Abstract
Different pollinators can exert different selective pressures on floral traits, depending on how they fit with flowers, which should be reflected in the patterns of variation and covariation of traits. Surprisingly, empirical evidence in support of this view is scarce. Here, we have studied whether the variation observed in floral phenotypic integration and covariation of traits in Narcissus species is associated with different groups of pollinators. Phenotypic integration was studied in two style dimorphic species, both with dimorphic populations mostly visited by long-tongued pollinators (close fit with flowers), and monomorphic populations visited by short-tongued insects (loose fit). For N. papyraceus, the patterns of variation and correlation among traits involved in different functions (attraction and fit with pollinators, transfer of pollen) were compared within and between population types. The genetic diversity of populations was also studied to control for possible effects on phenotypic variation. In both species, populations with long-tongued pollinators displayed greater phenotypic integration than those with short-tongued pollinators. Also, the correlations among traits involved in the same function were stronger than across functions. Furthermore, traits involved in the transfer of pollen were consistently more correlated and less variable than traits involved in the attraction of insects, and these differences were larger in dimorphic than monomorphic populations. In addition, population genetic parameters did not correlate with phenotypic integration or variation. Altogether, our results support current views of the role of pollinators in the evolution of floral integration.
Keywords: adaptation, genetic diversity, style dimorphism, phenotypic selection, plasticity, pollinator-mediated selection
1. Introduction
Most organisms display complex and integrated phenotypes with multiple traits involved in different and coordinated functions. This morphological complexity has long intrigued evolutionary biologists and it has stimulated discussions from both theoretical and empirical perspectives to understand how integrated phenotypes evolve [1–8]. Perhaps, one of the most influential works was that by Olson & Miller [1], which represented a turning point and inspired current views on phenotypic integration. These authors viewed integration as resulting mostly from the genetic and developmental programmes of organisms. As interpreted by Cheverud [3, p. 499], ‘the degree of interdependence in development and function among morphological characters is directly related to the degree of phenotypic morphological integration among these characters’. This perspective has stimulated research agendas, with most of the empirical case studies coming from animal biology [1,3,9–16] and less frequently from plant biology [2,17–20].
Adaptive evolution can also influence the strength and the patterns of phenotypic integration of traits. Following the ideas of correlation pleiades developed by Terentjev [21], Berg [2,22] developed the concept of integration as a result of natural selection. In these papers, plant–pollinator relationships were used as a theoretical framework to illustrate the mechanisms by which natural selection could shape the structure and intensity of correlations between traits. Specifically, flowers with tight relationships with their pollinators should undergo stronger selection and display less variation than those without ‘precise’ fit. These contrasting selection scenarios should be reflected in the strength of floral correlations, and by the magnitude of modularity and decoupling between groups of traits involved in different functions, such as floral and vegetative traits, the latter being unaffected by pollinators [18,23–29].
Berg's ideas have been expanded further in relation to the magnitude of intra-floral correlations with different functions and modularity [18,30–32]. The flower as a unit can be divided into semi-autonomous modules involved in the attraction of pollinators (e.g. flower size and display) and the transfer of pollen (e.g. pollen pick-up and delivery), and variation within these modules is usually restricted by genetic control and architectural constraints [17,19,20,32]. Despite these constraints, it could be expected that the adaptive peak of traits involved in the pollen transfer function may be narrower than those traits involved in the pollinator attraction function. Attraction traits are fundamental to receive visits and move pollen from anthers to stigmas [33,34], but more pollen should be transferred if pollinators pick up the pollen and touch the stigmas with the same body parts, which requires a precise position of these organs [35–37]. Furthermore, differences in the fitness surface between attraction traits and those involved in the pollen transfer should be larger in species and populations with close fit between flowers and pollinators (e.g. narrower adaptive peak for traits involved in pollen transfer than in attraction) than those with loose fit [2,18,29,38]. In addition, floral modularity will be favoured when traits involved in different functions experience different selective pressures [30].
Historical processes at lineage and population levels can influence trait correlation and covariation, but these have not been discussed much in the context of phenotypic integration [3,4,38–41]. Following the argument that genetic variation is a precondition for adaptive evolution [17,42], part of the variation in the strength of trait correlations could be explained by the variation in the gene pool of populations. This is supported by the fact that phenotypic correlation matrices and genetic correlation matrices do not differ much [17,19,39]. When studying the distribution of genetic diversity across species’ geographical ranges, genetic diversity is usually larger in central than peripheral populations [43]. Thus, population genetic processes might influence phenotypic variation strongly [44–47], which could in turn be reflected in the patterns of correlation and covariation of traits (but see [48]).
In this paper, we wished to test Berg's hypothesis of different patterns of flower integration when plants are under selection by different functional groups of pollinators, using Narcissus species and populations as a case study. The research on Narcissus has provided important evidence to understand the mechanisms by which shifts in pollinators can drive floral phenotypic variation and evolution. Many Narcissus species present style dimorphism, a sex polymorphism where populations present two floral morphs with either long- or short-style flowers (hereafter L and S flowers), and two anther levels (upper and lower) attached to the flower tube (the position of the upper and lower anther level does not differ between morphs; figure 1a). In a macroevolutionary context, changes in the polymorphism correlate with the evolution of long and narrow floral tubes, which seem to be the result of selection mediated by long-tongued nectarivorous insects [49]. Many style dimorphic Narcissus display great variation in the morph ratio, from dimorphic populations (L : S and L-biased) to L-monomorphic populations (although uncommon, S-biased populations can occur, [50–53]), and this variation is frequently associated with shifts in pollinators. For example, populations of N. papyraceus in the west of the Mediterranean Basin and N. tazetta in Israel can be either dimorphic (L : S, L > S and S > L in N. tazetta) and visited mostly by long-tongued nectarivorous pollinators, or L-monomorphic and highly L-biased (L > 95%) with short-tongued pollinivorous insects as main pollinators [29,50,53–55]. These variations in morph ratio can occur because, although the species are self-incompatible, crosses between different plants of the same morph render viable seeds [53,55,56]. Experimental manipulations have revealed that the maintenance of S flowers depends upon the presence of long-tongued insects, which transfer pollen (mostly from the lower anther level of L-flowers) to S-stigmas (short-tongued insects, such as syrphid flies, do not reach S-stigmas; [55,57–60]). Stigmas of long-styled flowers can receive pollen from either L- or S- anthers and both long- and short-tongued insects are able to deliver pollen. In Narcissus and other polymorphic species, the absence of one morph seems to be a derived condition [41,61–65]. Hence, it is reasonable to argue that L-monomorphic populations of N. papyraceus and N. tazetta are derived from dimorphic populations, although it is unclear how many times the polymorphism has been lost at the population level (but see [62]).
