Abstract
Women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer are candidates for treatment with the anti-HER2 antibody trastuzumab. Assessment of HER2 status in recurrent disease is usually made by core needle biopsy of a single lesion which may not be representative of the larger tumor mass or other sites of disease. Our long-range goal is to develop positron emission tomography (PET) of radiolabeled trastuzumab for systemically assessing tumor HER2 expression and identifying appropriate use of anti-HER2 therapies. The purpose of this study was to evaluate PET-CT of 64Cu-DOTA-trastuzumab for detecting and measuring tumor uptake of trastuzumab in patients with HER2-positive metastatic breast cancer.
Methods
Eight women with biopsy-confirmed HER2-positive metastatic breast cancer and no anti-HER2 therapy for ≥ 4 mo underwent complete staging, including 18F-fluorodeoxyglucose (FDG)/PET-CT. For 6 of the 8 patients, 64Cu-DOTA-trastuzumab injection (364-512 MBq, 5 mg trastuzumab) was preceded by trastuzumab infusion (45 mg). PET-CT (PET scan duration 1 h) was performed 21-25 (“Day 1”) and 47-49 (“Day 2”) h after 64Cu-DOTA-trastuzumab injection. Scan fields of view were chosen based on 18F-FDG/PET-CT. Lesions visualized relative to adjacent tissue on PET were considered PET-positive; analysis was limited to lesions identifiable on CT. Radiolabel uptake in prominent lesions was measured as maximum single-voxel standardized uptake value (SUVmax).
Results
Liver uptake of 64Cu was reduced approximately 75% with the 45 mg trastuzumab pre-dose, without significant effect on tumor uptake. The study included 89 CT-positive lesions; detection sensitivity was 77, 89 and 93% for Day 1, Day 2 and 18F-FDG, respectively. On average, tumor uptake was similar for 64Cu-DOTA-trastuzumab and 18F-FDG [SUVmax (mean, range): Day 1 (8.1, 3.0-22.5, n=48); Day 2 (8.9, 0.9-28.9, n=38); 18F-FDG (9.7, 3.3-25.4, n=56)], but the extent of same-lesion uptake was not correlated between the 2 radiotracers. No toxicities were observed, and estimated radiation dose from 64Cu-DOTA-trastuzumab was similar to 18F-FDG.
Conclusion
64Cu-DOTA-trastuzumab visualizes HER2-positive metastatic breast cancer with high sensitivity, and is effective in surveying disseminated disease. A 45 mg trastuzumab pre-dose provides a 64Cu-DOTA-trastuzumab biodistribution favorable for tumor imaging. 64Cu-DOTA-trastuzumab/PET-CT warrants further evaluation for assessing tumor HER2 expression and measuring delivery of trastuzumab-based therapy.
Keywords: 64Cu-labeled trastuzumab, HER2, breast cancer
Over-expression of human epidermal growth factor receptor 2 (HER2) is identified in 20% of breast cancers (1). Women with HER2-positive breast cancer are candidates for treatment with the humanized anti-HER2 antibody trastuzumab. When combined with chemotherapy, trastuzumab increases overall survival for all stages of HER2-positive breast cancer.
Accurate and comprehensive assessment of tumor HER2 status is critical in determining treatment. However, pathologic assessment of HER2 status suffers from inter-laboratory discordance and lack of a clear definition of positivity (2). Furthermore, confirmation of recurrent disease is usually made by core needle biopsy of an accessible lesion and may not represent the larger tumor mass or other sites of disease. Differences in HER2 expression between primary and metastatic tumors have been observed in as many as 20% of patients, especially when metastasis occurs after adjuvant or neoadjuvant therapy (3-5).
Systemic assessment of tumor receptor expression potentially can be obtained by imaging with targeted radiotracers. Radiolabeled trastuzumab has been used to image patients with HER2-positive breast cancer, initially with 111In and single-photon imaging (6-7), and more recently with 89Zr and positron emission tomography (PET) (8). While labeling with the positron-emitting isotope 124I is also a possibility, radiometals are preferred given the known cellular internalization of trastuzumab and subsequent rapid efflux of radiolabel from cells when trastuzumab is labeled with isotopes of I (9). Tumor visualization has been variable, perhaps because the women were on active trastuzumab treatment, which may have inhibited radiolabeled trastuzumab binding to HER2.
The positron-emitting isotope 64Cu is regularly available from Washington University, St. Louis, and we have extensive experience labeling antibodies with radiometals via the chelating agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (10). Based on our previous clinical study with 111In-MxDTPA-trastuzumab (7) and promising results in athymic mice bearing HER2-expressing human breast adenocarcinoma xenografts (11), we have obtained an investigational new drug (IND) application for 64Cu-DOTA-trastuzumab.
The primary objective of this Phase I study was to evaluate the feasibility and potential utility of computed tomography (CT)-supplemented PET scanning of 64Cu-DOTA-trastuzumab (64Cu-DOTA-trastuzumab/PET-CT) for lesion detection and uptake measurement in HER2-positive metastatic breast cancer. Similar to other antibodies (12), liver uptake of intravenously-administered trastuzumab is strongly dependent on antibody protein load. Thus, we sought to identify a trastuzumab dose that minimizes liver uptake of 64Cu-DOTA-trastuzumab. Additional goals were to compare 64Cu-DOTA-trastuzumab with the standard PET radiotracer, 18F-fluorodeoxyglucose (18F-FDG), and to confirm the safety of the 64Cu-DOTA-trastuzumab/PET-CT procedure.
