Abstract
Male rat sexual behavior has been intensively studied over the past 100 years, but few studies have examined how sexual behavior changes over the course of several days of interactions. In this experiment, adult male rats (n = 12) were given daily access to estrus females for 30 min per day for 15 consecutive days and control males did not interact with females. Ovariectomized females were induced into estrus with hormonal injections, and males interacted with a different female each day. The amount of sexual activity (mounts, intromissions, and ejaculations) was found to cycle with a period of approximately 4 days in most male rats. Additionally, blood was collected every other day following sexual interactions to assess serum testosterone levels. Testosterone was found to peak on the first day of interaction and then fell back to near the level of control rats that did not interact with females. Following the initial peak, testosterone concentrations fluctuated less in males exposed to females than in controls. Sexual activity was not found to predict testosterone concentration. We conclude that when male rats have daily sexual interactions, sexual behavior tends to show cyclic changes and testosterone is significantly elevated only on the first day of interactions.
Keywords: Sexual behavior, Rat, Mating, Testosterone, Ejaculation, Intromission
1. Introduction
Although the rats (Ratus norvegicus) most commonly used in scientific studies have been domesticated for more than one hundred years [1], their physiology and behavior are still not fully understood. Although multi-day cycles in female rat hormonal levels that correspond with changes in sexual behavior are well documented [1-5], to our knowledge no one has previously described male hormonal levels and sexual behavior across consecutive days. Past experiments documenting the sexual behavior and hormonal levels of male rats have focused mainly on males that reach sexual exhaustion during one prolonged interaction with one or more females [6-9]. A few studies have shown differences in sperm count and amount of ejaculate in a number of species across a period of days or weeks, often corresponding with the length of the female estrous cycle but have not investigated the possibility of cycles in male sexual behavior [10-12].
In male rats, sexual behavior consists of mounts (attempts to enter the female's vagina), intromissions (brief insertion of penis into the female's vagina for 200-400 ms), and ejaculations [1]. During a typical sexual interaction, the male performs ten to twelve intromissions and then a prolonged intromission in which the male ejaculates [13]. Typically a male rat can ejaculate six to seven times within four hours of contact with a receptive female [7, 8]. After being allowed to mate ad libitum, a male will eventually experience sexual exhaustion, which is defined by failure to copulate with an available sexually receptive female for at least 30 min [6, 8]. Sexual performance is not completely recovered until 15 days after the onset of sexual exhaustion. For most males, partial recovery begins 72 h following the onset of sexual exhaustion [6, 9], and some males will copulate within 24 h after exhaustion but these males do not achieve more than one ejaculation [8]. Sexual exhaustion is believed to be induced by high circulating levels of prolactin following multiple ejaculations [9]. Various social factors can also modify male rat sexual behavior. Males that are pre-exposed to a receptive female, but not allowed to copulate, are quicker to display sexual behavior when allowed to copulate with a different receptive female [14]. Among males that are housed together, the subordinate males will display less sexual behavior than the dominant males [15].
Sexual behavior in male rats is partially regulated by the hypothalamic-pituitary-gonadal (HPG) axis. Gonadotropin releasing hormone (GnRH) released from the hypothalamus triggers the release of luteinizing hormone (LH), which in turn stimulates the release of testosterone from the testes [16, 17]. Male rats reflexively release testosterone when they smell (anticipatory releases) or mate (ejaculatory release) with a novel receptive female; this testosterone release is in addition to normal surges called ‘spontaneous release’ which occur throughout the day [17-22]. LH is elevated 10 min after exposure to a female, and this is followed by the anticipatory release of testosterone at least 30 min after exposure [17, 20, 23, 24]. A caveat is that it appears that only male rats with prior sexual experience have significant testosterone release following sexual interactions [18]. Experiments with mice indicate that another LH surge cannot be triggered for 25-45 min after the initial LH surge [25]. Additionally, male mice that are not sexually exhausted and are given an injection of testosterone have a decreased latency to mount a receptive female introduced 60 min later [14]. Male rats that do not copulate with receptive females after multiple exposures do not have the normal hormonal surges following exposure to a female, which suggests that these LH surges may be necessary for motivating sexual behavior in rats [23]. Although a threshold level of testosterone is needed to stimulate sexual activity, experimental manipulation of testosterone in male rats suggests that the amount of sexual activity that a male engages in is not directly correlated with circulating testosterone levels [26, 27]. Testosterone levels return to baseline within 1.5 h after exposure to a receptive female [13, 23].