Figure 1.
Floral traits measured in (a) Narcissus papyraceus: flower diameter (1), corona diameter (2) and height (3), flower tube length (4) and width (5), style length (6), upper anther height (7) and lower anther (8) height in long- (L) and short-style (S) flowers, and (b) Narcissus tazetta: flower diameter (1), outer tepal length (2) and width (3), corona diameter (4) and height (5) and flower tube length (6). (Online version in colour.)
Most investigations on polymorphic species have focused on how pollinators select for and maintain discrete floral phenotypes [58,59,66–68], ignoring possible effects on the continuous variation (but see [69]). For example, species of Lithodora with closer reciprocal placement of anthers and stigmas display greater phenotypic integration values [70], and these patterns correspond to the efficiency of different pollinators [71]. Here, we wished to assess whether populations of N. papyraceus and N. tazzeta with contrasting functional groups of pollinators differed in their levels of floral integration and trait correlation. In dimorphic populations, long-tongued insects should exert strong selection, particularly on the flower tube length and the position of the anthers and the stigma, because these insects closely fit with the flower tube to reach the nectar (specialized pollinators sensu [2,22,18,72]). By contrast, selection exerted by short-tongued insects in L-monomorphic populations should be weaker on these traits. Short-tongued pollinators feed on the pollen from the upper anther level (they do not reach the nectar hidden at the bottom of the narrow flower tube) and their interaction with the flower is loose in terms of morphological fit (unspecialized pollinators sensu [2,22,18,72]). If the previous scenario holds, these different selective pressures should be reflected in the strength of phenotypic correlation and integration. In fact, in N. papyraceus, decoupling between floral and vegetative traits was greater in dimorphic populations than in L-monomorphic population [29], fitting Berg's predictions [2,22].
The first aim of this study was to assess whether phenotypic integration in dimorphic populations with long-tongued pollinators (hereafter LT pollinators) was greater than in L-monomorphic populations with short-tongued pollinators (hereafter ST pollinators) in N. papyraceus and N. tazetta. Secondly, modularity of N. papyraceus flowers was assessed by analysing the strength of correlations of sets of traits considered to play the same function with the correlations of traits involved in different functions. To test whether LT and ST pollinators could exert different selective pressures on floral traits, within and between population types, the phenotypic variation and phenotypic correlations of traits involved in the attraction of pollinators and access to the flower (i.e. flower diameter, corona diameter and height, flower tube length and width) was compared to that from traits involved in the transfer of pollen (i.e. style length, upper anther height and lower anther height). Finally, to control for possible population genetic constraints and marginal range effects on phenotypic integration (monomorphic populations are smaller and tend to occur more peripherally than dimorphic populations; [50,53]), the genetic diversity of dimorphic and L-monomorphic populations was studied using microsatellite markers. Population genetic parameters were used to explore possible associations with phenotypic integration, variation and correlation of floral traits. The comparisons across species and populations allowed validation of current views of selection on floral trait covariation and modularity caused by different pollinators [2,20,30,73].
2. Material and methods
(a). Population sampling for floral measurements
Flowers were collected from 17 populations of N. papyraceus (seven dimorphic and 10 L-monomorphic and highly L-biased, L > 95%, see [29,54]) and nine populations of N. tazetta (three dimorphic and six L-monomorphic and highly L-biased, L > 95%, see [50] for sampling details and table 1). For simplicity, we will call the group represented by L-monomorphic and highly L-biased populations as L-monomorphic. Flower measurements in N. papyraceus were taken by R.P.-B. (figure 1a) and included flower diameter (1), corona diameter (2) and height (3), flower tube length (4) and width (5), style length (6), upper (7) and lower (8) anther height in L and S flowers. Flower measurements in N. tazetta were taken by J.A. (figure 1b) and they included flower diameter (1), outer tepal length (2) and width (3), corona diameter (4) and height (5) and flower tube length (6). Details on pollinators, their ability to pick up and deliver pollen and select for L and S flowers can be found elsewhere [29,50,55,56].
Table 1.
Narcissus papyraceus and N. tazetta populations surveyed for flower measurements and analyses of phenotypic integration, and patterns of floral variation and phenotypic correlation (see the electronic supplementary material, appendices S1, S2 and S3). Estimates of population morph ratio were done on a larger sample size (see [29,49–51,53,54] for detailed information on population sampling). Confidence intervals (CIs) for the raw integration index were obtained by bootstrapping.