MATERIALS AND METHODS
Patient Selection
Women with metastatic HER2 positive breast cancer and no exposure to trastuzumab during the preceding 4 months or more were considered for study participation after undergoing a staging workup that included whole-body 18F-FDG/PET-CT. All candidates underwent biopsy of a metastatic lesion within 28 d prior to the 64Cu-DOTA-trastuzumab procedure to confirm recurrent, HER2-positive disease by immunohistochemical (IHC) staining and/or fluorescence in situ hybridization (FISH). Assessable disease outside the primary breast site, ipsilateral axillary region and biopsy site was also required. The study protocol was approved by the City of Hope institutional review board and radiation safety committee and an IND was accepted by the FDA. Informed consent was obtained from each study participant.
64Cu-DOTA-Trastuzumab Preparation
Trastuzumab is a recombinant humanized antibody that binds with high affinity to the extracellular domain of the HER2 receptor protein. Radiolabeled trastuzumab was prepared according to procedures defined in IND #109971. The antibody (Herceptin®, purchased from Genentech, South San Francisco, CA) was conjugated with the active ester of DOTA (Macrocyclics, Dallas, TX) under current good manufacturing (cGMP)-compliant conditions. Copper-64 (half life 12.8 h, 0.18 positrons/decay) was provided by the Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO. DOTA conjugated antibody was incubated with 64Cu for 45 min at 43°C, chased with 1 mM diethylenetriamine pentaacetic acid (DTPA), and purified on a size-exclusion, preparative column (Superdex-200). Radiolabeling efficiency was > 93%. Appropriate fractions were pooled, filtered and formulated with 1% human serum albumin for patient administration. The 64Cu-DOTA-trastuzumab preparations were sterile, with endotoxin levels < 0.05 EU/ml and immunoreactivity > 86%. DOTA-trastuzumab protein dose per 64Cu-DOTA-trastuzumab injection was approximately 5 mg.
Administration of Trastuzumab and 64Cu-DOTA-Trastuzumab
Patients were closely monitored for acute adverse reactions during trastuzumab administrations. 64Cu-DOTA-trastuzumab (364 to 512 MBq; mean 450 MBq) was infused intravenously in 25 ml of saline over 10 min. Patients receiving non-radiolabeled trastuzumab were infused intravenously with the antibody (45 mg in 50 ml of saline given over 15 min) immediately prior to radioactive injection.
Dijkers, et al., found that, compared with 10 mg, 50 mg of trastuzumab substantially reduced blood clearance and liver uptake of 89Zr-trastuzumab in trastuzumab-naïve patients (8). The first 4 patients in our study were randomly assigned to receive trastuzumab doses of 5 or 50 mg. When 64Cu-DOTA-trastuzumab/PET-CT of those patients confirmed the findings of Dijkers, et al., we adopted the 50 mg dose for the remainder of the study.
PET-CT Imaging
Imaging was performed with a GE Discovery STe 16 PET-CT scanner operated in 3-D mode (septa retracted). The PET axial field of view is 15.4 cm (image slice thickness 3.3 mm). PET images were reconstructed using an iterative, ordered subsets expectation maximization (OSEM) algorithm with Gaussian post-smoothing and standard corrections for non-uniform detector sensitivity, scanner dead time, random and scattered coincidence events. Correction for photon attenuation was based on co-registered CT acquired during the same examination. Measured spatial resolution of the PET images was approximately 9 mm full-width-at-half maximum.
Patients underwent a standard 18F–FDG/PET-CT examination ≤ 13 d prior to the 64Cu-DOTA-trastuzumab procedure. Patients fasted ≥ 6 h before injection of 18F–FDG. Serum glucose concentration measured at time of examination was high (184 mg/dl) for 1 patient and normal (< 120 mg/dl) for the others.
Injected 64Cu activity was limited to 555 MBq (15 mCi), based on radiation dose estimates calculated from the pharmacokinetics of 111In-MxDTPA-trastuzumab (7). One hour was chosen as a reasonable limit for PET scan duration. Within those constraints, disease location as judged from the preceding 18F–FDG/PET-CT examination was used in choosing the axial coverage for the 64Cu-DOTA-trastuzumab/PET-CT scans. The first (“Day 1”) 64Cu scan was performed 21-25 h post injection to allow radiolabeled antibody accumulation in tumor. A second (“Day 2”) scan was obtained 47-49 h post injection. Day 1 scans comprised of 3 or 4 (39 or 51 cm axial extent, 20 or 15 min per bed position) and Day 2 scans comprised of 1 or 2 (15 or 27 cm axial extent, 60 or 30 min per bed position) contiguous bed positions respectively, depending on patient body thickness. Signal-to-noise characteristics of the 64Cu-DOTA-trastuzumab images approximated those of the 18F–FDG scans (Figure 1).
FIGURE 1.