Aromatization of testosterone to estradiol in the medial preoptic area (mPOA) of the hypothalamus is essential for initiation of copulation, while dihydrotestosterone, another testosterone metabolite, is critical for controlling genital reflexes [28]. When mating is inhibited during sexual exhaustion, androgen receptor expression decreases and estrogen receptor expression increases within the mPOA [9]. When the male is recovering from sexual exhaustion, androgen receptors are over-expressed in several other brain regions that may contribute to sexual satiety and recovery including the bed nucleus of the stria terminalis, the medial amygdala, the lateral septum, and the ventromedial hypothalamic nucleus [9]. Thus, testosterone and its metabolites initiate male sexual behavior by acting on key brain regions. However, changes in male testosterone levels over the course of several consecutive days of exposure to females have not been previously studied.
For this research, we tested the sexual behavior and testosterone levels of male rats that were allowed to copulate with females for a limited period of time each day over the course of 15 days. We hypothesized that since the males would not reach exhaustion during the 30 min trials, they would remain sexually active on subsequent days of exposure to estrus females. Additionally, we hypothesized that the amount of sexual activity would be positively correlated with serum testosterone levels measured shortly after interactions with estrus females.
2. Materials and methods
2.1 Subjects
Twenty-four adult male and 16 adult female Long-Evans rats were obtained from Charles River Laboratories (St. Constant, Quebec, Canada). The rats were kept in the Middlebury College Animal Facility in temperature controlled rooms (21.1 ± 2.8°C) on a reversed 12:12 light-dark cycle (lights on at 20:00) to allow for sex testing and blood collection to occur during the rats’ dark cycle. All animals were pair housed and given ad libitum access to 2020X soy-protein-free rodent diet (Harlan Tekland, Indianapolis, IN) and tap water. One rat in the control group died of unknown causes on day 5 of sex testing, which reduced the number of control rats to 11 and caused one of the control rats to be single housed for the remaining 10 days of the experiment. The males were approximately 60 days old at the start of the experiment, while the females were approximately 120 days old because they had been used for another sexual behavior experiment prior to the current experiment. All procedures involving animals were approved by the Middlebury College Institutional Animal Care and Use Committee and were carried out in accordance with ethical guidelines set by the National Institutes of Health.
All females were ovariectomized using standard aseptic surgical procedures. Animals were anesthetized using isoflurane in oxygen (3.5-4.0% during induction and 2.0-2.5% during maintenance). Each ovary was extracted through an approximately 1 cm incision on the dorsal flank below the ribs and above the pelvis. Absorbable 4/0 chromic gut sutures (Ethicon, Somerville, NJ, USA) were used to suture the muscle layer and 4/0 ethilon sutures and/or surgical staples (Ethicon) were used to close the skin layer. The analgesic Ketofen (5 mg/kg body mass, s.c.) was given to each female just prior to starting surgery and again 12-24 h after surgery. Females were given at least one week of recovery from surgery prior to starting behavioral testing.
Hormone replacement was used to induce estrus in the ovariectomized females. This consisted of subcutaneous injections of estradiol benzoate (10 μg/rat) in 0.1 mL sesame oil 48 h before sex testing (13:00) and progesterone (500 μg/rat) in 0.1 mL sesame oil 4 h before sex testing (09:00). Prior to the current experiment, all females were used for sex testing in another experiment, which allowed us to select non-aggressive females that cons istently displayed lordosis during induced estrus. Additionally, the females were given one full cycle of induced estrus (four days) without interacting with a male prior to the start of sex testing for the current experiment.
2.2 Sex Testing
Male rats in the experimental group interacted with sexually receptive females for 30 min each day for 15 days. Males were handled for 4 min per day for four days prior to the start of sex testing. All sex testing took place during the rats’ dark cycle (13:00-15:00) under dim red lighting. Rats were placed in Plexiglas testing boxes (20×40×40 cm) with TEKFresh bedding (Madison, WI). Females were placed into the testing boxes for 5 min of habituation prior to adding the males, and no male was exposed to the same female more than once over the course of the experiment. The control males were not exposed to females at all during the course of the experiment. All trials were video recorded, and event-tracking software was used to quantify the frequency of behaviors for each trial (JWatcher ver. 1.0). The total number of attempted mounts (no pelvic thrusts), mounts (with pelvic thrusts), intromissions, ejaculations, attempted mounts by females (no pelvic thrusts), mounts by females (with pelvic thrusts), and wet dog shakes (a shaking of the body from side to side, indicative of anxiety) were recorded [20, 29, 30]. Hit rate was used as an index of copulatory efficiency: hit rate = number of intromissions/(number of intromissions + number of mounts) [31]. We also recorded the incidence of aggressive behaviors: boxing (repeated blows exchanged while both rats are upright) and pins (ventral/ventral contact is achieved as one rat forces and then immobilizes the other rat onto its back for at least two seconds) [20, 29].