| species and populations | sample size for flower measurements (L : S) | L morph (%) | phenotypic integration index (%) | raw phenotypic integration index | 95% CI |
|---|---|---|---|---|---|
| Narcissus papyraceus | |||||
| Morocco: Tánger-Tetuán, Oued Lediane | 100 (57 : 42) | 57 | 10.28 | 0.55 | 0.01–0.95 |
| Morocco: Tetuán-Larache, Souk el Arba Ayacha | 100 (95 : 100) | 96.3 | 3.76 | 0.23 | 0.01–0.38 |
| Morocco: Tánger-Tetuán, Ragaia | 100 (41 : 59) | 50 | 5.08 | 0.29 | 0.01–0.51 |
| Spain: Málaga, Casares-Manilva | 100 (87 : 13) | 87.4 | 14.99 | 0.79 | 0.04–1.37 |
| Spain: Málaga, San Pedro de Alcántara | 67 (66 : 1) | 98.53 | 13.39 | 0.73 | 0.01–1.23 |
| Spain: Cádiz, Tarifa-Bolonia | 100 (52 : 48) | 50 | 23.64 | 1.22 | 0.01–2.05 |
| Spain: Cádiz, Los Barrios | 100 (50 : 50) | 50 | 19.11 | 1.00 | 0.00–1.62 |
| Spain: Cádiz, El Bosque | 48 (32 : 16) | 66 | 19.36 | 1.05 | 0.01–1.74 |
| Spain: Huelva, Villanueva de los Castillejos | 100 (99 : 1) | 99 | 19.46 | 1.01 | 0.02–1.69 |
| Spain: Huelva, Hinojos, El Caoso | 24 | 100 | 8.15 | 0.57 | 0.13–0.94 |
| Spain: Huelva, Hinojos, Coto del Rey | 100 | 100 | 4.45 | 0.26 | 0.02–0.39 |
| Spain: Huelva, Almonte, El Rocío | 98 (95 : 3) | 98.5 | 5.44 | 0.31 | 0.01–0.55 |
| Spain: Sevilla, Aznalcázar | 98 | 100 | 8.33 | 0.46 | 0.02–0.78 |
| Spain: Córdoba, Carcabuey, Valdecañas | 100 | 100 | 5.59 | 0.32 | 0.01–0.57 |
| Portugal: Algarve, Barranco São Miguel | 60 (55 : 6) | 90.6 | 7.73 | 0.45 | 0.02–0.78 |
| Portugal: Algarve, Mesines-Alte | 100 | 100 | 10.49 | 0.57 | 0.01–0.98 |
| Portugal: Algarve, Tavira | 100 | 100 | 10.50 | 0.57 | 0.02–0.98 |
| Narcissus tazetta | |||||
| Israel: Yuvalim | 30 (15 : 15) | 96 | 9.88 | 0.63 | 0.05–1.15 |
| Israel: Stella Maris | 28 (25 : 3) | 95 | 13.4 | 0.89 | 0.12–1.5 |
| Israel: Megadim | 32 (30 : 2) | 100 | 15.7 | 0.94 | 0.08–1.7 |
| Israel: Nahal Mearot West | 16 | 100 | 10.1 | 0.92 | 0.08–1.53 |
| Israel: Nahal Mearot North | 11 | 100 | 20.43 | 1.68 | 0.08–0.04 |
| Israel: Nahal Ma'sad | 19 (17 : 2) | 89.5 | 9.41 | 0.83 | 0.06–1.41 |
| Israel: Yagur | 20 (10 : 10) | 54 | 21.66 | 1.55 | 0.03–2.42 |
| Israel: Kfar Yeoshua | 13 (3 : 10) | 20 | 19.55 | 1.56 | 0.12–2.77 |
| Israel: Kishon River | 34 (10 : 24) | 10 | 23.57 | 1.33 | 0.06–2.35 |
(b). Phenotypic integration in dimorphic and L-monomorphic populations of Narcissus papyraceus and N. tazetta
We used the method developed by Wagner [74] and Cheverud et al. [75] to calculate the phenotypic integration index for each species and population. The phenotypic integration index was estimated as the variance of the eigenvalues of the correlation matrix. Sample size varied among populations (table 1); hence, the integration index was corrected by subtracting the expected phenotypic integration under the assumption of random covariation of traits (see [26,29,74] for details). The integration index was expressed as percentage of the maximum value, which is the number of traits included [26]. In dimorphic populations, the phenotypic integration index was estimated by pooling the data from L and S flowers (style length and upper and lower anther height were not included in this analysis as these data were only available for N. papyraceus; see description of traits measured above, figure 1a,b). The average phenotypic integration between the two types of populations was analysed with an unpaired t-test. To control by the lack of independence (phenotypic integration index is based on a correlation matrix), we implemented a bootstrap procedure (n = 20.000 permutations with replacement; see [31,76] for details) in R [77] to detect significant differences.
(c). Patterns of phenotypic variation and phenotypic correlations in Narcissus papyraceus
To evaluate if N. papyraceus flowers could be divided into different functional modules, we tested whether the average of the correlation coefficients of the set of traits included within the same function (attraction: diameter and corona diameter; access: corona height and flower tube length and width; pollen transfer: style length, upper and lower anther position) was larger than the average of the correlation coefficients between traits belonging to different functions. These comparisons were conducted within population type.
The phenotypic variation of traits involved in pollinator attraction and access, and pollen pick-up and deposition was analysed within and between population types. Within population type, pairwise comparisons were used to test for differences in the average coefficient of variation (hereafter CV) between groups of traits (attraction versus pollinator access and fit, attraction versus pollen pick-up and deposition, and pollinator access and fit versus pollen pick-up and deposition). Between populations, pairwise comparisons were implemented to test for differences on the average CV of the same type of trait (e.g. differences in the CV of attraction traits in dimorphic versus L-monomorphic populations).
The strength of the correlation coefficient of sets of traits included in the same function was also studied. Within population type, the correlations of traits involved in pollen transfer (the style length–flower tube length correlation, the upper anther height–flower tube correlation and the lower anther height–flower tube correlation) were compared against the average correlations among traits involved in attraction or access (diameter, corona diameter and height, and flower width). In addition, comparisons were established to detect possible differences between population types in the style length–flower tube length correlation, the upper and lower anther height–flower tube length correlations and the average correlations among traits involved in attraction or access.
We used the resampling procedure described above to detect significant differences in all the comparisons.
(d). Genetic diversity in Narcissus papyraceus populations
Leaf tissue was collected from 15 to 20 individuals chosen randomly, totalling 164 N. papyraceus plants in six dimorphic populations and four L-monomorphic populations (tables 1 and 2). Sampled plants were separated from each other by at least 1 m. Leaf tissue was dried out in silica gel and later frozen at −80°C until DNA extraction. DNA was isolated following Bernartzky & Tanksley's [78] protocol without mercaptoethanol. All samples were genotyped according to eight nuclear microsatellite markers previously tested for polymorphism (A5, A109, A116, A121, B7, B104, B109 and B112; [79]). We performed polymerase chain reactions (PCR) in 25 μl of reaction mixture containing 50 ng of template DNA, 1× PCR buffer, 1.5 mM MgCl2, 0.1 μM fluorescently labelled (6-FAMTM, VIC, NEDTM and PET dyes) forward primer, 0.1 μM reverse primer, 0.05 mM each dNTP and 1.25 U Taq polymerase. PCRs were performed in a Biometra Gradient Thermal Cycler (Biometra, Göttingen, Germany), with an initial 5 min of denaturation at 94°C, 45 cycles at 94°C for 30 s, annealing at different temperatures depending on the marker (57°C for A109 and B7; 58°C for A116, A121 and B109; 59°C for B104 and B112) for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 5 min. Polymerase chain reaction products were analysed on an ABI 3130 × 1 Genetic Analyser and sized using GeneMapper v. 4.0 (Applied Biosystems, Foster City, CA, USA) and GeneScanTM 500 LIZ size standard.
