Abstract
Although most studies on integration and modularity have focused on variation among individuals within populations or species, this is not the only level of variation for which integration and modularity exist. Multiple levels of biological variation originate from distinct sources: genetic variation, phenotypic plasticity resulting from environmental heterogeneity, fluctuating asymmetry from random developmental variation and, at the interpopulation or interspecific levels, evolutionary change. The processes that produce variation at all these levels can impart integration or modularity on the covariance structure among morphological traits. In turn, studies of the patterns of integration and modularity can inform about the underlying processes. In particular, the methods of geometric morphometrics offer many advantages for such studies because they can characterize the patterns of morphological variation in great detail and maintain the anatomical context of the structures under study. This paper reviews biological concepts and analytical methods for characterizing patterns of variation and for comparing across levels. Because research comparing patterns across level has only just begun, there are relatively few results, generalizations are difficult and many biological and statistical questions remain unanswered. Nevertheless, it is clear that research using this approach can take advantage of an abundance of new possibilities that are so far largely unexplored.
Keywords: allometry, covariation, evolution, geometric morphometrics, modularity, morphological integration
1. Introduction
Although the underlying ideas were already present in the thinking of nineteenth-century pioneers such as Georges Cuvier and Charles Darwin [1] and the modern concept was formulated in the 1950s [2], it is mainly in the past two decades that morphological integration and modularity have become prominent subjects in evolutionary biology [3–7]. Most empirical studies have focused on integration within populations, investigating how the variation among individuals is structured. This is only one aspect of the problem, however, because the concept of integration also applies at different levels [4], including genetic and environmental integration [8,9], integration of fluctuating asymmetry within individuals [9–15] and evolutionary integration across taxa in a clade [16–21]. Most plants, because of their modular body plans, offer additional opportunities to study integration among structures such as leaves or flowers within and among individuals in a population [22,23]. The distinction of different levels makes it possible to compare patterns of integration between them, in order to gain insight into evolutionary and developmental processes from the resemblance of patterns across the levels of integration [8–12,16,24–27].
In a similar way, different levels have long been distinguished for allometry, according to the origin of variation in size that is the basis for the allometric relationship [28–31]. Ontogenetic allometry is associated with size increase due to growth in individuals of a single species, evolutionary allometry is associated with size differences among species and static allometry is due to variation of size within a population and in a single growth stage. A variety of comparisons have been made across levels of allometry [30–32], increasingly with the new methods of geometric morphometrics [25,33–35]. These comparisons across levels of allometry can serve as models for analyses of morphological integration and modularity.
This review aims to summarize the levels of integration and modularity that have been considered in most detail, to outline the conceptual background for these levels and to provide information on the methods and experimental or comparative context. Such analyses incorporating multiple levels of variation are increasingly widespread and are a promising approach for studies of integration and modularity [25,36].
2. The concepts of integration and modularity
The concepts of morphological integration and modularity are inherently connected [4]. Integration is the tendency of different traits to vary jointly, in a coordinated manner, throughout a morphological structure or even a whole organism. Modularity exists if integration is concentrated within certain parts or regions of a structure, the modules, but is relatively weak between these modules. Morphological modularity therefore means that integration in a structure is compartmentalized, with strong within-module and weak between-module integration [37]. This conceptual link between integration and modularity goes back to the original formulation of integration, as the ‘ρ-groups’ and ‘F-groups’ discussed by Olson & Miller [2] closely correspond to what are now called static and functional modules. Whether a structure is integrated or modular at a particular level of integration depends on the processes that produce the integration at that level. As a consequence, the levels of integration and modularity are the same and can be discussed jointly.
Hallgrímsson et al. [38] suggested that integration is best viewed as a dispositional concept, the propensity of a system to produce covariation, rather than covariation of traits as it is directly observable. Such dispositional concepts were proposed, for instance, by Wagner & Altenberg [3] who distinguished ‘variability’, the propensity of a system, from ‘variation’, the actual variation in a population. Wagner and Altenberg introduced this distinction in direct analogy to the difference between the concepts of solubility of a substance versus a particular solution. The concept of solubility has great predictive power because a set of experiments with one batch of the substance and solvent, say sodium chloride and water, can make precise predictions on the behaviour of the same substance and solvent in other instances. This powerful generalization from one set of experiments to a wide range of other instances is possible because different batches of the same substance and solvent are identical in the relevant properties and thus behave in essentially the same way from one instance to another when mixed in the same ratio and under the same conditions (temperature, pressure, etc.). This is not necessarily the case for biological individuals and populations, as has been pointed out repeatedly (e.g. Mayr's [1] vehement criticism of ‘typological’ thinking). Biological individuals and populations are unique and historically contingent, and extrapolation from one instance to another is always fraught with substantial uncertainty and imprecision. The dispositional properties of any one individual or population can therefore only be ascertained by observation of the actual properties in specific instances.
Instead of treating integration as a dispositional concept, it might be more helpful to think about this problem from a multilevel perspective. The propensity for traits to covary at a particular level is often due to the association of processes at a lower level, and thus depends on the standing stock of variation and covariation at that level. For instance, the propensity of a set of traits in a population to evolve in a correlated manner stems from genetic covariation in the population, because of pleiotropy or linkage disequilibrium between loci affecting different traits. In turn, pleiotropy can originate because loci segregating in the population have allelic effects on multiple traits simultaneously, for instance, because gene products of these loci are involved in multiple developmental pathways, or because there are developmental interactions between the pathways that give rise to different traits [4]. This hierarchical nature of integration underscores the importance of multilevel studies of integration and modularity [24,25].
3. Levels of integration
Depending on the processes responsible for integration and modularity, several levels of integration can be distinguished (figure 1). Analyses across levels have been central to analyses of morphological integration and modularity since Olson & Miller [2] introduced the concept of integration and compared ‘ρ-groups’ and ‘F-groups’, which they defined as sets of highly correlated or functionally related measurements. These comparisons were later expanded by adding further levels, including genetic, environmental and evolutionary integration [8,22,24,26].
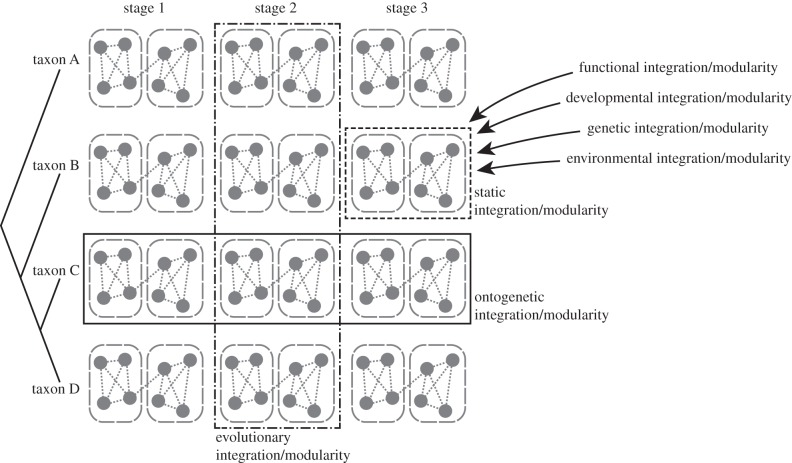
Figure 1.
