, Leyva-Cobian F, Palomino P, Lahoz C. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur. J. Immunol. 1992;22:1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 46.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc. Natl. Acad. Sci. USA. 1989;86:1348–1351. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weller PF. The immunobiology of eosinophils. N. Engl. J. Med. 1991;324:1110–1118. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- 48.Gleich GJ, Adolphson MS, Leiferman KM. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 49.Del Prete G. Human Thl and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy (Cph.) 1992;47:450–455. doi: 10.1111/j.1398-9995.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 50.Scott P, Pearce E, Cheever AW, Coffman RL, Sher A. Role of cytokines and CD4+ T cell subsets in the regulation of parasite immunity and disease. Immunol. Rev. 1989;112:161–183. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]