2.3 Testosterone Assay
Blood samples were taken every other day beginning two days before the first day of sex testing (baseline) and continuing until the final day of sex testing (8 samples total per rat). To draw blood, the animals were placed in a plastic restrainer, and the topical anesthetic Cetacaine (Cetylite Industries, Inc., Pennsauken, NJ) was applied to the tail. Approximately 1 mm of the tail tip was then cut using a sterile blade and 0.3 ml of blood was collected into a microcentrifuge tube within 6 min. Following each blood collection, the tail tip was pinched to induce clotting. For rats in the sex group, blood was collected within 15 min of the end of sex testing. Samples were stored overnight at 4°C to coagulate. The following day, samples were centrifuged for 15 min at 9000 g and serum was extracted and stored at −20°C.
Serum testosterone was assayed using testosterone coated-tube radioimmunoassay kits (Siemens Healthcare Diagnostics, Inc., Washington D.C.), which had a lower limit of detection of 0.04 ng/ml. The testosterone antibody had 3.4% cross-reactivity with 5α-dihydrotestosterone, <0.5% cross-reactivity with other androgens and <0.1% cross-reactivity with glucocorticoids, estrogens, or progesterone. The average inter-assay percent coefficient of variation was 2.95%, based on 11 samples that were run using two separate kits. The average intra-assay percent coefficient of variation for all samples that were run in duplicate was 3.92%. Only 11 samples were not run in duplicate due to insufficient serum.
2.4 Data Analysis
For all analyses, SPSS 19.0 was used with a significance level of α=0.05. Repeated measures analyses of variance (ANOVA) were used to determine how each behavior changed over the 15 days of testing, and when significant differences over time were detected Fisher's LSD test was used to compare each day to the day with the highest average incidence of the behavior in question (reference day). The use of a reference day more clearly showed the daily patterns in the data than what could be obtained by reporting all possible pairwise comparisons among days. Due to a video recording error on day 15 of sex testing, two rats had only 21 min of their 30 min interactions recorded; data from the recorded portion of these two samples was used for all analyses, potentially underestimating behaviors for day 15. Repeated measures ANOVAs were used to determine how testosterone concentration changed over the course of testing and how treatment affected testosterone concentration. When there was a significant day×treatment interaction, t-tests were performed to compare the two groups on each day. For all the testosterone analyses, only data from 10 control animals was utilized due to insufficient blood collection for one animal.
A mixed General Linear Model was used to examine the relationship between behavioral variables (attempted mounts, mounts, intromissions, ejaculations, and wet dog shakes) and testosterone concentration across sex days, which allowed us to apply multivariate regression while accounting for repeated measurements. The day of sex testing was considered a fixed factor to allow for the apparent non-linear relationship between day of sexual activity and testosterone concentration using an autoregressive covariance structure. The data were analyzed with each behavior and day as main effects as well as with behavior×day interaction terms.
3. Results
3.1 Testosterone
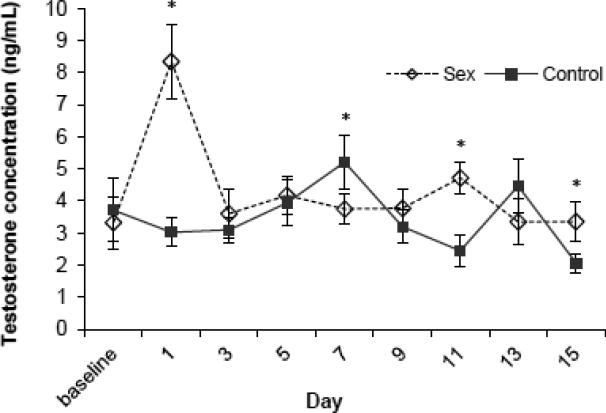
Serum testosterone levels showed significant changes during the 15 days of sexual interactions [F (8,160) = 3.92, P<0.0005], with a major peak in testosterone occurring among the sex treatment rats on day 1 of testing (Fig. 1). There was also a significant day×treatment interaction [Fig. 1; F (8,160) =5.89, P < 0.0005], but the main effect of treatment was not significant (P = 0.177). For days 1, 11 and 15, the rats in the sex treatment had significantly higher testosterone levels than did the control rats (all P < 0.036), and for day 7 the control rats had significantly higher testosterone levels than did the rats in the sex treatment (P = 0.045).