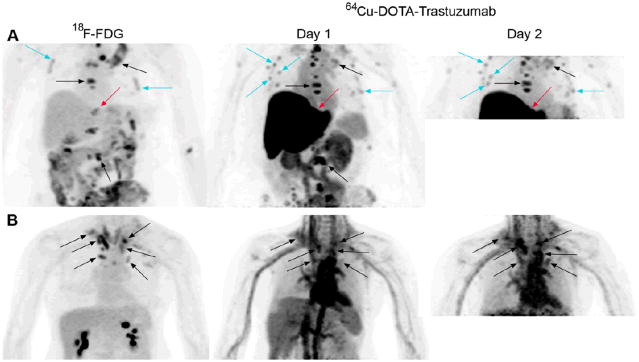
Visualization of HER2-positive metastatic breast cancer by positron emission tomography (PET) of 64Cu-DOTA-trastuzumab. Images are maximum intensity projections with upper intensity thresholds corresponding to SUV = 10 g/ml. (A) PET scans of 18F-fluorodeoxglucose (FDG), 64Cu-DOTA-trastuzumab 23 h post-injection (“Day 1”), and 64Cu-DOTA-trastuzumab 48 h post-injection (“Day 2”) in a patient (“Patient A”) who received a trastuzumab dose of 5 mg. Black arrows point out several of many corresponding CT-positive bone lesions seen both with 18F-FDG and 64Cu-DOTA-trastuzumab, while turquoise arrows denote a few of many instances of 64Cu-DOTA-trastuzumab, or 64Cu-DOTA-trastuzumab and 18F-FDG, focal uptake in rib regions too small to be evaluated on associated CT. Red arrows indicate an intrahepatic lesion seen with 18F-FDG but obscured by intense liver uptake in the 64Cu-DOTA–trastuzumab scans. (B) 18F-FDG, 64Cu-DOTA-trastuzumab 24 h post-injection, and 64Cu-DOTA-trastuzumab 48 h post-injection scans of a patient (“Patient B”) given a trastuzumab dose of 50 mg. Arrows denote a number of lymph nodes visualized in both the 18F-FDG and 64Cu-DOTA–trastuzumab scans. Liver uptake of 64Cu was much lower for Patient B than for Patient A (compare Day 1 images).
Image Analysis
PET-CT examinations were interpreted by a nuclear medicine-boarded radiologist (PSC). Tumor assessment was limited to lesions with identifiable anatomic correlates on CT. Lesions with image intensity visually different than adjacent tissue were considered to have been “detected” on PET. Due to possible 18F–FDG or non-specific antibody uptake secondary to biopsy, biopsied tumor sites were not included in the analysis.
Radiolabel uptake in as many as 10 of the most prominent lesions per patient, as well as selected non-tumor tissues and organs, was measured in terms of standardized uptake value (SUV = tissue activity per cm3 × body weight (g)/injected activity decay-corrected to time of scan). Tumor uptake was parameterized as single-voxel maximum SUV (SUVmax) and background-adaptive whole-tumor average SUV (13). We found whole-tumor SUV to be closely and linearly correlated with SUVmax (r2 ≥ 0.97, P < 0.001). Therefore, tumor uptake results are presented only in terms of SUVmax.
Uptake analysis for blood, liver, spleen, kidney and heart wall consisted of averaging the mean SUVs of circular or elliptical regions of interest of fixed size placed well within the tissue’s or organ’s PET image boundaries on 3 contiguous image slices. Blood measurements were obtained from PET images of the cardiac ventricles; heart wall was visualized as a region of relatively low uptake adjacent to the ventricles.
Pharmacokinetic Analysis and Radiation Dose Estimates
Copper-64 activity concentration was measured in peripheral venous samples acquired 0-1, 23-24 and 47-48 h post injection from patients who received a 50 mg trastuzumab dose. Radiation dose estimates for these patients were obtained by combining blood activity and organ uptake measurements from the current study with blood and organ time-activity measurements (0-168 h) from our previous clinical study with 111In-MxDTPA-trastuzumab (7). Details of the radiation dose calculations are given in the Supplemental Material (available only online at http://jnm.snmjournals.org).
Human Anti-Trastuzumab Antibody Response
Serum samples obtained just prior to trastuzumab/64Cu-DOTA-trastuzumab infusion and 1, 3 and 6 mo later, when possible, were evaluated for immune responses using a size-exclusion HPLC shift assay. Samples (125 μl) were incubated with radiolabeled DOTA-trastuzumab (111In, 9 μCi/μg, 0.1 μCi) and then run on a Superose-6 size exclusion column at 0.4 ml/min in PBS/0.05% NaN3. A change in the elution pattern of the radiolabeled trastuzumab consistent with higher molecular weight was considered positive for an anti-antibody response.
Statistical Analysis
Lesion detection sensitivities were compared by 2-sided Fisher’s Exact Test. Comparison of tumor uptake between trastuzumab doses and among lesion sites employed analysis of variance (ANOVA) to evaluate both dose/lesion site and patient effects, using Holm’s method to adjust for multiple comparisons. Linear regression analysis was used to demonstrate (a) correlation between whole-tumor SUV and SUVmax, and (b) lack of correlation between 18F-FDG and 64Cu-DOTA-trastuzumab. The effect of trastuzumab dose on organ uptake was evaluated by Wilcoxon rank sum test. P values < 0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Eight of 10 women considered for study participation met the eligibility criteria. Biopsies of 2 patients previously treated for early-stage HER2-positive breast cancer showed recurrent disease to be HER2 negative. Participating patients are characterized in Table 1.
TABLE 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Trastuzumab protein dose
|
All patients | |
|---|---|---|---|
| 5 mg | 50 mg | ||
| No. of patients | 2 | 6 | 8 |
|
| |||
| Age (years) | |||
| median | 60 | 54 | 56 |
| range | 44-75 | 39-69 | 39-75 |
|
| |||
| No. of patients | |||
| Prior anti-HER2 therapy | |||
| none | 1 | 1 | |
| adjuvant trastuzumab | 1 | 2 | 3 (14, 18, 18)† |
| trastuzumab for metastasis | 1 | 3 | 4 (4, 6, 14, 18)† |
|
| |||
| Hormone receptor and HER2 status of recurrent disease | |||
| ER and/or PR positive | 1 | 3 | 4 |
| ER and PR negative | 1 | 3 | 4 |
| HER2 | |||
| IHC3+ | 2 | 5 | 7 |
| IHC2+/FISH positive | 1 | 1 | |
|
| |||
| Sites of metastatic disease | |||
| bone | 2 | 4 | 6 |
| lymph nodes | 2 | 5 | 7 |
| liver | 2 | 2 | 4 |
| lung | 1 | 1 | 2 |
| pleural effusion | 1 | 1 | |
| breast | 2 | 2 | |
months since last anti-HER2 therapy administration
ER = estrogen receptor; PR = progesterone receptor;
IHC = immunohistochemistry; FISH = fluorescence in situ hybridization
Lesion Detection Sensitivity of 64Cu-DOTA-Trastuzumab/PET-CT
Figure 1 illustrates 64Cu-DOTA-trastuzumab image quality and tumor visualization compared with 18F-FDG. Tumor-to-non tumor contrast for 64Cu-DOTA-trastuzumab was generally high (Fig. 1, Patient A). Exceptions occurred for lymph nodes in the cervical, clavicular and mediastinal regions due to high blood pool activity (Fig. 1, Patient B), and in the liver for the 5 mg trastuzumab dose (Fig. 1, Patient A). Visualization of lymph nodes in regions of high blood activity improved between Day 1 and Day 2, but changed little for other lesion sites between the two scans (Figure 2).