Table 2.
Genetic diversity parameters (±s.d.) for each of the selected Narcissus papyraceus populations. Percentage of polymorphic loci (PL), mean number of alleles (na), genetic diversity (HS), allelic richness (RS) and the fixation index (FIS) per locus.
| population | sample size | PL | na | HS | RS | FIS |
|---|---|---|---|---|---|---|
| Morocco: Tánger-Tetuán, Ragaia | 20 | 100 | 11.4 (1.2) | 0.81 (0.06) | 3.2 (0.2) | 0.40 (0.07) |
| Spain: Málaga, Casares-Manilva | 15 | 87.5 | 7.6 (1.5) | 0.68 (0.10) | 2.8 (0.3) | 0.45 (0.08) |
| Spain: Cádiz, Tarifa-Bolonia | 18 | 100 | 9.0 (1.1) | 0.75 (0.06) | 3.0 (0.2) | 0.38 (0.10) |
| Spain: Cádiz, Los Barrios | 15 | 100 | 8.6 (0.9) | 0.74 (0.06) | 3.0 (0.2) | 0.37 (0.09) |
| Spain: Cádiz, El Bosque | 15 | 100 | 7.6 (1.0) | 0.69 (0.10) | 2.8 (0.3) | 0.42 (0.10) |
| Spain: Huelva, Villanueva de los Castillejos | 16 | 100 | 5.8 (0.7) | 0.71 (0.06) | 2.8 (0.2) | 0.47 (0.12) |
| Spain: Huelva, Hinojos, El Caoso | 19 | 100 | 8.4 (1.0) | 0.77 (0.05) | 3.0 (0.2) | 0.55 (0.08) |
| Spain: Sevilla, Aznalcázar | 15 | 100 | 8.4 (1.3) | 0.69 (0.09) | 2.9 (0.3) | 0.46 (0.08) |
| Spain: Córdoba, Carcabuey, Valdecañas | 15 | 87.5 | 6.9 (1.5) | 0.65 (0.10) | 2.7 (0.3) | 0.27 (0.10) |
| Portugal: Algarve, Barranco São Miguel | 16 | 87.5 | 5.0 (1.0) | 0.52 (0.10) | 2.3 (0.3) | 0.46 (0.13) |
For each N. papyraceus population, the mean number of alleles per locus (na), the mean genetic diversity (HS), the fixation index (FIS) and the proportion of polymorphic loci (PL) was calculated using GENALEX v. 6 [80]. Allelic richness (RS) was estimated with HP-RARE v. 1 [81]. Non-parametric Kruskal–Wallis one-way ANOVA was used to detect possible differences in the population genetic parameters between dimorphic and L-monomorphic populations. The relationship between population genetic parameters and the phenotypic integration index, the average CV of floral traits and the average coefficient of correlation of floral traits was analysed with Spearman's rank correlation.
3. Results
(a). Phenotypic integration in Narcissus papyraceus and N. tazetta populations
Our results supported the hypothesis that dimorphic populations visited by LT pollinators should display higher integration values than L-monomorphic mostly visited by ST pollinators. Table 1 includes the phenotypic integration index and 95% CI estimates for N. papyraceus and N. tazetta populations. The magnitude of the phenotypic integration index in N. papyraceus ranged from 3.7 to 23.6%, and dimorphic populations showed greater integration than L-monomorphic populations (dimorphic populations mean (95% CI): 15.6% (9.1, 20.7); L-monomorphic populations: 10.2% (7.1, 13.5), p = 0.02, figure 2). Phenotypic integration values for N. tazetta ranged from 9.9 to 23.6% (table 1), and they also showed that dimorphic populations displayed larger integration values (18.6% (9.4, 23.6)) than L-monomorphic populations (13.2% (9.9, 20.4)), but the significance of the differences were marginal (p = 0.07, figure 2).
Figure 2.
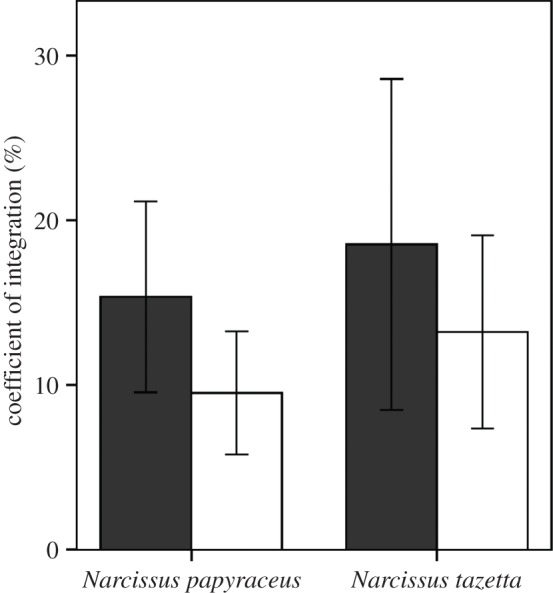
Means and 95% CI of the phenotypic integration index in dimorphic (black bars) and L-monomorphic (white bars) populations with mainly long- and short-tongued pollinators, respectively, in N. papyraceus and N. tazetta.
(b). Patterns of variation and phenotypic correlation in groups of traits with shared function in Narcissus papyraceus
Electronic supplementary material, appendices S1, S2 and S3, include the coefficients of variation and correlation for N. papyraceus populations (estimates for L and S morph were calculated separately, and differences in the coefficients among floral traits for L and S flowers were not significant, results not shown).