Fig. 1.
Serum testosterone levels (mean ± SEM) in male rats in the sex group (n = 12) and the control group (n = 10) collected two days before sex testing (baseline) and every other day during sex testing. There was a significant day effect (P<0.0005) as well as a significant day×treatment interaction (P<0.0005). Post-hoc tests indicated significant difference between sex and control groups on that specific day (*P<0.05).
3.2 Sexual Behavior
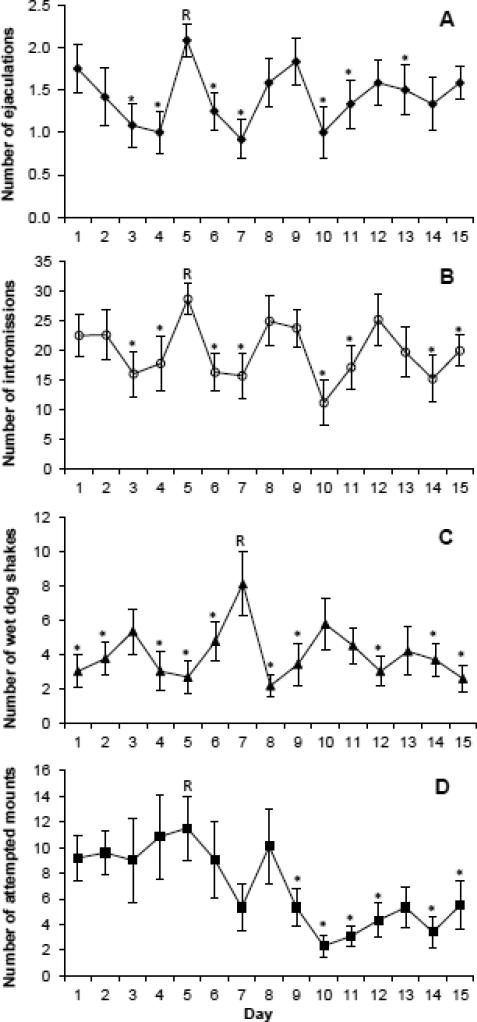
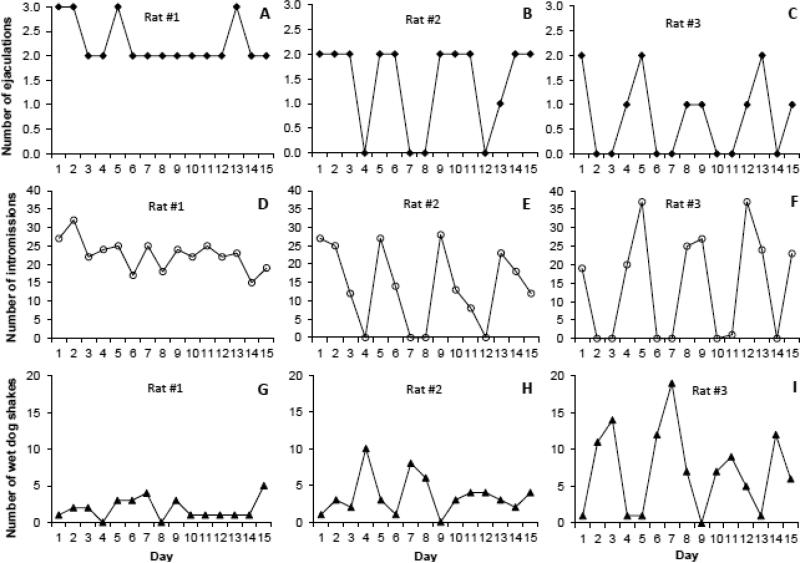
Number of ejaculations [F (14,154) = 2.13, P = 0.013; Fig. 2A] and intromissions [F (14,154) = 2.09, p = 0.015; Fig. 2B] changed significantly over the course of testing. The males showed cyclic changes in the number of intromissions and ejaculations based on comparisons to the reference day with the highest frequency of these behaviors (Fig. 2A and 2B). These cycles in sexual activity are even clearer when examining the behavior of individual rats (Fig. 3). While some rats did not show cyclicity in their behavior, most showed cycles with peaks in activity approximately every 3-4 days (Fig. 3). Wet dog shakes also varied significantly across days in a cyclic manner [F (14,154) = 2.47, p = 0.004; Fig. 2C]. Attempted mounts decreased significantly over the course of testing [F (14,154) = 2.49, p = 0.003; Fig 2D]. There were no significant changes across sex testing days for hit rate (p = 0.076), mounts (p = 0.314), boxing (p = 0.616), pins by males (p = 0.536), pins by females (p = 0.489). Female mount attempts (p = 0.058) and female mounts (p = 0.086) approached significance due to increased frequency of these behaviors on days when males were less sexually active.