Different levels of integration and modularity. The diagram contains four related species, each with three ontogenetic stages. Ontogenetic integration and modularity concern the variation across stages within each species, whereas the evolutionary level focuses on the variation among species at any given stage. The static level is within one species and stage. Functional, developmental, genetic and environmental integration and modularity are usually studied in a static context, that is, at one particular ontogenetic stage for a given species. Pooled within-group analyses can be used to summarize patterns.
The following overview of levels is not meant to be a complete enumeration of all possible levels, but focuses on those that have been used in empirical studies, particularly those using geometric morphometrics. The first five levels in this compilation concern variation within homogeneous samples of individuals and the components of integration that reflect the processes producing integration and modularity: static integration and the developmental, functional, genetic and environmental components that contribute to it. These components are usually studied in a static context (figure 1), that is, in a single population and at a particular ontogenetic stage (most often adults). The remaining levels concern analyses across multiple growth stages or species: ontogenetic and evolutionary integration.
(a). Static integration
Of the levels named in figure 1, the most frequently used in empirical studies of integration and modularity is the static level; this is simply the level variation of variation among individuals in a homogeneous sample, where all specimens are from the same species and ontogenetic stage. This level of variation is so widely used in studies of integration that it usually is not named at all. For consistency, I adopted the name from the corresponding level of allometry [28–31].
Even in comparisons of integration or modularity among species or other groups, the patterns that are being compared are most often computed at the static level [15,39–44]. This level of variation is therefore the basis for many evolutionary studies.
(b). Developmental integration
Developmental integration is due to interactions between developmental processes that give rise to different traits, and therefore produce covariation between them. A variety of different developmental mechanisms can produce such interactions, but all of them share the key property that variation can be passed directly from a single origin to multiple traits [4,38,45–47]. Integration by such direct interactions among developmental processes has implications for evolution that differ from other mechanisms producing covariation [47]. Developmental integration is usually studied as a component of static integration.
To quantify and characterize developmental integration in morphometric data, it is useful to analyse covariation in signed fluctuating asymmetry [48,49]. Because fluctuating asymmetry originates from random variation in developmental processes, the asymmetries of different traits are correlated only if there are direct interactions between developmental pathways that produce the traits [46,47]. This approach has been applied in a broad range of animal systems [10,12–15,27,36,37,48–55]. Whereas this reasoning applies widely to motile organisms, including most animals, caution is necessary in applying it to sessile organisms like most plants, where a part of fluctuating asymmetry may be due to plastic responses to heterogeneity in microenvironmental factors [25,56].
Because the data used for this type of analysis are a snapshot at a particular stage, the patterns of integration are the cumulative effect of all developmental processes up to that stage. Because processes acting at various stages of development can have different patterns of integration and modularity, involving differing sets of traits, that overlie each other in various manners, the cumulative effects of all these processes may not have a clear modular structure even if all individual processes are strictly modular. Hallgrímsson et al. [38] used the metaphor of a ‘palimpsest’ for this situation, where processes at later stages partly overwrite the patterns of integration laid down by earlier processes, without completely extinguishing the earlier patterns. The total effect of these partly incongruent modular covariance structures, overlaid on each other, may produce the impression of a mainly integrated system where no individual hypothesis of modularity holds, even though all contributing processes may act in a clearly modular manner. For instance, the patterns of integration due to postnatal bone remodelling under mechanical loading of functional parts may not be the same as the patterns due to the embryonic origins of the same structures. Such apparently integrated patterns are found in many empirical studies, and there is evidence that patterns of integration change during ontogeny [38,57–61]. Disentangling these effects of sequential processes that are overlaid on top of each other remains a serious challenge for morphometric studies of integration and modularity that has not yet been solved adequately.
The qualifier ‘developmental’ can lead to misunderstandings, and it is important to keep in mind that developmental integration, as it is defined here, does not subsume any type of integration that is linked to development in any way, but exclusively the integration that is due to interactions among developmental processes that form the different traits. This restriction rules out several possible sources of information. Although developmental processes are intimately connected to various genes, genetic approaches such as quantitative trait locus mapping provide estimates of pleiotropy [62], but cannot be used to infer developmental interactions because pleiotropic effects may arise because products of a locus are involved in separate developmental processes that take place in different locations and at different times in development, and does not necessarily involve any direct interactions between them [47]. Likewise, studies of ontogenetic integration, with data concerning changes in traits during ontogeny, do not provide reliable information on developmental integration because ontogenetic changes can occur in many traits simultaneously during ontogeny even though there may be no interactions between the corresponding developmental processes. To infer developmental interactions from these approaches, they would have to be combined with manipulative experiments. To my knowledge, the only observational approach to infer developmental interactions is the analysis of correlations in fluctuating asymmetry [46,47].
(c). Functional integration
Functional integration is based on associations between parts interacting in some functional context. For instance, the upper and lower jaws interact in biting, chewing or gnawing, and need to be coordinated to achieve proper occlusion in order to perform those functions effectively [17,63]. Information about functional implications of morphological changes can be obtained from studies combining biomechanical and morphometric approaches [64]. It is intuitive that changes in such systems, and the coordination among parts within them, are likely to have significant fitness consequences. Functional considerations have played a key role in studies of morphological integration since their inception, as the ‘F-groups’ of Olson & Miller [2] were sets of traits related to a common function.
More recently, some authors have argued that developmental systems may evolve adaptively so that patterns of developmental and functional integration should match [3,65]. This ‘matching hypothesis’ can be tested particularly well in systems where functional and developmental units are incongruent, either because a single developmental unit is subdivided into sections that perform different functions or because several parts with distinct developmental origins contribute jointly to a function [66]. Under these circumstances, the matching hypothesis predicts that developmental processes would evolve to match the functional structure of the system. Empirical tests of the matching hypothesis have been relatively rare so far. A morphometric study of integration in the forewings of male crickets found no developmental or genetic modularity corresponding to the functional subdivision of the wing into regions with distinct roles in sound production [67]. More often, however, functional and developmental units in a structure are at least broadly congruent, so that functional and developmental considerations provide similar predictions for morphological integration and modularity and explicit tests of the matching hypothesis are difficult or impossible.
An excellent example of a study that links functional considerations to developmental and evolutionary integration is the analysis of variation in the predatory appendages of mantis shrimp [26,55]. An intraspecific analysis found that the functional subdivision into engine, amplifier and tool also corresponds to the pattern of developmental integration, where those three elements form separate modules [55]. A comparative analysis across the clade showed that the tool behaves as an evolutionary module separate from engine and amplifier [26]. Also, the manner in which the predatory appendage functions has an influence on integration: the subclade of ‘smashers’ has more strongly integrated part and a lower rate of evolution than other functional groups [26].
The function of a structure can have direct effects on its integration and modularity through processes such as bone remodelling, in which mechanical forces applied during use have consequences for the morphology of a structure [68–70]. In turn, such plastic responses can have evolutionary consequences.