The comparisons of the correlation coefficients of sets of traits included in the same function and in different functions supported the hypothesis of floral modularity in N. papyraceus. In dimorphic populations, the average correlation coefficient of sets of traits involved in the same function was larger than the average correlation coefficient of traits belonging to different functions (correlation coefficient of sets of traits within function: 0.64 (0.60, 0.67); correlation coefficient of sets of traits between functions: 0.33 (0.31, 0.36), p < 0.0001). The same results were found in L-monomorphic populations (correlation coefficient within function: 0.55 (0.51, 0.59); correlation coefficient between functions: 0.21 (0.18, 0.23), p < 0.0001).
The comparisons of the CV aimed at testing whether patterns of floral phenotypic variability in dimorphic populations with LT pollinators differed from those found in L-monomorphic populations with ST pollinators. In dimorphic populations, the CV of floral traits involved in the attraction of pollinators (12.9 (10.5, 11.9)) was significantly larger than the CV of floral traits involved in the access and fit with pollinators (11. 7 (10.9, 12.6), p = 0.02) and than the CV of floral traits involved in pollen pick-up and deposition (11.2 (10.5,12.1), p = 0.0036, figure 3). By contrast, the CV of traits involved in pollinator access and fit did not differ from the CV of traits involved in pollen pick-up and deposition (p = 0.23, figure 3). In L-monomorphic populations, the CV of floral traits to attract pollinators (11.6 (11.0, 12.8)) did not differ from the CV of floral traits involved in the access and fit with pollinators (10.9 (10.2, 11.8), p = 0.11, figure 3), whereas differences between the CV of floral traits involved in pollen pick-up and deposition (9.6 (9.1, 9.9)) and traits involved in attraction and pollinator access and fit were significant (p < 0.0001 and p = 0.0002, respectively, see figure 3). Comparisons between population types showed that, on average, the CV of attraction traits and traits involved in pollen pick-up and deposition were larger in dimorphic populations than in L-monomorphic populations (p = 0.05 and p < 0.0001, respectively); in contrast, differences in the CV of traits involved in the fit with pollinators were not significant (p = 0.13, figure 3).
Figure 3.
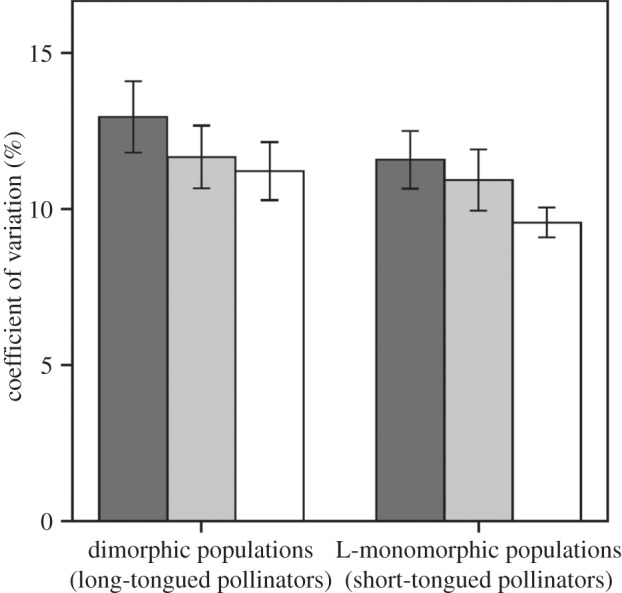
Mean and 95% CI of the coefficient of variation of floral traits involved in the pollinator attraction (black bars), pollinator access and fit (grey bars) and in pollen pick-up and deposition (white bars) in dimorphic and L-monomorphic populations of N. papyracueus.
In dimorphic and L-monomorphic populations, the style length–flower tube length correlation, and the anther height–flower tube correlation, both upper and lower anther level, were larger than the average phenotypic floral correlations among attraction traits and these differences were significant (dimorphic populations: style versus attraction correlations, p = 0.02; upper anther versus attraction correlations, p < 0.0001; lower anther versus attraction correlations, p < 0.0001; L-monomorphic populations: style versus attraction correlations, p < 0.0001, upper anther versus attraction correlations, p < 0.0001, lower anther versus attraction correlation, p < 0.0001, figure 4). These results supported the prediction that the fitness surface for traits involved in pollen pick-up and delivery should be steeper than in traits involved in the attraction of pollinators. Comparisons between population types showed that upper anther–flower tube correlations (dimorphic populations: 0.85 (0.82, 0.88); L-monomorphic populations: 0.78 (0.73, 0.82)) and lower anther height–flower tube length correlations (dimorphic populations: 0.73 (0.68, 0.76); L-monomorphic populations: 0.64 (0.58, 0.68)) were larger in dimorphic than L-monomorphic populations, and the differences were significant (p = 0.01 for both comparisons, figure 4). By contrast, style length–flower tube length correlations did not differ between dimorphic and L-monomorphic populations (dimorphic populations: 0.57 (0.48, 0.66); L-monomorphic populations: 0.51 (0.46, 0.57), p = 0.12). The average floral correlations among attraction traits were significantly larger in dimorphic than L-monomorphic populations (dimorphic populations: 0.46 (0.40, 0.52); L-monomorphic populations: 0.33 (0.25, 0.41), p = 0.005, figure 4). These comparisons between population types agreed with the expectation that the adaptive peak of floral traits should be narrower when selection is mediated by specialized LT-pollinators than by generalized ST-pollinators.
Figure 4.