FIGURE 2.
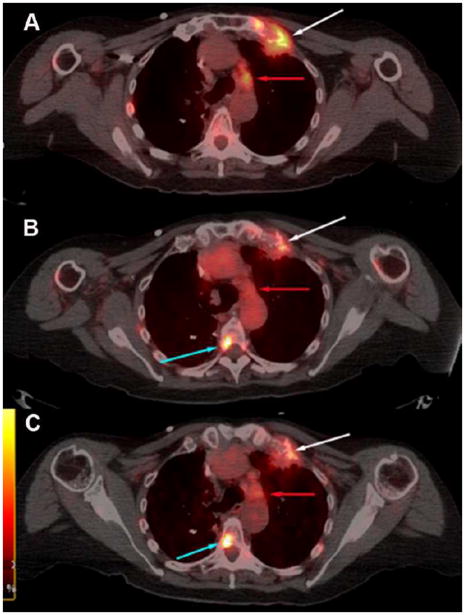
Visualization of bone and nodal metastases. Shown are transaxial PET-CT fusion images of (A) 18F-FDG, (B) 64Cu-DOTA-trastuzumab 23 h post injection and (C) 64Cu-DOTA-trastuzumab 48 h post injection from a patient given 50 mg of trastuzumab. Upper intensity thresholds (white color) correspond to SUV = 10 g/ml. Lesion to non-lesion contrast improved modestly between Day 1 and Day 2 after 64Cu-DOTA-trastuzumab injection. A lesion growing out of the left first rib (white arrows) is well visualized on the Day 1 64Cu scan and little changed on Day 2. A spinal metastasis (turquoise arrows) not seen with 18F-FDG is well visualized on both the Day 1 and Day 2 64Cu scans. On the other hand, a nodal metastasis (red arrows) seen with 18F-FDG is visualized only on Day 2 with 64Cu-DOTA-trastuzumab.
Lesion detection statistics are summarized in Table 2. All 8 patients had CT-positive lesions that were detected with 64Cu-DOTA-trastuzumab/PET. There were no statistically significant differences in 64Cu lesion detection sensitivity between 5 and 50 mg trastuzumab doses (data not shown). On Day 1, 64Cu detection sensitivity was lower for lymph nodes than for bone lesions. Overall, detection sensitivity for 64Cu-DOTA-trastuzumab on Day 1 was lower than for 18F-FDG, with the difference being due primarily to the low sensitivity of lymph nodes in regions of high blood activity. There were 7 instances in which a CT-positive lesion was detected with 18F-FDG but not 64Cu-DOTA-trastuzumab on either Day 1 or Day 2. In 6 instances (3 bone, 2 liver, 1 node), a CT-positive lesion was detected with 64Cu-DOTA-trastuzumab but not with 18F-FDG.
TABLE 2.
Detection of CT-Positive Lesions with Positron Emission Tomography
| Lesion site | 18F-FDG |
64Cu-DOTA-trastuzumab*
|
|
|---|---|---|---|
| Day 1 | Day 2 | ||
| All | 83 of 89 (93%) | 61 of 79 (77%)† | 54 of 61 (89%) |
| Bone | 35 of 38 (92%) | 33 of 36 (92%) | 19 of 20 (95%) |
| Lymph nodes | 30 of 31 (97%) | 20 of 31 (65%)‡,§ | 19 of 23 (88%) |
| Liver | 8 of 10 (80%) | 1 of 3 (33%) | 8 of 8 (100%) |
| Lung | 5 of 5 (100%) | 4 of 4 (100%) | 4 of 5 (80%) |
| Pleural effusion | 2 of 2 (100%) | 0 of 2 (0%) | 1 of 2 (50%) |
| Breast | 3 of 3 (100%) | 3 of 3 (100%) | 3 of 3 (100%) |
Combined data for 5 and 50 mg trastuzumab doses
P < 0.01 relative to 18F-FDG, all sites
P < 0.01 relative to 18F-FDG, lymph nodes
P < 0.05 relative to 64Cu-DOTA-trastuzumab Day 1, bone
All other instances of focal 64Cu-DOTA-trastuzumab activity were either correlated with CT-positive lesions or identifiably associated with intravascular activity. 64Cu-DOTA-trastuzumab was not prone to false positive findings. In one patient with numerous bone metastases, 64Cu-DOTA-trastuzumab, or both 64Cu-DOTA-trastuzumab and 18F-FDG, produced “hot spots” in rib regions too small to be assessed on associated CT (Fig. 1, Patient A).