(d). Genetic integration
The genetic component of integration and modularity is important because it is a crucial determinant of the potential for evolutionary change in a structure. Genetic integration between parts means that selection on one part will produce a correlated response in other parts, whereas genetic modularity can facilitate independent evolutionary changes in different parts [3,7,8]. Studies combining geometric morphometrics and quantitative genetics have investigated correlated responses by simulating selection for a highly localized shape change and observing whether the evolutionary response affects other parts as well [67,71–73].
One of the consequences of morphological integration is that variation is concentrated mostly in some directions in shape space, whereas other directions have less variation, if any. The direction associated with the most genetic variation, the first principal component of the genetic covariance matrix, has been discussed as a ‘line of least resistance’ [74] (or a plane or subspace of least resistance, if two or more dimensions are similarly dominant [73]). These directions are important because the direction of the evolutionary response to selection is deflected from the original direction of selection towards the direction of the line of least resistance [73,74]. Another question is whether there are directions in shape space where there is no genetic variation at all, which would constitute absolute genetic constraints because selection or drift cannot produce evolutionary change in those directions. A study that specifically addressed this question with a large experimental design in Drosophila found no evidence for such absolute constraints [75].
A different approach to genetic integration is to search for patterns of integration and modularity in the estimated effects of quantitative trait loci affecting the shape of a structure [62,76] or in the effects of mutations that affect the development of the structure under study [38,77–81]. To quantify the effects of naturally occurring mutations, it is possible to use mutation accumulation lines and to analyse the patterns of covariation among lines [82]. Altogether, these different approaches provide a multifaceted view of genetic integration and modularity of morphological structures.
(e). Environmental integration
Environmental integration can have somewhat different meanings, depending on analytical contexts, which may have different biological implications. On the one hand, it can denote the integration of the residual component in a quantitative genetic analysis [8,9] and, on the other hand, it also can mean the integration in the phenotypic variation induced by environmental differences, following a reaction norm perspective [83]. These two perspectives are not necessarily opposed, but differ in the study designs required to implement them and in how they treat variation that cannot be attributed to known environmental factors. The reaction norm perspective, where integration results from coordinated plastic responses in several traits to variation in environmental factors, is conceptually clearer in that it is better focused on a causative agent and better delineated from other levels of integration.
There are few explicit studies of environmental integration or modularity so far, but there is clearly great potential in this area, as well as substantial challenges [83,84]. Patterns of environmental integration depend on the types of the reaction norms for different traits (e.g. linear or nonlinear) and on whether different traits respond in a similar manner to the same environmental factor, and a further question is whether the reaction norms for different environmental factors and any given trait are similar or different in the resulting morphological changes. Overall, the approach must consider reaction norms as mapping from a multidimensional space of environmental factors to a multidimensional space of morphological traits. The structure of the reaction norms, in terms of covariation across environmental factors and across traits, will determine the resulting patterns of integration and modularity.
Because plasticity is a response of the developmental system to variation in environmental factors, environmental integration is linked to developmental integration. Moreover, if plasticity is adaptive and morphological changes serve to optimize the performance of some function under varying environmental conditions, there is also a clear link to functional integration.
(f). Ontogenetic integration
Ontogenetic integration, the integration of traits across ontogenetic stages in a single population (figure 1), can be expected to be quite strong because most parts of organisms change in a coordinated manner during ontogeny. Therefore, integration in a mixed-age, intraspecific sample can be expected to be mainly of ontogenetic origin [85]. Furthermore, because growth brings about large size increases, ontogenetic integration is also closely related to ontogenetic allometry, which is an important integrating factor in many organisms [37,86]. Accordingly, many studies that concern ontogenetic integration have been conducted under the heading of allometry or even heterochrony, and studies that address ontogenetic integration often put the main emphasis on those topics [85,87,88].
A number of studies have analysed ontogenetic trajectories of morphological structures and compared them among related taxa [88–90]. These studies can shed light on the developmental processes by which morphological differences between taxa arise, and thus provide links between ontogenetic and evolutionary integration.
(g). Evolutionary integration
Integration and modularity at the evolutionary level, concerning the covariation among evolutionary changes in different traits, originate from a range of processes including drift, mutation, selection and gene flow [91]. To study evolutionary integration and modularity, comparative approaches are required to take into account the phylogenetic structure of the data at this level. Comparative methods such as independent contrasts or, equivalently, phylogenetic generalized least squares, can be combined with the usual morphometric methods for analysing morphological integration and modularity [18]. This combination of methods has been used in a range of studies, using analyses such as multivariate regressions to estimate evolutionary allometry [18,25,35,92,93], partial least-squares analysis for examining patterns of integration among parts [16–21,26] and tests of modularity [18,21,52].
Several studies have found correspondences between static and evolutionary integration or modularity in diverse organisms [16,25,52]. Such correspondences are sometimes interpreted as favouring a scenario of neutral evolution by drift, but these results should be viewed with some caution because of the various assumptions that are used in the analyses [94,95]. Also, recent studies have supported the hypothesis that modularity is associated with increased evolutionary rates or greater disparity [26,96].
4. Integration in organisms with modular body plans
A variety of additional levels of integration is conceivable for organisms that have modular body plans, consisting of relatively simple elements that are multiply repeated in different positions and possibly in several variants. Note that, in this context, the terms ‘module’ and ‘modular’ are used in a different sense from the rest of this article, denoting repeated parts rather than complexes of parts that are internally integrated but relatively independent of other such complexes (yet the repeated parts of an organism with modular body plan usually are also internally integrated and relatively independent of each other, and thus are modules in both senses). The majority of plants and colonial animals fall into this category.
The key feature of modular body plans is that there can be variation within individuals, and that this variation is often substantial [23]. For instance, leaves, flowers and fruits of plants can be considered modules, and there tends to be notable variation among the copies of each type of module in each plant. Also, copies of the same modules in different locations can perform different functions and be morphologically differentiated (e.g. aquatic or aerial leaves, sun or shade leaves), and there can be systematic differences among modules initiated at different ages in the ontogeny of the whole organism [97,98]. Accordingly, there also can be intra-individual integration, and investigating this integration may involve developmental, functional and other considerations. A long-standing hypothesis is that flowers with specialized pollination are more highly integrated than vegetative parts or flowers pollinated by wind or non-specialized insects [99], a hypothesis that continues to stimulate new research [44,83,84].
Many modules, such as flowers and compound leaves, are themselves modular structures consisting of multiple parts arranged in specific ways. Depending on the number and arrangement of parts, these structures can show a range of types of symmetry. Among flowering plants, for instance, there is a wide range of floral symmetries [100,101]. Floral symmetry is subject to various selection regimes because different pollinators prefer different floral shapes and symmetries [102,103]. There are morphometric methods that can analyse these complex types of symmetry and extract different components of symmetric variation and asymmetry [56,104]. These different components of variation may have different biological significance. For instance, in analyses of algae with complex cellular symmetry, the symmetric component shows the differentiation among taxa particularly well, whereas different asymmetry components are associated with growth or developmental instability [105,106]. This type of analysis is particularly promising for investigating floral shape, but has not been used yet in this context.