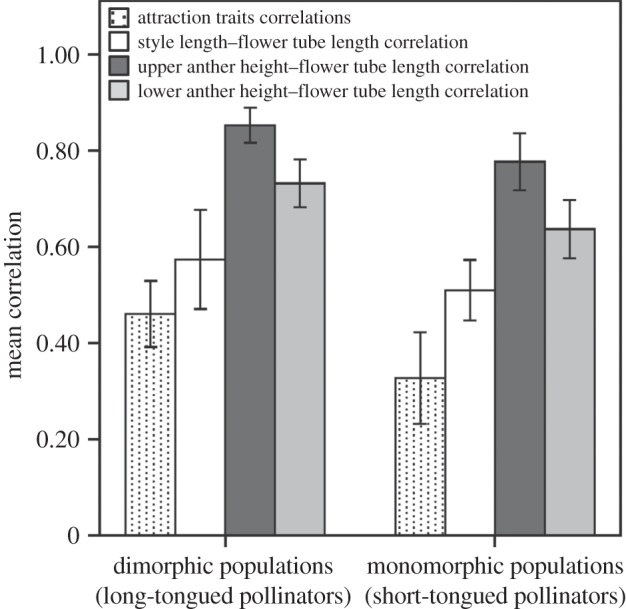
Mean and 95% CI of the correlation coefficients among traits involved in the attraction of pollinators and traits involved in the pollination function (style length–flower tube length correlation, upper anther height–flower tube correlation and lower anther height–flower tube correlation) in dimorphic and L-monomorphic populations of Narcissus papyraceus.
(c). Genetic diversity in Narcissus papyraceus populations
The percentage of PL among N. papyraceus populations varied between 87.5 and 100% (table 2). The mean number of alleles per locus (na) ranged between 5.0 and 11.4, and genetic diversity (HS) between 0.52 and 0.51. The allelic richness (RS) varied between 2.3 and 3.2, and the fixation indices FIS were all positive and ranged from 0.27 to 0.55. The non-parametric Kruskal–Wallis one-way ANOVA showed that dimorphic and L-monomorphic populations did not differ in the population genetic parameters estimated (PL: H = 0.071, na: H = 0.736, HS: H = 0.011, RS: H = 0.191 and FIS: H = 1.183, in all cases d.f. = 1 and p > 0.2). The Spearman's correlation coefficients between HS and the integration index (ρ = −0.024), the mean CV (ρ = 0.248) and the average correlation coefficients (ρ = 0.041) for all floral traits were not significant (N = 10 and p > 0.5 in all estimates).
4. Discussion
(a). Patterns of phenotypic integration in Narcissus species
Narcissus papyraceus and N. tazetta both have dimorphic populations with mostly long-tongued diurnal and nocturnal pollinators, and highly L-biased and L-monomorphic populations pollinated mainly by short-tongued syrphid flies [29,51,53–55]. Shifts from long- to short-tongued pollinators seem to select against the S morph and favour the L morph [59,60]. Thus, a steeper fitness surface could be expected in populations where flowers have close fit with pollinators (long-tongued insects) than in populations in which pollinators fit loosely with flowers (short-tongued insects), and this should be reflected in the patterns of phenotypic integration [2,18]. Our results confirmed this expectation: the phenotypic integration index in dimorphic populations was larger than in L-monomorphic or highly L-biased populations, and these trends were consistent across the two species.
The phenotypic integration observed in the two species could reflect possible effects of common ancestry [38,40,41]. However, N. papyraceus and N. tazetta are not sister species [49,82], and other species with different stylar condition (fixed monomorphism in N. serotinus or dimorphism in N. broussonetii) are in the same clade. Assuming that legitimate pollinators in dimorphic species and populations are long-tongued insects, as the floral syndrome suggests [49], and that L-monomorphism with pollination by short-tongued insects is a derived condition [49,59,83], similar levels of integration in dimorphic populations (15.6% for N. papyraceus and 18.5% for N. tazetta), which differ greatly from the 10-fold variation in other species of the clade (3–30%; R Pérez-Barrales, R Santos-Gally and J Arroyo 2013, unpublished data), may reflect factors other than common ancestry. More evidence in support of pollinators as drivers of floral integration includes the similar patterns of variation in population morph ratio, and the similar shifts in pollinators and patterns of floral integration in two species at the edges of the Mediterranean Basin (ca 4000 km distance), which are unlikely to be caused by phylogenetic effects. Nevertheless, detailed evolutionary reconstruction of flower phenotypic integration would help to elucidate this question.
Colonization of rocky habitats with severe temperature fluctuations, which determine early blooming, has been proposed as a cause for the shift of pollinators in N. tazetta populations [50]. An expansion to inland from coastal ranges seems to have played a similar role in N. papyraceus populations [84]. Hence, the lower integration in L-monomorphic populations could also be explained by (i) historical effects, if all L-monomorphic populations represent a single evolutionary event, and they have inherited the patterns of trait correlation and covariation (but see discussion above); and (ii) a reduction in genetic variation associated with the colonization of marginal ranges [43]. At present, we do not have sufficient phylogeographic information for these species to trace the colonization history of populations, and therefore reconstruct variation of integration across the ranges. However, population neutral genetic variation based on microsatellite markers did not differ in dimorphic and L-monomorphic populations of N. papyraceus; neither was significant the non-parametric correlations with integration, average floral variation and average correlation among traits (see discussion below). This evidence suggests that population genetic processes other than selection may have played a minor role in the patterns observed [85,86] and that low integration values are not due to a reduction in population genetic variation. However, our interpretations must be taken cautiously due to the limited number of populations in which we could relate phenotypic and genetic variation.
(b). Patterns of floral variation, modularity and correlation in Narcissus papyraceus
Traits involved in attraction of pollinators (flower diameter, corona diameter and height) were significantly more variable than traits putatively involved in pollen pick-up and delivery (style length, upper and lower anther height) in dimorphic and L-monomorphic populations. This is not surprising because, regardless of the level of pollination specialization, developmental canalization and selection for precision in the pollination function reduces phenotypic variation [35–37,87,88]. By contrast, access and fit traits (flower tube width and length) displayed different patterns: their CV was lower than attraction traits and similar to pollination traits in dimorphic populations; while in L-monomorphic populations, the CV of access and fit traits was within the same range as attraction traits and substantially larger than pollination traits (figure 3). Small values for the CV of traits involved in pollen pick-up and delivery (e.g. access and fit, position of sexual organs) might reflect stronger directional selection or steeper stabilizing selection caused by long-tongued pollinators compared with short-tongued pollinators [38,87,88].