Fig. 2.
Number of behaviors (mean ± SEM) per day for males that engaged in 30 min sexual interactions each day (n=12). For each behavior, there were significant changes over the days of testing (all P≤0.013). Post hoc tests were used to compare the number of behaviors on each day to the reference day (R), which was the day with the highest average incidence of each behavior (*P<0.05).
Fig. 3.
Number of instances of each behavior across 15 days of 30 min sex trials for Rat #1, which did not show clear cyclicity (A, D, G), and for Rats #2 and #3, which showed clear cycles in behaviors (B, E, H and C, F, I).
Mixed model analyses revealed a significant (positive on all days relative to the last day of testing) relationship between serum testosterone concentration and day of sex testing (all P ≤ 0.0005) with no significant main effects of wet dog shakes, mounts, intromissions, ejaculations or attempted mounts on testosterone concentration (all P > 0.45) when each were analyzed separately. When interaction terms were added to each model (behavior×day), the only interaction term approaching significance was intromission×day [F (7,60.5) = 1.957, p = 0.076], and in this model there was no main effect of intromissions (p = 0.546) and day still had a significant effect on testosterone concentration [F (7,65.7) = 3.787, p = 0.006].
4. Discussion
When male rats were exposed to a sexually receptive female for 30 min every day for 15 days, we observed evidence of cyclicity in the amount of sexual behavior. Specifically there were three to four day cycles in the number of ejaculations, intromissions and wet dog shakes. The majority of males were found to ‘cycle’ in this way, but there were some animals that did not show cyclicity in their sexual activity. A decrease over the 15 days in attempted mounts illustrates that males learned how to accurately mount a female. We also found that there was a sharp peak in testosterone following the first day of access to sexually receptive females, but following this change serum testosterone leveled off to near baseline and was more stable from day to day than that observed for control males. We did not find any significant relationships between the amount of sexual behavior and testosterone levels when accounting for testosterone changes over the course of the experiment.
The cyclicity that we observed for male sexual behavior has not been reported previously. Sexual exhaustion has mainly been examined it in the context of one continuous exposure to one or more estrus females [8, 9]. Studies of this type find that males can ejaculate 6-8 times before they are sexually exhausted [7, 8, 28]. The very low levels of sexual behavior on day three of sex testing in our study suggest that two consecutive days of sexual encounters for 30 min each are enough to cause sexual exhaustion in most male rats. This extreme cyclicity was unexpected given that a pervious study with a similar timeline for sex testing did not report any significant changes in sexual behaviors over the course of the experiment [32], but the researchers did mention that rats did not ejaculate on every day. However, a variety of experiments indicate that males show cycles between days in the amount of ejaculate produced per ejaculation [10-12]. In an experiment using male mice, daily samples of exfoliated cells from the urethra indicated a cyclical variation in cell types consistent with the length of the female estrus cycle [10]. Additional evidence of cycles in male rodent fecundity comes from an analysis of male rats fitted with girdles to prevent penile grooming, which indicated that spontaneously ejaculated seminal plugs varied in dry weight with a mean period of 4.4 days [12]. Cyclicity in male rat sexual behavior and sperm count may be adaptive since it approximates the female rat estrus cycle of approximately four days [11, 12, 33].
Given that sexual activity heightens HPG axis activity, which in turn decreases immune function, it may be beneficial for males to decrease sexual activity and HPG axis activity during a females’ non-receptive period [34]. Other potential costs of sustained sexual activity that may contribute to natural cyclicity include distraction from other activities necessary for survival (e.g., feeding and predator avoidance) and reduced fertility due to too many ejaculations [35]. Males have limited stores of sperm and cannot ejaculate repeatedly without time to replace depleted stores of sperm and other ejaculate materials [36-38].