For composite structures such as flowers or compound leaves, it is possible to consider integration in the whole structure or in individual parts. This adds further levels of integration that can be investigated, in addition to the levels discussed in the preceding section. For instance, in compound leaves of plants, geometric morphometric analyses of integration focusing on the whole leaves or on individual leaflets found that patterns of evolutionary and static integration were similar, but that the patterns for fluctuating asymmetry were more distinct [25]. Similar analyses for flowers, examining how shape variation in individual petals or other parts combines to variation in the overall shape and symmetry of the whole flower, have not yet been conducted but are a promising area for future research.
5. Methods for multilevel studies of integration and modularity
A wide range of morphometric methods have been used to study morphological integration and modularity (for recent reviews, see [5,86]). For instance, the amount of integration can be quantified using the scaled variance of eigenvalues [44,107], patterns of covariation between parts can be examined with partial least-squares analysis [10,13,18,85] and hypotheses of modularity can be tested by comparing the strength of covariation between hypothesized modules with that in alternative partitions of landmarks [15,18,37,43,52,76]. In addition to these ‘standard tools’ for analysing morphological integration and modularity, some morphometric methods are specifically designed to compare patterns of integration in different covariance matrices, a task that is especially relevant for comparisons across multiple levels. The remainder of this section focuses exclusively on these.
(a). Matrix correlation
Because patterns covariation are usually characterized as covariance matrices of shape coordinates, a common task in multilevel analyses of integration and modularity is the comparison of covariance matrices. A simple measure of the resemblance between covariance matrices is matrix correlation, the correlation between corresponding entries in two covariance matrices. Matrix correlations have long been used in analyses of morphological integration using traditional morphometrics [8] and have been adapted for use in geometric morphometrics [9,11,13,25,53]. For instance, a multilevel analysis of integration in the leaves of cinquefoil plants found that the patterns of static integration within species and of evolutionary integration were remarkably similar, whereas the pattern of fluctuating asymmetry was more distinct [25]. Whereas the matrix correlation provides a fairly intuitive measure of how much two covariance matrices resemble each other, it provides no information at all about the particular features that differ or are similar between them.
(b). Examining patterns of variation and covariation
Perhaps the most widespread approach for comparing patterns of integration is to conduct analyses such as principal component analyses or partial least squares and then to compare the results of these analyses. The advantage of this approach is that specific aspects of variation or covariation can be emphasized, depending on the choice of the analyses. For example, in some structures, the main patterns of overall variation, represented by the shape changes associated with first few principal components, strongly resemble the main patterns of covariation between parts, which can be obtained as the shape changes associated with the first few partial least-squares axes [10,12,13,16,18,85]. This indicates that the patterns of covariation between parts are also the dominant patterns of overall variation in the structure under study, and therefore emphasize the strength of integration. The shape changes of principal components and partial least-squares axes can also be compared among levels, such as fluctuating asymmetry, static or evolutionary integration [10,12,13,15,25,27,48].
(c). Ordination of covariance matrices
If there are multiple groups included in a study, perhaps with several levels of integration, pairwise comparisons of covariance matrices via matrix correlation or comparisons of the patterns of variation are no longer feasible. Instead, the relationships among covariance matrices can be examined in ordinations of the covariance matrices using principal coordinate analysis [42,60,78,108–111]. The distance measures for the resemblance among covariance matrices can either be derived from the matrix correlation [42,78,108,109] or from considerations concerning the space of all covariance matrices [111,112]. Direct comparisons between analyses using different distance measures have shown some common features, but also some marked differences [60,110]. More work is required to understand the properties of these analyses and of the different distance measures used in them. Perhaps more fundamentally, principal coordinate analysis has one serious drawback: because it starts from a matrix of distances among the objects, covariance matrices in this context, and generates a coordinate system from them, there is no information on the features of variation that are associated with the new coordinate axes (unlike principal components of shape data, for instance, where each axis is associated with a particular shape change).
6. Examples of comparisons across levels
So far, there are only few studies that systematically compare integration at multiple levels, but there are many studies that contain comparisons of patterns at two or three levels, which indicate that true multilevel studies of integration and modularity hold considerable promise.
A large number of studies has compared static integration among individuals to fluctuating asymmetry and obtained widely variable results, indicating that the relationship between static and developmental integration differs according to taxa and other circumstances [10–15,36,37,48–53,110]. Genetic integration has mostly been compared to static phenotypic integration, reflecting the importance of the genetic and phenotypic covariance matrices in quantitative genetic theory [67,71,73]. Evolutionary integration has been compared to static within-taxon integration [16,25,95] and ontogenetic variation [35,85,87,88], and through links to ecological variables such as diet or direct biomechanical arguments, functional considerations have also been incorporated [63,113]. Systematic multilevel studies incorporating more than two levels simultaneously are rare. Some of these are studies of within-individual, within- and among-population variation [22], analyses of variation among individuals, among populations and among species [24], or comparisons of fluctuating asymmetry, intra-taxon variation and evolutionary integration [25,52]. Such studies are relatively easily feasible, as long as there are multiple specimens per species and left and right sides are available for each specimen (either separately or as symmetric structures such as skulls or leaves). Therefore, I hope there will soon be many more of these studies.
7. Conclusion and outlook
Multilevel studies of morphological integration and modularity have only just begun, and their potential is not yet explored fully. The promise of the approach comes from the possibility to infer possible processes that are involved in generating the variation that is observable at different levels. In the study of allometry, such reasoning has a long tradition [30–32], but for morphological integration and modularity, this type of study has not been pursued systematically. Also, a number of methodological questions remain, and new morphometric methods that combine statistical rigour and biological interpretability would be a most helpful addition to the existing toolkit. It remains to be seen whether such methods can be developed.