Effects of Trastuzumab Protein Dose
Blood clearance was slowed and liver uptake of 64Cu-DOTA-trastuzumab was markedly decreased in patients pre-infused with trastuzumab (45 mg) (Figure 3). However, with only 2 patients at the lower protein dose, SUV differences between the 50 and 5 mg trastuzumab doses were not statistically significant (P = 0.10 and 0.05 on Days 1 and 2 for blood; P = 0.10 and 0.07 on Days 1 and 2 for liver). Trastuzumab pre-dosing dramatically improved visualization of hepatic metastases (Figure 4) and had little effect on 64Cu-DOTA-trastuzumab uptake in heart wall (Fig. 3), kidney or spleen (data not shown).
FIGURE 3.
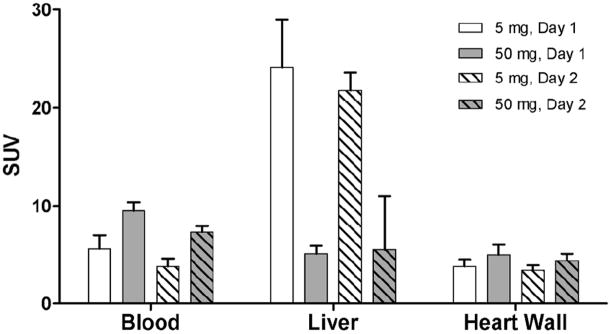
Effect of trastuzumab protein dose on the pharmacokinetics and biodistribution of 64Cu-DOTA–trastuzumab. Error bars indicate standard deviations. 5 mg trastuzumab dose: n = 2. 50 mg trastuzumab dose: Day 1, n = 5; Day 2, n= 6.
FIGURE 4.
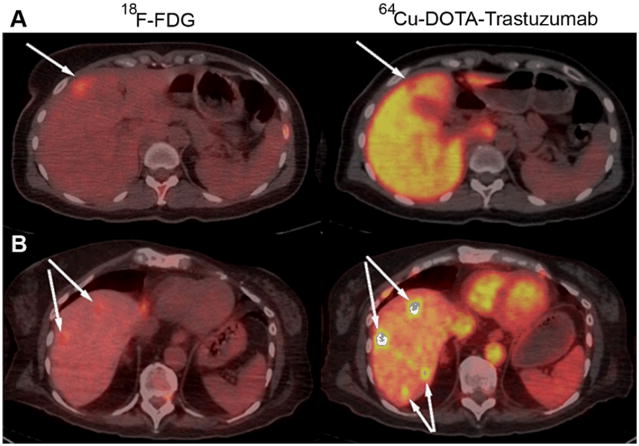
Visualization of hepatic metastases. (A) Fused transaxial PET-CT images of 18F-FDG and 64Cu-DOTA-trastuzumab 21 h post injection for a patient (“Patient A”) given 5 mg of trastuzumab. Upper intensity thresholds (white color) correspond to SUV = 10 g/ml for 18F-FDG and 40 g/ml for 64Cu-DOTA-trastuzumab. (B) Fused transaxial PET-CT images of 18F-FDG and 64Cu-DOTA-trastuzumab 47 h post injection for a patient (“Patient B”) given 50 mg of trastuzumab. Upper intensity thresholds correspond to SUV = 10 g/ml. Arrows indicate detected lesions. For Patient A, an anterior hepatic lesion is visualized as a cold spot on the 64Cu-DOTA-trastuzumab scan. Liver SUV was 27.6 g/ml in Patient A compared with 5.5 g/ml in Patient B, for whom hepatic lesions are dramatically visualized as hot spots. For Patient B, note that 2 lesions not seen with 18F-FDG are detected with 64Cu-DOTA-trastuzumab.
No statistically significant difference in tumor uptake of 64Cu-DOTA-trastuzumab was observed between the 2 trastuzumab doses. Tumor SUVmax was generally higher for 5 mg than 50 mg dose on Day 1 (mean ± SD = 11.3 ± 5.9 compared with 6.7 ± 2.4, P = 0.01), but trended in the other direction on Day 2 (mean ± SD = 5.9 ± 3.7 compared with 9.6 ± 5.9, P = 0.11). When ANOVA included both a patient effect (i. e., accounted for varying numbers of lesions among different patients) and a dose effect, there was no significant trastuzumab dose effect on either day.
Heterogeneity of 64Cu-DOTA-Trastuzumab Uptake in Tumors
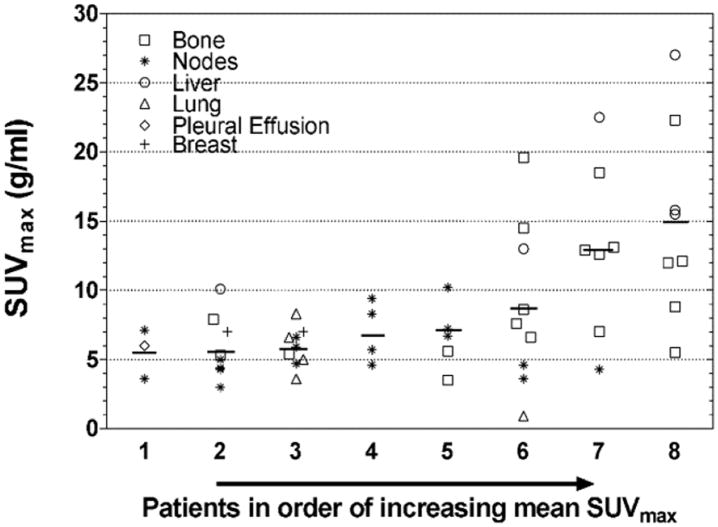
Uptake varied widely both among and within patients (Figure 5). For the data included in Fig. 5, mean SUVmax ranged from 5.5 to 15.0 g/ml among the 8 patients. Within patients, SUVmax varied between 2- and 5-fold in 7 patients and 22-fold in 1 patient. The variability was, in part, associated with lesion site (Figure 6).