Comparisons of correlation coefficients of traits involved in attraction and transfer of pollen, as well as correlations of traits across functions revealed interesting patterns. The hypothesis of floral modularity in N. papyraceus was supported. In both dimorphic and L-monomorphic populations, correlations of sets of traits associated with attraction, access and pollen transfer were larger than correlations of traits involved in different functions. From a developmental and genetic perspective this is expected, as shown for both plants [25,26,32] and animals [2,3]. Floral modularity can be selected for, and this can cause low floral integration [30,32]. In this study, we lack fitness estimates to measure the adaptive value of traits involved in different functions, and hence cannot say that modularity is the cause of low integration. Interestingly, dimorphic and L-monomorphic populations displayed similar modularity but different phenotypic integration.
Despite the fact that selection for modularity may act against high integration, the comparisons of groups of traits related to attraction, access and transfer of pollen in dimorphic and L-monomorphic populations agreed with the hypothesis that selection by different pollinators can generate differences in levels of integration. Within population type, the correlations between organs involved in pollen pick-up and deposition (upper and lower anther length, style length and flower tube length) were consistently larger than the average correlations among floral traits involved in attraction. In addition, the correlation coefficient was substantially larger for the anther height–flower tube correlation than the style length–flower tube length correlation (figure 4). An important aspect of Narcissus flowers is that the filaments are fused to the flower tube. Hence, the high correlations observed for anther position and flower tube length both in dimorphic and L-monomorphic populations might reflect important developmental constraints, genetic correlation and pleiotropy (see discussion above, [89–91]). However, we detected differences in the strength of correlations as predicted by Berg [2,18], and the results fitted the expectations that LT-pollinators exert stronger selection on anther position than ST-pollinators. Furthermore, differences between population types in the average correlation between anther height and flower tube length were larger for the lower anther level than the upper anther level. This may reflect two processes that are not mutually exclusive: (i) selection generated by LT-pollinators for a precise position of the lower anther level to donate pollen to S-stigmas in dimorphic populations [58,92]; and/or (ii) relaxation of selection on the lower anther level in L-monomorphic populations because, unlike long-tongued insects, short-tongued pollinators interact only with the upper anther level (R Pérez-Barrales 2003, personal observation; [60]).
In contrast to anther height and flower tube length, the correlation between style length and flower tube length did not differ between population types (although the average correlation was smaller in L-monomorphic than in dimorphic populations, figure 4). In addition to pollinators, the position of the stigma may be constrained by additional factors. For example, avoidance of self-interference through stigma clogging can affect stigma position in self-incompatible species and increase the deviation from optimal position for pollen arrival [35–37,93,94].
5. Concluding remarks
In a previous paper, we documented a pattern of floral phenotypic integration in N. papyraceus consistent with the role of different pollinators in different populations. Here, we expanded our results using N. tazetta, a different species from a distant geographical range, but displaying similar variation in pollinators. We also studied patterns of variation and correlation of traits involved in different functions, and incorporated a population genetic dataset to assess possible effects of demographic population processes on the phenotypic patterns described. Taken together, the results suggest that pollinator-mediated selection plays an important role in the phenotypic integration of N. papyraceus and N. tazetta flowers: selection probably maintains the correlation structure in dimorphic populations pollinated by long-tongued pollinators, whereas this structure is weakened when these pollinators are mostly substituted by short-tongued pollinators in other populations. Our findings agree with a number of studies supporting the idea that plant species with specialized pollinators present larger values of floral integration than species with generalized pollinators [2,22,18,29,38]. Notwithstanding this, our study did not include female and male fitness estimates to quantify the adaptive value of integration, nor could we assess the adaptive value of the traits taking part in attraction, access and pollen transfer with different functional groups of pollinators. Future research will require combining phenotypic selection studies with developmental and quantitative genetics to better understand how pollinators can select for integrated phenotypes.
Supplementary Material
Acknowledgements
We are grateful to Scott Armbruster for his suggestions and two anonymous reviewers for useful comments. J. H. Peterkin improved the English style of the manuscript.
Funding statement
This work was supported by funds from the Spanish Ministerio de Ciencia e Innovación (grant nos. CGL-2006–13847-C02–01 and CGL-2009 12565) and PAIDI (Andalousian regional government grant no. P09-RNM-5280). R.P-B. was supported by a Juan de la Cierva grant from the Spanish Ministerio de Ciencia e Innovación.
References
- 1.Olson EC, Miller RL. 1958. Morphological integration. Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14, 171–180. ( 10.2307/2405824) [DOI] [Google Scholar]
- 3.Cheverud JM. 1982. Phenotypic, genetic and environmental morphological integration in the cranium. Evolution 36, 499–516. ( 10.2307/2408096) [DOI] [PubMed] [Google Scholar]
- 4.Cheverud JM. 1988. A comparison of genetic and phenotypic correlations. Evolution 42, 958–968. ( 10.2307/2408911) [DOI] [PubMed] [Google Scholar]
- 5.Arnold SJ. 1992. Constraints in phenotypic evolution. Am. Nat. 140, S85–S107. ( 10.1086/285398) [DOI] [PubMed] [Google Scholar]