References
- 1.Mayr E. 1982. The growth of biological thought: diversity, evolution, and inheritance. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Olson EC, Miller RL. 1958. Morphological integration. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Wagner GP, Altenberg L. 1996. Complex adaptations and the evolution of evolvability. Evolution 50, 967–976. ( 10.2307/2410639) [DOI] [PubMed] [Google Scholar]
- 4.Klingenberg CP. 2008. Morphological integration and developmental modularity. Annu. Rev. Ecol. Evol. Syst. 39, 115–132. ( 10.1146/annurev.ecolsys.37.091305.110054) [DOI] [Google Scholar]
- 5.Goswami A, Polly PD. 2010. Methods for studying morphological integration and modularity. In Quantitative methods in paleobiology (eds Alroy J, Hunt G.), pp. 213–243. Ithaca, NY: Paleontological Society. [Google Scholar]
- 6.Wagner GP, Pavlicev M, Cheverud JM. 2007. The road to modularity. Nat. Rev. Genet. 8, 921–931. ( 10.1038/nrg2267) [DOI] [PubMed] [Google Scholar]
- 7.Klingenberg CP. 2010. Evolution and development of shape: integrating quantitative approaches. Nat. Rev. Genet. 11, 623–635. ( 10.1038/nrg2829) [DOI] [PubMed] [Google Scholar]
- 8.Cheverud JM. 1982. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution 36, 499–516. ( 10.2307/2408096) [DOI] [PubMed] [Google Scholar]
- 9.Willmore KE, Klingenberg CP, Hallgrímsson B. 2005. The relationship between fluctuating asymmetry and environmental variance in rhesus macaque skulls. Evolution 59, 898–909. ( 10.1111/j.0014-3820.2005.tb01763.x) [DOI] [PubMed] [Google Scholar]
- 10.Klingenberg CP, Zaklan SD. 2000. Morphological integration between developmental compartments in the Drosophila wing. Evolution 54, 1273–1285. ( 10.1111/j.0014-3820.2000.tb00560.x) [DOI] [PubMed] [Google Scholar]
- 11.Debat V, Alibert P, David P, Paradis E, Auffray J-C. 2000. Independence between developmental stability and canalization in the skull of the house mouse. Proc. R. Soc. Lond. B 267, 423–430. ( 10.1098/rspb.2000.1017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingenberg CP, Badyaev AV, Sowry SM, Beckwith NJ. 2001. Inferring developmental modularity from morphological integration: analysis of individual variation and asymmetry in bumblebee wings. Am. Nat. 157, 11–23. ( 10.1086/317002) [DOI] [PubMed] [Google Scholar]
- 13.Klingenberg CP, Mebus K, Auffray J-C. 2003. Developmental integration in a complex morphological structure: how distinct are the modules in the mouse mandible? Evol. Dev. 5, 522–531. ( 10.1046/j.1525-142X.2003.03057.x) [DOI] [PubMed] [Google Scholar]
- 14.Ivanović A, Kalezić ML. 2010. Testing the hypothesis of morphological integration on a skull of a vertebrate with a biphasic life cycle: a case study of the alpine newt. J. Exp. Zool. B Mol. Dev. Evol. 314, 527–538. ( 10.1002/jez.b.21358) [DOI] [PubMed] [Google Scholar]
- 15.Jojić V, Blagojević J, Vujošević M. 2011. B chromosomes and cranial variability in yellow-necked field mice (Apodemus flavicollis). J. Mammal. 92, 396–406. ( 10.1644/10-MAMM-A-158.1) [DOI] [Google Scholar]
- 16.Monteiro LR, Bonato V, dos Reis SF. 2005. Evolutionary integration and morphological diversification in complex morphological structures: mandible shape divergence in spiny rats (Rodentia, Echimyidae). Evol. Dev. 7, 429–439. ( 10.1111/j.1525-142X.2005.05047.x) [DOI] [PubMed] [Google Scholar]
- 17.Hautier L, Lebrun R, Cox PG. 2012. Patterns of covariation in the masticatory apparatus of hystricognathous rodents: implications for evolution and diversification. J. Morphol. 273, 1319–1337. ( 10.1002/jmor.20061) [DOI] [PubMed] [Google Scholar]
- 18.Klingenberg CP, Marugán-Lobón J. 2013. Evolutionary covariation in geometric morphometric data: analyzing integration, modularity and allometry in a phylogenetic context. Syst. Biol. 62, 591–610. ( 10.1093/sysbio/syt025) [DOI] [PubMed] [Google Scholar]
- 19.Chamero B, Buscalioni ÁD, Marugán-Lobón J. 2013. Pectoral girdle and forelimb variation in extant Crocodylia: the coracoid–humerus pair as an evolutionary module. Biol. J. Linn. Soc. 108, 600–618. ( 10.1111/j.1095-8312.2012.02037.x) [DOI] [Google Scholar]
- 20.Bastir M, Rosas A, Stringer CB, Cuétara JM, Kruszynski R, Weber GW, Ross CF, Ravosa MJ. 2010. Effects of brain and facial size on basicranial form in human and primate evolution. J. Hum. Evol. 58, 424–431. ( 10.1016/j.jhevol.2010.03.001) [DOI] [PubMed] [Google Scholar]
- 21.Santana SE, Lofgren SE. 2013. Does nasal echolocation influence the modularity of the mammal skull? J. Evol. Biol. 26, 2520–2526. ( 10.1111/jeb.12235) [DOI] [PubMed] [Google Scholar]
- 22.Armbruster WS. 1991. Multilevel analysis of morphometric data from natural plant populations: insights into ontogenetic, genetic, and selective correlations in Dalechampia scandens. Evolution 45, 1229–1244. ( 10.2307/2409730) [DOI] [PubMed] [Google Scholar]
- 23.Herrera CM. 2009. Multiplicity in unity: plant subindividual variation and interactions with animals. Chicago, IL: University of Chicago Press [Google Scholar]
- 24.Armbruster WS, Pélabon C, Hansen TF, Mulder CPH. 2004. Floral integration, modularity, and accuracy: distinguishing complex adaptations from genetic constraints. In Phenotypic integration: studying the ecology and evolution of complex phenotypes (eds Pigliucci M, Preston K.), pp. 23–49. New York, NY: Oxford University Press. [Google Scholar]
- 25.Klingenberg CP, Duttke S, Whelan S, Kim M. 2012. Developmental plasticity, morphological variation and evolvability: a multilevel analysis of morphometric integration in the shape of compound leaves. J. Evol. Biol. 25, 115–129. ( 10.1111/j.1420-9101.2011.02410.x) [DOI] [PubMed] [Google Scholar]
- 26.Claverie T, Patek SN. 2013. Modularity and rates of evolutionary change in a power-amplified prey capture system. Evolution 67, 3191–3207. ( 10.1111/evo.12185) [DOI] [PubMed] [Google Scholar]
- 27.Laffont R, Renvoisé E, Navarro N, Alibert P, Montuire S. 2009. Morphological modularity and assessment of developmental processes within the vole dental row (Microtus arvalis, Arvicolinae, Rodentia). Evol. Dev. 11, 302–311. ( 10.1111/j.1525-142X.2009.00332.x) [DOI] [PubMed] [Google Scholar]
- 28.Cock AG. 1966. Genetical aspects of metrical growth and form in animals. Q. Rev. Biol. 41, 131–190. ( 10.1086/404940) [DOI] [PubMed] [Google Scholar]
- 29.Gould SJ. 1966. Allometry and size in ontogeny and phylogeny. Biol. Rev. 41, 587–640. ( 10.1111/j.1469-185X.1966.tb01624.x) [DOI] [PubMed] [Google Scholar]