FIGURE 5.
Inter- and intra-patient heterogeneity of 64Cu-DOTA-trastuzumab tumor uptake. Short horizontal lines indicate intra-patient mean SUVmax. Data are from Day 1 (n=49), or Day 2 (n = 7) for lesions not included in the Day 1 scan. Six of the Day 2 lesions are for Patient 8, for whom data from 2 of the 3 scanned bed positions on Day 1 were lost due to scanner malfunction. The other Day 2 lesion is for Patient 6. Patients 6 and 7 received trastuzumab doses of 5 mg; the others received 50 mg.
FIGURE 6.
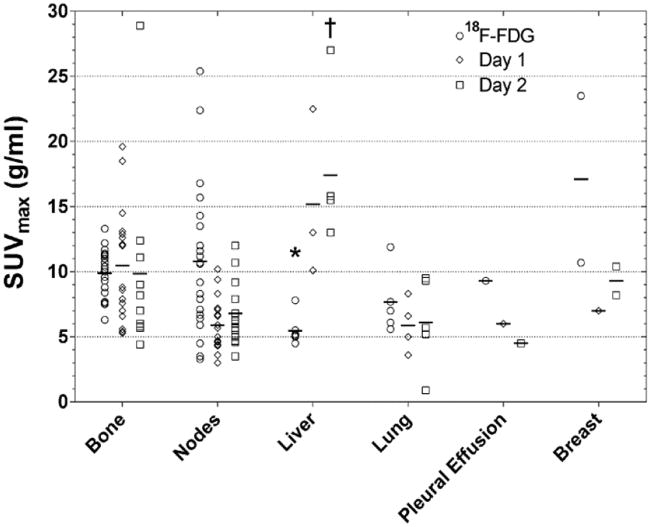
Tumor uptake of 18F-FDG and 64Cu-DOTA-trastuzumab (Day 1 and Day 2) vs. lesion site. Short horizontal lines indicate intra-site averages. * < 18F-FDG bone, P < 0.01; † > Day 2 bone, P < 0.02.
Tumor Uptake Compared Between 64Cu-DOTA-Trastuzumab and 18F-FDG
Uptakes of 64Cu-DOTA-trastuzumab and 18F-FDG were comparable when averaged over all lesions. For combined 5 and 50 mg trastuzumab doses, SUVmax results (mean, median, range) were: 18F-FDG (9.7, 9.3, 3.3-25.4, n=56); 64Cu-DOTA-trastuzumab Day 1 (8.1, 7.0, 3.0-22.5, n=48); 64Cu-DOTA-trastuzumab Day 2 (9.0, 7.5, 0.9-28.9, n=38).
Analysis of variance including both lesion site and patient effects indicated significant lesion site effects for both 64Cu-DOTA-trastuzumab and 18F-FDG (Fig. 6). Pair-wise comparisons between sites showed 18F-FDG uptake in liver metastases to be less than in bone metastases (P < 0.01), while 64Cu-DOTA-trastuzumab uptake on Day 2 was higher in liver metastases than in bone metastases (P < 0.02).
Same-lesion maximum SUVs for 64Cu-DOTA-trastuzumab and 18F-FDG were uncorrelated (P ≥ 0.4, correlation coefficients = - 0.1). SUVmax ratios (64Cu-DOTA-trastuzumab to 18F-FDG) varied from 0.2 to 4.3 (Figure 7).
FIGURE 7.
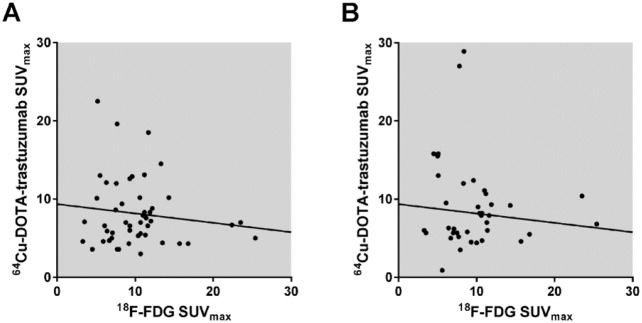
Tumor uptakes of 64Cu-DOTA-trastuzumab and 18F-FDG are uncorrelated. The plots compare maximum single-voxel standardized uptake values (SUVmax) within the same tumor between 18F-FDG and 64Cu-DOTA-trastuzumab Day 1 (A) and Day 2 (B). Slopes of the indicated linear regression lines are not significantly different from zero (P = 0.4, correlation coefficient = -0.1 for Day 1; P = 0.5, correlation coefficient = -0.1 for Day 2). tras = trastuzumab.
Patient Safety
Trastuzumab infusion and 64Cu-DOTA-trastuzumab/PET-CT were well tolerated, with no unanticipated toxicity or adverse side effects observed. Anti-trastuzumab antibody response assays were negative for 6 patients. Minor increases in higher molecular weight complexes were observed in the HPLC shift assays of 2 patients at baseline and 6 mo, or baseline, 3 and 6 mo after their 64Cu-DOTA-trastuzumab/PET-CT procedures. Estimated radiation doses (Table 3) were well within the range of those for established radionuclear imaging procedures.
TABLE 3.
| Equivalent or effective dose per unit injected activity (mSv/MBq) |
Equivalent or effective dose per PET examination (mSv)‡ |
|
|---|---|---|
| Heart wall | 0.16 | 71 |
| Kidneys | 0.09 | 42 |
| Liver | 0.12 | 53 |
| Red marrow | 0.04 | 17 |
| Spleen | 0.10 | 45 |
| Whole body | 0.02 | 10 |
|
| ||
| Effective dose | 0.03 | 12 |
Trastuzumab protein dose = 50 mg.