There was also cyclicity in the number of wet dog shakes, which can be used as a proxy for 5-HT2A receptor activation in the brain [30]. The cyclicity of wet dog shakes makes sense when taken into in conjunction with the cyclicity of sexual behavior since these have been shown to be negatively correlated [31, 39, 40]. This relationship has been best demonstrated by Watson and Gorzalka (1990) who found that the male rats that were the most sexually active also had the lowest incidence of wet dog shakes. The increase in wet dog shakes during periods of low sexual activity may be due to increased corticosterone, which also influences serotonin activity in the brain and inhibits sexual behavior [31, 40].
This experiment does not provide evidence of a strong relationship between the amount of sexual activity and testosterone. The single peak in testosterone was a surprising result given that studies of single sexual encounters find that testosterone levels remain elevated for up to 1.5 h following exposure to receptive females [23]. Additionally, the peak in testosterone on the first day of interactions is contrary to research by Bonilla-Jaime et al. (2006), which indicated that only males with prior sexual experience had an increase in testosterone levels. None of our male rats had sexual experience prior to starting the experiment. These differences may be due to difference in rat strain, as Wistar rats were used by Bonilla-Jamie et al. (2006). Additionally, ‘experienced’ males had not had contact with a female for 3 days before the final sexual encounter in the Bonilla-Jaime et al. (2006) experiment. Perhaps there are peaks in testosterone in rats that have intermittent exposure to estrus females, whereas daily exposure eliminates these peaks.
The single initial peak in testosterone observed on the first day of sexual interaction may be beneficial as compared to a daily spike because of the negative impacts of high levels of testosterone: elevated aggression [41], lowered immune responses [42], and increased risk taking [43-45]. Additionally, the single increase in testosterone may induce activational effects in the brain that make further increases in testosterone unnecessary as long as access to estrus females continues. Another possible explanation for our results is that since sex testing took place at the same time every day, the males likely became conditioned to the testing context and may have had anticipatory releases of testosterone before even being placed with the females, and therefore their testosterone concentrations may have returned to baseline by the time of blood collection [46].
Following the initial spike in testosterone, the male rats exposed to females showed more stable serum testosterone levels than did the control males. Although a certain level of testosterone is needed to induce sexual activity, there is little evidence that additional increases in testosterone correlate with increased sexual behavior [26, 41]. In contrast the control males had small fluctuations in testosterone concentration between days, possibly indicating responsiveness to environmental factors such as cage cleaning or any other stressors. It may be that having a stable testosterone level to activate sexual activity is beneficial when females are present, but the lack of response to environmental factors (for example food availability or stressors) when females are not present might be detrimental to survival [47-50].
Given the previously unreported cyclicity of male rat sexual activity, more research should be done to determine the prevalence of this pattern. Given the limited research on male sexual cycles involving physiological changes in the amount of ejaculate, it would be valuable to repeat such experiments in conjunction with measures of sexual activity and testosterone to investigate whether changes in the amount of ejaculate and sperm count are positively correlated with the amount of sexual behavior exhibited. Among sperm-limited species, it may be common for males to show cyclicity in sexual activity and amount of ejaculate that correspond with behavioral estrus cycles in females. One would also predict that synchronous estrus among females would favor more cyclcity among males relative to species with nonsynchronous estrus.
Research Highlights.
Male sexual behaviors had a 3-4 day cycle over 15 days of 30 min interactions.
Serum testosterone increased on the first day of sexual behavior interactions.
Serum testosterone returned to baseline on subsequent days of sexual interactions.
Serum testosterone levels were not related to amount of sexual behavior.