- 30.Cheverud JM. 1982. Relationships among ontogenetic, static, and evolutionary allometry. Am. J. Phys. Anthropol. 59, 139–149. ( 10.1002/ajpa.1330590204) [DOI] [PubMed] [Google Scholar]
- 31.Klingenberg CP, Zimmermann M. 1992. Static, ontogenetic, and evolutionary allometry: a multivariate comparison in nine species of water striders. Am. Nat. 140, 601–620. ( 10.1086/285430) [DOI] [Google Scholar]
- 32.Pélabon C, Bolstad GH, Egset CK, Cheverud JM, Pavlicev M, Rosenqvist G. 2013. On the relationship between ontigenetic and static allometry. Am. Nat. 181, 195–212. ( 10.1086/668820) [DOI] [PubMed] [Google Scholar]
- 33.Drake AG, Klingenberg CP. 2008. The pace of morphological change: historical transformation of skull shape in St. Bernard dogs. Proc. R. Soc. B 275, 71–76. ( 10.1098/rspb.2007.1169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisensee KE, Jantz RL. 2011. Secular change in craniofacial morphology of the Portuguese using geometric morphometrics. Am. J. Phys. Anthropol. 145, 548–559. ( 10.1002/ajpa.21531) [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez PN, Perez SI, Bernal V. 2011. Ontogenetic allometry and cranial shape diversification among human populations from South America. Anat. Rec. 294, 1864–1874. ( 10.1002/ar.21454) [DOI] [PubMed] [Google Scholar]
- 36.Jojić V, Blagojević J, Vujošević M. 2012. Two-module organization of the mandible in the yellow-necked mouse: a comparison between two different morphometric approaches. J. Evol. Biol. 25, 2489–2500. ( 10.1111/j.1420-9101.2012.02612.x) [DOI] [PubMed] [Google Scholar]
- 37.Klingenberg CP. 2009. Morphometric integration and modularity in configurations of landmarks: tools for evaluating a priori hypotheses. Evol. Dev. 11, 405–421. ( 10.1111/j.1525-142X.2009.00347.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallgrímsson B, Jamniczky HA, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS. 2009. Deciphering the palimpsest: studying the relationship between morphological integration and phenotypic covariation. Evol. Biol. 36, 355–376. ( 10.1007/s11692-009-9076-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrera CM, Cerdá X, García MB, Guitán J, Medrano M, Rey PJ, Sánchez-Lafuente AM. 2002. Floral integration, phenotypic covariance structure and pollinator varition in bumblebee-pollinated Helleborus foetidus. J. Evol. Biol. 15, 108–121. ( 10.1046/j.1420-9101.2002.00365.x) [DOI] [Google Scholar]
- 40.Goswami A. 2006. Cranial modularity shifts during mammalian evolution. Am. Nat. 168, 270–280. ( 10.1086/505758) [DOI] [PubMed] [Google Scholar]
- 41.Goswami A. 2006. Morphological integration in the carnivoran skull. Evolution 60, 169–183. ( 10.1111/j.0014-3820.2006.tb01091.x) [DOI] [PubMed] [Google Scholar]
- 42.Jamniczky HA, Hallgrímsson B. 2009. A comparison of covariance structure in wild and laboratory muroid crania. Evolution 63, 1540–1556. ( 10.1111/j.1558-5646.2009.00651.x) [DOI] [PubMed] [Google Scholar]
- 43.Sanger TJ, Mahler DL, Abzhanov A, Losos JB. 2012. Roles for modularity and constraint in the evolution of cranial diversity among Anolis lizards. Evolution 66, 1525–1542. ( 10.1111/j.1558-5646.2011.01519.x) [DOI] [PubMed] [Google Scholar]
- 44.Gómez JM, Perfectti F, Klingenberg CP. 2014. The role of pollinator diversity in the evolution of corolla-shape integration in a pollination-generalist plant clade. Phil. Trans. R. Soc. B 369, 20130257 ( 10.1098/rstb.2013.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riska B. 1986. Some models for development, growth, and morphometric correlation. Evolution 40, 1303–1311. ( 10.2307/2408955) [DOI] [PubMed] [Google Scholar]
- 46.Klingenberg CP. 2003. Developmental instability as a research tool: using patterns of fluctuating asymmetry to infer the developmental origins of morphological integration. In Developmental instability: causes and consequences (ed. Polak M.), pp. 427–442. New York, NY: Oxford University Press. [Google Scholar]
- 47.Klingenberg CP. 2005. Developmental constraints, modules and evolvability. In Variation: a central concept in biology (eds Hallgrímsson B, Hall BK.), pp. 219–247. Burlington, MA: Elsevier. [Google Scholar]
- 48.Klingenberg CP, McIntyre GS. 1998. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 52, 1363–1375. ( 10.2307/2411306) [DOI] [PubMed] [Google Scholar]
- 49.Klingenberg CP, Barluenga M, Meyer A. 2002. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56, 1909–1920. ( 10.1111/j.0014-3820.2002.tb00117.x) [DOI] [PubMed] [Google Scholar]
- 50.Young RL, Badyaev AV. 2006. Evolutionary persistence of phenotypic integration: influence of developmental and functional relationships on complex trait evolution. Evolution 60, 1291–1299. ( 10.1111/j.0014-3820.2006.tb01206.x) [DOI] [PubMed] [Google Scholar]
- 51.Jamniczky HA, Hallgrímsson B. 2011. Modularity in the skull and cranial vasculature of laboratory mice: implications for the evolution of complex phenotypes. Evol. Dev. 13, 28–37. ( 10.1111/j.1525-142X.2010.00453.x) [DOI] [PubMed] [Google Scholar]
- 52.Drake AG, Klingenberg CP. 2010. Large-scale diversification of skull shape in domestic dogs: disparity and modularity. Am. Nat. 175, 289–301. ( 10.1086/650372) [DOI] [PubMed] [Google Scholar]
- 53.Breuker CJ, Patterson JS, Klingenberg CP. 2006. A single basis for developmental buffering of Drosophila wing shape. PLoS ONE 1, e7 ( 10.1371/journal.pone.0000007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zelditch ML, Wood AR, Swiderski DL. 2009. Building developmental integration into functional systems: function-indiced integration of mandibular shape. Evol. Biol. 36, 71–87. ( 10.1007/s11692-008-9034-7) [DOI] [Google Scholar]
- 55.Claverie T, Chan E, Patek SN. 2011. Modularity and scaling in fast movements: power amplification in mantis shrimp. Evolution 65, 443–461. ( 10.1111/j.1558-5646.2010.01133.x) [DOI] [PubMed] [Google Scholar]
- 56.Savriama Y, Gómez JM, Perfectti F, Klingenberg CP. 2012. Geometric morphometrics of corolla shape: dissecting components of symmetric and asymmetric variation in Erysimum mediohispanicum (Brassicaceae). New Phytol. 196, 945–954. ( 10.1111/j.1469-8137.2012.04312.x) [DOI] [PubMed] [Google Scholar]
- 57.Zelditch ML, Mezey JG, Sheets HD, Lundrigan BL, Garland T., Jr 2006. Developmental regulation of skull morphology II: ontogenetic dynamics of covariance. Evol. Dev. 8, 46–60. ( 10.1111/j.1525-142X.2006.05074.x) [DOI] [PubMed] [Google Scholar]