Calculations employed averaged time-activity curves (8 patients) from our 111In-MxDTPA-trastuzumab study (7), which were normalized to averaged blood and organ uptake data from the current 64Cu-DOTA-trastuzumab study (6 patients).
Assumes 64Cu injected activity = 450 MBq, the average in the current study.
DISCUSSION
Patient HER2 status and trastuzumab exposure history were more clearly prescribed in the current investigation than in prior imaging studies with trastuzumab (6-8). All patients had biopsy confirmation of HER2 positivity at time of study, and none had received anti-HER2 therapy for at least 4 months prior to imaging.
We have clearly shown that, in spite of the relatively short half life of the radiolabel, 64Cu-DOTA-trastuzumab/PET-CT can effectively detect and quantify tumor uptake in patients with known HER2-positive disease. Other than brain, all anatomic sites common to metastatic breast cancer were included in the patient cohort. Lesions were visualized in all 8 patients examined and were seen in bone, lymph nodes, liver, lung, pleural effusions and breast. Detection sensitivity was 77% on Day 1 and 89% on Day 2 (Table 2). Tumor uptake was substantial by 24 h and, on average, increased modestly between 24 and 48 h. Detection of lymph nodes in the neck, upper thorax and mediastinum is difficult at 24 h due to high blood background, but improves by 48 h (Fig. 2). The chief limitation of 64Cu-DOTA-trastuzumab/PET-CT is that, due to the 13 h half life of 64Cu, it does not provide whole-body coverage with acceptable signal-to-noise ratio and scan duration. Nonetheless, as demonstrated here, 64Cu-DOTA-trastuzumab/PET can be used effectively in disseminated, HER2-positive breast cancer when disease location is defined in advance by 18F-FDG/PET and/or CT.
A second major objective was to establish a trastuzumab protein load that minimizes liver uptake without inhibiting tumor uptake of 64Cu-DOTA-trastuzumab. We observed that adding 45 mg of trastuzumab to the 5 mg of DOTA-trastuzumab delivered with the radioactive injection approximately doubled blood SUV and reduced liver uptake by 75-80% on Days 1 and 2 after radiotracer injection (Fig. 3). These observations are quantitatively similar to those reported by Dijkers, et al., for 89Zr-trastuzumab given with trastuzumab loads of 10 and 50 mg (8).
Comparison with our 111In-MxDTPA-trastuzumab study (trastuzumab load 4-8 mg/kg) suggests that increasing beyond 50 mg trastuzumab dose would not yield further improvement in the pharmacokinetics or biodistribution of 64Cu-DOTA-trastuzumab. We observed no statistically significant difference in tumor uptake between 5 mg and 50 mg doses in this small study. However, other investigations have demonstrated that a significant fraction of tumor binding sites can be occupied at antibody loading doses ≪ 4-8 mg/kg (14). Furthermore, the dissociation constant for 111In-DTPA-trastuzumab-HER2 binding is ~ 10 nM (15), a concentration that very likely would be exceeded in tumors at a trastuzumab load of 4-8 mg/kg. This suggests that HER2 saturation may have contributed to the relatively low tumor detection sensitivity in our 111In-MxDTPA-trastuzumab study (4 lesions visualized in 3 of 7 patients with known lesions).
Heterogeneity of tumor HER2 expression within and among patients is poorly understood (3-5) and may be elucidated by imaging studies with radiolabeled trastuzumab. The high degree of tumor positivity observed in the current study suggests that most lesions in HER2-positive patients have HER2 expression adequate to render them detectable with 64Cu-DOTA-trastuzumab/PET-CT. On the other hand, tumor uptake was also highly variable among and within patients (Fig. 5). That heterogeneity suggests a potential role for 64Cu-DOTA-trastuzumab/PET-CT in the selection of patients for trastuzumab-based therapy.
Implementation and evaluation of the 64Cu-DOTA-trastuzumab PET examinations on 18F-FDG scans to determine region of interest. Tumor uptake and detection sensitivity were only modestly lower for 64Cu-DOTA-trastuzumab than for 18F-FDG. Most lesions were positively visualized with both radiotracers, and 6 CT-positive tumors were detected with 64Cu-DOTA-trastuzumab and not with 18F-FDG. Same-lesion maximum SUVs for 64Cu-DOTA-trastuzumab and 18F-FDG were uncorrelated, and their ratios (64Cu-DOTA-trastuzumab to 18F-FDG) varied by a factor of 22 (Fig. 7). Tumor uptake of 18F-FDG reflects density of glycolytic activity, which in turn depends on viable cell density and tissue oxygenation status (16-18). In breast cancer, tumor uptake of 18F-FDG is positively correlated with tumor aggressiveness (19). For 64Cu-DOTA-trastuzumab, the unproven assumption is that tumor uptake is closely related to HER2 receptor density, which in turn is positively correlated with tumor growth rate and aggressiveness (20). Thus the observed lack of correlation between same-tumor SUVmax for 18F-FDG and 64Cu-DOTA-trastuzumab is perhaps surprising and bears further investigation, given that uptakes of both tracers are thought to be associated with tumor aggressiveness.
The procedures used in this study were well tolerated. As expected, based on the extensive history of the antibody in the treatment of breast cancer, there were no toxicity associated with the trastuzumab or 64Cu-DOTA-trastuzumab administrations. Two patients had assay results that might indicate low-level anti-antibody responses following the 64Cu-DOTA-trastuzumab procedure. However, both patients had positive pre-64Cu-DOTA-trastuzumab baseline assays and intermittently positive assays thereafter. This suggests positivity resulted from something other than the 64Cu-DOTA-trastuzumab procedure, such as prior treatment with trastuzumab and/or the presence of circulating antigen (i. e., HER2 extracellular domain) in the serum, a possibility that we are currently evaluating.