Acknowledgements
We thank Vicki Major and the animal care staff for their assistance. This project was funded by Middlebury College and the Vermont Genetics Network (INBRE grant number 2P20RR016462) from the INBRE Program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this project are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Memoirs of the University of California. 1922;6:1–148. [Google Scholar]
- 2.McClintock MK. Estrous synchrony and its mediation by airborne chemical communication (rattus norvegicus). Horm Behav. 1978;10:264–76. doi: 10.1016/0018-506x(78)90071-5. [DOI] [PubMed] [Google Scholar]
- 3.Mcclintock MK, Adler NT. The role of the female during copulation in wild and domestic norway rats (Rattus norvegicus). Behaviour. 1978;67:67–96. [Google Scholar]
- 4.Schank JC. Do norway rats (rattus norvegicus) synchronize their estrous cycles?. Physiol Behav. 2001;72:129–39. doi: 10.1016/s0031-9384(00)00395-4. [DOI] [PubMed] [Google Scholar]
- 5.Schank JC. Oestrous and birth synchrony in norway rats, rattus norvegicus. Anim Behav. 2001;62:409–15. doi: 10.1016/s0031-9384(00)00395-4. [DOI] [PubMed] [Google Scholar]
- 6.Beach FFA, Jordan L. Sexual exhaustion and recovery in the male rat. Q J Exp Psychol. 1956;8:121–33. [Google Scholar]
- 7.Mas M, Fumero B, Fernandezvera JR, Gonzalezmora JL. Neurochemical correlates of sexual exhaustion and recovery as assessed by in-vivo microdialysis. Brain Res. 1995;675:13–19. doi: 10.1016/0006-8993(95)00029-p. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Manzo G. Blockade of the establishment of the sexual inhibition resulting from sexual exhaustion by the coolidge effect. Behav Brain Res. 1999;100:245–254. doi: 10.1016/s0166-4328(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 9.Phillips-Farfan BV, Fernandez-Guasti A. Endocrine, neural and pharmacological aspects of sexual satiety in male rats. Neurosci Biobehav Rev. 2009;33:442–455. doi: 10.1016/j.neubiorev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Jäurnebrand K, Kihlströum JE. A cyclic variation in the exfoliation of cells from the urethra of the male mouse. Acta Physiol Scand. 1969;75:518–22. doi: 10.1111/j.1748-1716.1969.tb04406.x. [DOI] [PubMed] [Google Scholar]
- 11.Kihlstrom JE. A sex cycle in the male. Experientia. 1966;22:630–2. doi: 10.1007/BF01895296. [DOI] [PubMed] [Google Scholar]
- 12.Kihlström JE. Periodic variation in the amount of semen yielded at spontaneous ejaculations of the male rat. Acta Physiol Scand. 1965;65:61–4. [Google Scholar]
- 13.Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neil JD, editors. The physiology of reproduction. Raven Press Ltd.; New York: 1994. Chapter 35. [Google Scholar]
- 14.James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musuculus). Physiol Behav. 2002;75:287–294. doi: 10.1016/s0031-9384(01)00666-7. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard RJ, Dullogg L, Markham C, Nishimura O, Compton JN, Jun A, Han C, Blanchard DC. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol Behav. 2001;72:245–254. doi: 10.1016/s0031-9384(00)00403-0. [DOI] [PubMed] [Google Scholar]
- 16.Neave N. Hormones and Behavior: a psychological approach. Cambridge University Press; Cambridge, U.K.: 2008. [Google Scholar]
- 17.Nyby JG. Reflexive testosterone release: A model system for studying the nongenomic effects of testosterone upon male behavior. Front Neuroendocrinol. 2008;29:199–210. doi: 10.1016/j.yfrne.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49:376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Kamel F, Wright WW, Mock EJ, Frankel AI. The influence of mating and related stimuli on plasma levels of luteinizing hormone, follicle stimulating hormone, prolactin, and testosterone in the male rat. Endocrinology. 1977;101:421–429. doi: 10.1210/endo-101-2-421. [DOI] [PubMed] [Google Scholar]
- 20.Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: A review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- 21.Purvis K, Haynes NB. Effect of the odour of female rat urine on plasma testosterone concentrations in male rats. J Reprod Fert. 1978;53:63–65. doi: 10.1530/jrf.0.0530063. [DOI] [PubMed] [Google Scholar]
- 22.Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- 23.Kamel F, Frankel AI. Hormone release during mating in the male rat: Time course, relation to sexual behavior, and interaction with handling procedures. Endocrinology. 1978;103:2172–2179. doi: 10.1210/endo-103-6-2172. [DOI] [PubMed] [Google Scholar]
- 24.Richardson HN, Nelson ALA, Ahmed EI, Parfitt DB, Romeo RD, Sisk CL. Female pheromones stimulate release of luteinizing hormone and testosterone without altering GnRH mRNA in adult male syrian hamsters (mesocricetus auratus). Gen Comp Endocrinol. 2004;138:211–217. doi: 10.1016/j.ygcen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Coquelin A, Bronson FH. Episodic release of luteinizing hormone in male mice: Antagonism by a neural refractory period. Endocrinology. 1981;109:1605–1610. doi: 10.1210/endo-109-5-1605. [DOI] [PubMed] [Google Scholar]