- 58.Mitteroecker P, Bookstein FL. 2009. Examining modularity via partial correlations: a rejoinder to a comment by Paul Magwene. Syst. Biol. 58, 346–348. ( 10.1093/sysbio/syp040) [DOI] [PubMed] [Google Scholar]
- 59.Willmore KE, Leamy L, Hallgrímsson B. 2006. Effects of developmental and functional interactions on mouse cranial variability through late ontogeny. Evol. Dev. 8, 550–567. ( 10.1111/j.1525-142X.2006.00127.x) [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez PN, Hallgrímsson B, Oyhenart EE. 2011. Developmental plasticity in covariance structure of the skull: effects of prenatal stress. J. Anat. 218, 243–257. ( 10.1111/j.1469-7580.2010.01326.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez PN, Oyhenart EE, Hallgrímsson B. 2011. Effects of environmental perturbations during postnatal development on the phenotypic integration of the skull. J. Exp. Zool. B Mol. Dev. Evol. 316, 547–561. ( 10.1002/jez.b.21430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klingenberg CP, Leamy LJ, Cheverud JM. 2004. Integration and modularity of quantitative trait locus effects on geometric shape in the mouse mandible. Genetics 166, 1909–1921. ( 10.1534/genetics.166.4.1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figueirido B, Tseng ZJ, Martín-Serra A. 2013. Skull shape evolution in durophagous carnivorans. Evolution 67, 1975–1993. ( 10.1111/evo.12059) [DOI] [PubMed] [Google Scholar]
- 64.O'Higgins P, Cobb SN, Fitton LC, Gröning F, Phillips R, Liu J, Fagan MJ. 2011. Combining geometric morphometrics and functional simulation: an emerging toolkit for virtual functional analyses. J. Anat. 218, 3–15. ( 10.1111/j.1469-7580.2010.01301.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheverud JM. 1984. Quantitative genetics and developmental constraints on evolution by selection. J. Theor. Biol. 110, 155–171. ( 10.1016/S0022-5193(84)80050-8) [DOI] [PubMed] [Google Scholar]
- 66.Breuker CJ, Debat V, Klingenberg CP. 2006. Functional evo-devo. Trends Ecol. Evol. 21, 488–492. ( 10.1016/j.tree.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 67.Klingenberg CP, Debat V, Roff DA. 2010. Quantitative genetics of shape in cricket wings: developmental integration in a functional structure. Evolution 64, 2935–2951. ( 10.1111/j.1558-5646.2010.01030.x) [DOI] [PubMed] [Google Scholar]
- 68.Zelditch ML, Wood AR, Bonett RM, Swiderski DL. 2008. Modularity of the rodent mandible: integrating bones, muscles, and teeth. Evol. Dev. 10, 756–768. ( 10.1111/j.1525-142X.2008.00290.x) [DOI] [PubMed] [Google Scholar]
- 69.Renaud S, Auffray J-C, de la Porte S. 2010. Epigenetic effects on the mouse mandible: common features and discrepancies in remodeling due to muscular dystrophy and response to food consistency. BMC Evol. Biol. 10, 28 ( 10.1186/1471-2148-10-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young RL, Badyaev AV. 2010. Developmental plasticity links local adaptation and evolutionary diversification in foraging morphology. J. Exp. Zool. B Mol. Dev. Evol. 314, 434–444. ( 10.1002/jez.b.21349) [DOI] [PubMed] [Google Scholar]
- 71.Klingenberg CP, Leamy LJ. 2001. Quantitative genetics of geometric shape in the mouse mandible. Evolution 55, 2342–2352. ( 10.1111/j.0014-3820.2001.tb00747.x) [DOI] [PubMed] [Google Scholar]
- 72.Martínez-Abadías N, Paschetta C, de Azevedo S, Esparza M, González-José R. 2009. Developmental and genetic constraints on neurocranial globularity: insights from analyses of deformed skulls and quantitative genetics. Evol. Biol. 36, 37–56. ( 10.1007/s11692-008-9045-4) [DOI] [Google Scholar]
- 73.Martínez-Abadías N, Esparza M, Sjøvold T, González-José R, Santos M, Hernández M, Klingenberg CP. 2012. Pervasive genetic integration directs the evolution of human skull shape. Evolution 66, 1010–1023. ( 10.1111/j.1558-5646.2011.01496.x) [DOI] [PubMed] [Google Scholar]
- 74.Schluter D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766–1774. ( 10.2307/2410734) [DOI] [PubMed] [Google Scholar]
- 75.Mezey JG, Houle D. 2005. The dimensionality of genetic variation for wing shape in Drosophila melanogaster. Evolution 59, 1027–1038. ( 10.1111/j.0014-3820.2005.tb01041.x) [DOI] [PubMed] [Google Scholar]
- 76.Burgio G, Baylac M, Heyer E, Montagutelli X. 2012. Exploration of the genetic organization of morphological modularity on the mouse mandible using a set of interspecific recombinant congenic strains between C57BL/6 and mice of the Mus spretus species. G3–Genes Genomes Genet. 2, 1257–1268. ( 10.1534/g3.112.003285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hallgrímsson B, Brown JJY, Ford-Hutchinson AF, Sheets HD, Zelditch ML, Jirik FR. 2006. The brachymorph mouse and the developmental-genetic basis for canalization and morphological integration. Evol. Dev. 8, 61–73. ( 10.1111/j.1525-142X.2006.05075.x) [DOI] [PubMed] [Google Scholar]
- 78.Debat V, Milton CC, Rutherford S, Klingenberg CP, Hoffmann AA. 2006. Hsp90 and the quantitative variation of wing shape in Drosophila melanogaster. Evolution 60, 2529–2538. ( 10.1554/06-045.1) [DOI] [PubMed] [Google Scholar]
- 79.Debat V, et al. 2011. Developmental stability: a major role for Cyclin G in Drosophila melanogaster. PLoS Genet. 7, e1002314 ( 10.1371/journal.pgen.1002314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martínez-Abadías N, Heuzé Y, Wang Y, Jabs EW, Aldridge K, Richtsmeier JT. 2011. FGF/FGFR signaling coordinates skull development by modulating magnitude of morphological integration: evidence from Apert syndrome mouse models. PLoS ONE 6, e26425 ( 10.1371/journal.pone.0026425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parsons TE, Schmidt EJ, Boughner JC, Jamniczky HA, Marcucio RS, Hallgrímsson B. 2011. Epigenetic integration of the developing brain and face. Dev. Dyn. 240, 2233–2244. ( 10.1002/dvdy.22729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Houle D, Fierst J. 2012. Properties of spontaneous multational variance and covariance for wing size and shape in Drosophila melanogaster. Evolution 67, 1116–1130. ( 10.1111/j.1558-5646.2012.01838.x) [DOI] [PubMed] [Google Scholar]
- 83.Pélabon C, Osler NC, Diekmann M, Graae BJ. 2013. Decoupled phenotypic variation between floral and vegetative traits: distinguishing between developmental and environmental correlations. Ann. Bot. 111, 935–944. ( 10.1093/aob/mct050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pélabon C, Armbruster WS, Hansen TF. 2011. Experimental evidence for the Berg hypothesis: vegetative traits are more sensitive than pollination traits to environmental variation. Funct. Ecol. 25, 247–257. ( 10.1111/j.1365-2435.2010.01770.x) [DOI] [Google Scholar]