Estimated radiation doses for 64Cu-DOTA-trastuzumab (Table 3) are moderate compared with 18F-FDG and other imaging procedures with radiolabeled antibodies. For the mean administered activity in this study (450 MBq) and 50 mg trastuzumab dose, estimated effective dose and maximum organ (heart wall) equivalent dose for 64Cu-DOTA-trastuzumab are 12 and 71 mSv, respectively. 18F-FDG has effective and critical organ (bladder wall) equivalent doses of 11 and 72 mSv, respectively, for the typical injected activity of 555 MBq (15 mCi) (21). Monoclonal antibodies labeled with 111In incur effective and critical organ (spleen and liver) equivalent doses of approximately 40 and 200 mSv, respectively, for the typical injected activity of 185 MBq (5 mCi) (21). Dijkers, et al., estimated a “radiation dose” (presumably effective dose) of 18 mSv from a 37 MBq (1 mCi) injection of 89Zr-trastuzumab (8).
CONCLUSION
We have shown that, in patients with HER2-positive metastatic breast cancer, tumors rapidly accumulate 64Cu-DOTA-trastuzumab to high concentrations, thus supporting both detection and measurement of tumor uptake by 1 d post injection. The rapid uptake, supplemented by prior knowledge of tumor location afforded by 18FDG/PET-CT, makes 64Cu-DOTA-trastuzumab effective for surveying disseminated disease in spite of the limited half life of 64Cu. We have confirmed that 50 mg trastuzumab protein dose provides a 64Cu-DOTA-trastuzumab biodistribution favorable for tumor imaging. This study demonstrates that 64Cu-DOTA-trastuzumab/PET-CT is a practical and acceptably safe procedure in patients with metastatic breast cancer.
We will next broaden the study to include patients with metastatic breast cancer classified as HER2-negative on pre-scan biopsy and thus correlate tumor uptake of 64Cu-DOTA-trastuzumab with HER2 expression. Beyond that, we envision using 64Cu-DOTA-trastuzumab/PET-CT to individualize treatment regimens that include trastuzumab and other HER2 directed therapies.
Supplementary Material
Acknowledgments
Jose Reyes, CNMT, performed the PET-CT scans. Blood samples were assayed for radioactivity and anti-trastuzumab immune response by Nicole Bowles, BA, and Jing Guo, BS, respectively. The production of 64Cu at Washington University School of Medicine is supported by the Department of Energy.
This work was supported by the Department of Defense (Grant # 1024511) and the National Cancer Institute (Grant # P30 CA33572).
Footnotes
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
References
- 1.Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 3.Simon R, Nocito A, Hübscher T, et al. Patterns of HER2/neu amplification and over-expression in primary and metastatic breast cancer. JNCI. 2001;93:1141–1146. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- 4.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 6.Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24:2276–2282. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 7.Wong JYC, Raubitschek A, Yamauchi D, et al. A pretherapy biodistribution and dosimetry study of indium-111-radiolabeled trastuzumab in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. Cancer Biother Radiopharm. 2010;25:387–394. doi: 10.1089/cbr.2010.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 9.Lub-de Hooge M, Kosterink JGW, Perik PJ, et al. Preclinical characterization of 111In-DTPA-trastuzumab. Br J Pharmacol. 2004;143:99–106. doi: 10.1038/sj.bjp.0705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Bading J, Yazaki PJ, et al. A versatile bifunctional chelate for radiolabeling humanized anti-CEA antibody with In-111 and Cu-64 at either thiol or amino groups: PET imaging of CEA-positive tumors with whole antibodies. Bioconj Chem. 2008;19:89–96. doi: 10.1021/bc700161p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bading JR, Ng TSC, Jacobs RE, Raubitschek A, Colcher D. 64Cu-DOTA-Herceptin: radio-synthesis and initial evaluation in vivo. J Nucl Med. 2009;50(suppl 2):404P. abstract. [Google Scholar]
- 12.Patt YZ, Lamki LM, Haynie TP, et al. Improved tumor localization with increasing dose of indium-111-labeled anti-carcinoembryonic antigen monoclonal antibody ZCE-025 in metastatic colorectal cancer. J Clin Oncol. 1988;6:1220–1230. doi: 10.1200/JCO.1988.6.8.1220. [DOI] [PubMed] [Google Scholar]
- 13.Cheebsumon P, Yaqub M, van Velden FH, Hoekstra OS, Lammertsma AA, Boellaard R. Impact of [18F]FDG PET imaging parameters on automatic tumour delineation: need for improved tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38:2136–2144. doi: 10.1007/s00259-011-1899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donoghue JA, Smith-Jones PM, Humm JL, et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J Nucl Med. 2011;52:1878–1885. doi: 10.2967/jnumed.111.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. 111In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48:1357–1368. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 16.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos R, van der Hoeven JJM, van der Wall E, et al. Biologic correlates of 18Fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–387. doi: 10.1200/JCO.2002.20.2.379. [DOI] [PubMed] [Google Scholar]
- 18.Minn H, Clavo AC, Wahl RL. Influence of hypoxia on tracer accumulation in squamous-cell carcinoma: in vitro evaluation for PET imaging. Nucl Med Biol. 1996;23:941–946. doi: 10.1016/s0969-8051(96)00134-5. [DOI] [PubMed] [Google Scholar]
- 19.Groheux D, Giachetti S, Moretti JL, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 20.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;35:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 21.International Commission on Radiological Protection. Ann ICRP. 1-2. Vol. 38. ICRP Publication 106; 2008. Radiation dose to patients from radiopharmaceuticals - Addendum 3 to ICRP Publication 53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.