- 26.Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–86. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- 27.Smith ER, Damassa DA, Davidson JM. Plasma testosterone and sexual behavior following intracerebral implantation of testosterone propionate in the castrated male rat. Horm Behav. 1977;8:77–87. doi: 10.1016/0018-506x(77)90022-8. [DOI] [PubMed] [Google Scholar]
- 28.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43:205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 30.Yap C, Taylor D. Involvement of 5-HT<SUB>2</SUB>receptors in the wet-dog shake behaviour induced by 5-hydroxytryptophan in the rat. Neuropharmacology. 1983;22:801–4. doi: 10.1016/0028-3908(83)90123-5. [DOI] [PubMed] [Google Scholar]
- 31.Brotto LA, Gorzalka BB, Hanson LA. Effects of housing conditions and 5-HT2A activation on male rat sexual behavior. Physiol Behav. 1998;63:475–479. doi: 10.1016/s0031-9384(97)00482-4. [DOI] [PubMed] [Google Scholar]
- 32.Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an intial elevation in stress hormones. PLoS One. 2010;5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers JB. Hormonal control of sexual receptivity during the estrous cycle of the rat. Physiol Behav. 1970;5:831–5. doi: 10.1016/0031-9384(70)90167-8. [DOI] [PubMed] [Google Scholar]
- 34.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr. Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 35.Bancroft J. Central inhibition of sexual response in the male: A theoretical perspective. Neuroscience & Biobehavioral Reviews. 1999;23:763–84. doi: 10.1016/s0149-7634(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 36.Dewsbury DA. Ejaculate cost and male choice. The American Naturalist. 1982;119:601–630. [Google Scholar]
- 37.Pessah H, Kochva E. The secretory activity of the seminal vesicles in the rat after copulation. Biol Reprod. 1975;13:557–560. doi: 10.1095/biolreprod13.5.557. [DOI] [PubMed] [Google Scholar]
- 38.Tlachi-López JL, Eguibar JR, Fernández-Guasti A, Lucio RA. Copulation and ejaculation in male rats under sexual satiety and the coolidge effect. Physiol Behav. 2012;106:626–30. doi: 10.1016/j.physbeh.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Watson NV, Gorzalka BB. Relationship of spontaneous wet dog shakes and copulatory behavior in male rats. Parmacol. Biochem. Behav. 1990;37:825–829. doi: 10.1016/0091-3057(90)90569-4. [DOI] [PubMed] [Google Scholar]
- 40.Gorzalka BB, Hanson LA. Sexual behavior and wet dog shakes in the male rat: Regulation by corticosterone. Behav Brain Res. 1998;97:143–151. doi: 10.1016/s0166-4328(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 41.Lumia AR, Thorner KM, McGinnis MY. Effects of chronically high doses of the anabolic androgenic steroid, testosterone, on intermale aggression and sexual behavior in male rats. Physiol Behav. 1994;55:331–5. doi: 10.1016/0031-9384(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed SA, Penhale W, Talal N. Sex hormones, immune responses, and autoimmune diseases. mechanisms of sex hormone action. The American journal of pathology. 1985;121:531. [PMC free article] [PubMed] [Google Scholar]
- 43.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABA(a) receptors in the rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 44.Edinger KL, Frye CA. Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol Behav. 2007;92:443–453. doi: 10.1016/j.physbeh.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Svensson AI, Akesson P, Engel JA, Soderpalm B. Testosterone treatment induces behavioral disinhibition in adult male rats. Parmacol Biochem Behav. 2003;75:481–490. doi: 10.1016/s0091-3057(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 46.Graham J, Desjardins C. Classical conditioning: Induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–41. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- 47.Badger TM, Lynch EA, Fox PH. Effects of fasting on luteinizing hormone dynamics in the male rat. J Nutr. 1985;115:788–797. doi: 10.1093/jn/115.6.788. [DOI] [PubMed] [Google Scholar]
- 48.Howland BE, Skinner KR. Effect of starvation on gonadotropin secretion in intact and castrated male rats. Can J Physiol Pharmacol. 1973;51:759–762. doi: 10.1139/y73-115. [DOI] [PubMed] [Google Scholar]
- 49.Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Martinez-Garcia R, Velazquez-Moctezuma J. Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44:327–337. doi: 10.1016/j.yhbeh.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Holmäng A, Björntrop P. The effects of testosterone on insulin sensitivity in male rats. Acta Physiol Scand. 1992;146:505–10. doi: 10.1111/j.1748-1716.1992.tb09452.x. [DOI] [PubMed] [Google Scholar]