- 85.Bookstein FL, Gunz P, Mitteroecker P, Prossinger H, Schaefer K, Seidler H. 2003. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J. Hum. Evol. 44, 167–187. ( 10.1016/S0047-2484(02)00201-4) [DOI] [PubMed] [Google Scholar]
- 86.Klingenberg CP. 2013. Cranial integration and modularity: insights into evolution and development from morphometric data. Hystrix 24, 43–58. [Google Scholar]
- 87.Drake AG. 2011. Dispelling dog dogma: an investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape. Evol. Dev. 13, 204–213. ( 10.1111/j.1525-142X.2011.00470.x) [DOI] [PubMed] [Google Scholar]
- 88.Mitteroecker P, Gunz P, Bookstein FL. 2005. Heterochrony and geometric morphometrics: a comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evol. Dev. 7, 244–258. ( 10.1111/j.1525-142X.2005.05027.x) [DOI] [PubMed] [Google Scholar]
- 89.Bulygina E, Mitteroecker P, Aiello L. 2006. Ontogeny of facial dimorphism and patterns of individual development within one human population. Am. J. Phys. Anthropol. 131, 432–443. ( 10.1002/ajpa.20317) [DOI] [PubMed] [Google Scholar]
- 90.Kimmel CB, Hohenlohe PA, Ullmann B, Currey M, Cresko WA. 2012. Developmental dissociation in morphological evolution of the stickleback opercle. Evol. Dev. 14, 326–337. ( 10.1111/j.1525-142X.2012.00551.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Armbruster WS, Schwaegerle KE. 1996. Causes of covariation of phenotypic traits among populations. J. Evol. Biol. 9, 261–276. ( 10.1046/j.1420-9101.1996.9030261.x) [DOI] [Google Scholar]
- 92.Figueirido B, Serrano-Alarcón FJ, Slater GJ, Palmqvist P. 2010. Shape at the cross-roads: homoplasy and history in the evolution of the carnivoran skull towards herbivory. J. Evol. Biol. 23, 2579–2594. ( 10.1111/j.1420-9101.2010.02117.x) [DOI] [PubMed] [Google Scholar]
- 93.Perez SI, Klaczko J, Rocatti G, dos Reis SF. 2011. Patterns of cranial shape diversification during the phylogenetic branching process of New World monkeys (Primates: Platyrrhini). J. Evol. Biol. 24, 1826–1835. ( 10.1111/j.1420-9101.2011.02309.x) [DOI] [PubMed] [Google Scholar]
- 94.Weaver TD, Roseman CC, Stringer CB. 2007. Were Neandertal and modern human cranial differences produced by natural selection or genetic drift? J. Hum. Evol. 53, 135–145. ( 10.1016/j.jhevol.2007.03.001) [DOI] [PubMed] [Google Scholar]
- 95.Smith HF. 2011. The role of genetic drift in shaping modern human cranial evolution: a test using microevolutionary modeling. Int. J. Evol. Biol. 2011, 145262 ( 10.4061/2011/145262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goswami A, Polly PD. 2010. The influence of modularity on cranial morphological disparity in Carnivora and Primates (Mammalia). PLoS ONE 5, e9517 ( 10.1371/journal.pone.0009517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chitwood DH, Headland LR, Kumar R, Peng J, Maloof JN, Sinha NR. 2012. The developmental trajectory of leaflet morphology in wild tomato species. Plant Physiol. 158, 1230–1240. ( 10.1104/pp.111.192518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones CS. 1992. Comparative ontogeny of a wild cucurbit and its derived cultivar. Evolution 46, 1827–1847. ( 10.2307/2410034) [DOI] [PubMed] [Google Scholar]
- 99.Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14, 171–180. ( 10.2307/2405824) [DOI] [Google Scholar]
- 100.Endress PK. 2001. Evolution of floral symmetry. Curr. Opin. Plant Biol. 4, 86–91. ( 10.1016/S1369-5266(00)00140-0) [DOI] [PubMed] [Google Scholar]
- 101.Jabbour F, Nadot S, Damerval C. 2009. Evolution of floral symmetry: a state of the art. C. R. Biol. 332, 219–231. ( 10.1016/j.crvi.2008.07.011) [DOI] [PubMed] [Google Scholar]
- 102.Gómez JM, Perfectti F, Camacho JPM. 2006. Natural selection on Erysimum mediohispanicum flower shape: insights into the evolution of zygomorphy. Am. Nat. 168, 531–545. ( 10.1086/507048) [DOI] [PubMed] [Google Scholar]
- 103.Gómez JM, Perfectti F, Bosch J, Camacho JPM. 2009. A geographic selection mosaic in a generalized plant–pollinator–herbivore system. Ecol. Monogr. 79, 245–263. ( 10.1890/08-0511.1) [DOI] [Google Scholar]
- 104.Savriama Y, Klingenberg CP. 2011. Beyond bilateral symmetry: geometric morphometric methods for any type of symmetry. BMC Evol. Biol. 11, 280 ( 10.1186/1471-2148-11-280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neustupa J. 2013. Patterns of symmetric and asymmetric morphological variation in unicellular green microalgae of the genus Micrasterias (Desmidiales, Viridiplantae). Fottea 13, 53–63. [Google Scholar]
- 106.Savriama Y, Neustupa J, Klingenberg CP. 2010. Geometric morphometrics of symmetry and allometry in Micrasterias rotata (Zygnemophyceae, Viridiplantae). Nova Hedwigia Suppl. 136, 43–54. ( 10.1127/1438-9134/2010/0136-0043) [DOI] [Google Scholar]
- 107.Young NM. 2006. Function, ontogeny and canalization of shape variance in the primate scapula. J. Anat. 209, 623–636. ( 10.1111/j.1469-7580.2006.00639.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Debat V, Cornette R, Korol AB, Nevo E, Soulet D, David JR. 2008. Multidimensional analysis of Drosophila wing variation in Evolution Canyon. J. Genet. 87, 407–419. ( 10.1007/s12041-008-0063-x) [DOI] [PubMed] [Google Scholar]
- 109.Debat V, Debelle A, Dworkin I. 2009. Plasticity, canalization, and developmental stability of the Drosophila wing: joint effects of mutations and developmental temperature. Evolution 63, 2864–2876. ( 10.1111/j.1558-5646.2009.00774.x) [DOI] [PubMed] [Google Scholar]
- 110.Breno M, Leirs H, Van Dongen S. 2011. No relationship between canalization and developmental stability of the skull in a natural population of Mastomys natalensis (Rodentia: Muridae). Biol. J. Linn. Soc. 104, 207–216. ( 10.1111/j.1095-8312.2011.01702.x) [DOI] [Google Scholar]
- 111.Mitteroecker P, Bookstein FL. 2009. The ontogenetic trajectory of the phenotypic covariance matrix, with examples from craniofacial shape in rats and humans. Evolution 63, 727–737. ( 10.1111/j.1558-5646.2008.00587.x) [DOI] [PubMed] [Google Scholar]
- 112.Dryden IL, Koloydenko A, Zhou D. 2009. Non-Euclidean statistics for covariance matrices, with applications to diffusion tensor imaging. Ann. Appl. Stat. 3, 1102–1123. ( 10.1214/09-AOAS249) [DOI] [Google Scholar]
- 113.Monteiro LR, Nogueira MR. 2010. Adaptive radiations, ecological specialization, and the evolutionary integration of complex morphological structures. Evolution 64, 724–744. ( 10.1111/j.1558-5646.2009.00857.x) [DOI] [PubMed] [Google Scholar]