- 6.Armbruster WS, Schwaegerle KE. 1996. Causes of covariation of phenotypic traits among populations. J. Evol. Biol. 9, 261–276. ( 10.1046/j.1420-9101.1996.9030261.x) [DOI] [Google Scholar]
- 7.Pigliucci M, Preston K. 2004. Phenotypic integration: studying the ecology and evolution of complex phenotypes. New York, NY: Oxford University Press. [Google Scholar]
- 8.Hallgrimsson B, Brian KH. 2011. Epigenetics: linking genotype and phenotype in development and evolution. Berkeley, CA: University of California Press. [Google Scholar]
- 9.Courtney JM. 2012. The integrated phenotype. Integr. Comp. Biol. 52, 64–76. ( 10.1093/icb/ics043) [DOI] [PubMed] [Google Scholar]
- 10.Klingenberg CP, Zaklan SD. 2007. Morphological integration between developmental compartments in the Drosophila wing. Evolution 54, 1273–1285. ( 10.1111/j.0014-3820.2000.tb00560.x) [DOI] [PubMed] [Google Scholar]
- 11.Sanchez JA, Lasker HR. 2003. Patterns of morphological integration in marine modular organisms: supra-module organization in branching octocoral colonies. Proc. R. Soc. Lond. B 270, 2039–2044. ( 10.1098/rspb.2003.2471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingenberg CP, Leamy LJ, Cheverud JM. 2004. Integration and modularity of quantitative trait locus on geometric shape in the mouse mandible. Genetics 166, 1909–1921. ( 10.1534/genetics.166.4.1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami A. 2006. Cranial modularity shifts during mammalian evolution. Am. Nat. 168, 270–280. ( 10.1086/505758) [DOI] [PubMed] [Google Scholar]
- 14.Pavlicev M, Kenney-Hunt JP, Norgard EA, Roseman CC, Wolf JB, Cheverud JM. 2008. Genetic variation in pleiotropy: differential epistasis as a source of variation in the allometric relationship between long bone lengths and body weight. Evolution 62, 199–213. [DOI] [PubMed] [Google Scholar]
- 15.Badayaev AV. 2010. The beak of the other finch: coevolution of genetic covariance structure and developmental modularity during adaptive evolution. Phil. Trans. R. Soc. B 365, 1111–1126. ( 10.1098/rstb.2009.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanger TJ, Mahler DL, Abzhanov A, Losos JB. 2012. Roles for modularity and constraint in the evolution of cranial diversity among Anolis lizards. Evolution 66, 1525–1542. ( 10.1111/j.1558-5646.2011.01519.x) [DOI] [PubMed] [Google Scholar]
- 17.Waitt DE, Levin DA. 1998. Genetic and phenotypic correlations in plants: a botanical test of Cheverud's conjecture. Heredity 80, 310–319. ( 10.1046/j.1365-2540.1998.00298.x) [DOI] [Google Scholar]
- 18.Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Velásques Runk JL. 1999. Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a reevaluation of Berg's correlation-pleiades concept. Am. J. Bot. 86, 39–55. ( 10.2307/2656953) [DOI] [PubMed] [Google Scholar]
- 19.Conner JK. 2002. Genetic mechanisms of floral trait correlations in a natural population. Nature 420, 407–410. ( 10.1038/nature01105) [DOI] [PubMed] [Google Scholar]
- 20.Pélabon C, Osler NC, Dielmann N, Graae BJ. 2013. Decoupled phenotypic variation between floral and vegetative traits: distinguishing between developmental and environmental correlations. Ann. Bot. 111, 935–944. ( 10.1093/aob/mct050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terentjev PV. 1931. Biometrische untersuchungen über die morphologischen Merk-male von Rana ridibunda Pall. (Amphinia, Salientia). Biometrika 23, 23–51. [Google Scholar]
- 22.Berg RL. 1959. A general evolutionary principle underlying the origin of developmental homeostasis. Am. Nat. 93, 103–105. ( 10.1086/282061) [DOI] [Google Scholar]
- 23.Conner J, Via S. 1993. Patterns of phenotypic and genetic correlations among morphological and life-history traits in wild radish, Raphanus raphanistrum. Evolution 47, 704–711. ( 10.2307/2410086) [DOI] [PubMed] [Google Scholar]
- 24.Conner JK, Sterling A. 1996. Selection for independence of floral and vegetative traits: evidence from correlation patterns in five species. Can. J. Bot. 74, 642–644. ( 10.1139/b96-080) [DOI] [Google Scholar]
- 25.Herrera J. 2001. The variability of organs differentially involved in pollination, and correlations of traits in Genisteae (Leguminosae: Papilionoideae). Ann. Bot. 88, 1027–1037. ( 10.1006/anbo.2001.1541) [DOI] [Google Scholar]
- 26.Herrera CM, Cerdá X, García MB, Guitián J, Medrano M, Rey PJ, Sánchez-Lafuente AM. 2002. Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. J. Evol. Biol. 15, 108–121. ( 10.1046/j.1420-9101.2002.00365.x) [DOI] [Google Scholar]
- 27.Murren CJ. 2002. Phenotypic integration in plants. Plant Species Biol. 17, 89–99. ( 10.1046/j.1442-1984.2002.00079.x) [DOI] [Google Scholar]
- 28.Hansen TF, Pelabon C, Armbruster WS. 2007. Comparing variational properties of homologous floral and vegetative characters in Dalechampia scandens: testing the Berg hypothesis. Evol. Biol. 34, 86–98. ( 10.1007/s11692-007-9006-3) [DOI] [Google Scholar]
- 29.Pérez-Barrales R, Arroyo J, Armbruster WS. 2007. Differences in pollinator faunas may generate geographic differences in floral morphology and integration in Narcissus papyraceus (Amaryllidaceae). Oikos 116, 1904–1918. ( 10.1111/j.0030-1299.2007.15994.x) [DOI] [Google Scholar]
- 30.Ordano M, Fornoni J, Boege K, Dominguez CA. 2008. The adaptive value of phenotypic floral integration. New Phytol. 179, 1183–1192. ( 10.1111/j.1469-8137.2008.02523.x) [DOI] [PubMed] [Google Scholar]
- 31.Conner JK, Sterling A. 1995. Testing hypotheses of functional relationships—a comparative survey of correlation patterns among floral traits in 5 insect-pollinated plants. Am. J. Bot. 82, 1399–1406. ( 10.2307/2445866) [DOI] [Google Scholar]
- 32.Bissell EK, Diggle PK. 2010. Modular genetic architecture of floral morphology in Nicotiana: quantitative genetic and comparative phenotypic approaches to floral integration. J. Evol. Biol. 23, 1744–1758. ( 10.1111/j.1420-9101.2010.02040.x) [DOI] [PubMed] [Google Scholar]
- 33.Rush S, Conner JK, Jenneten P. 1995. The effects of natural variation in pollinator visitation on rates of pollen removal in wild radish Raphanus raphanistrum (Brassicaceae). Am. J. Bot. 82, 1522–1526. ( 10.2307/2446180) [DOI] [Google Scholar]
- 34.Engel EC, Irwin RE. 2003. Linking pollinator visitation rate and pollen receipt. Am. J. Bot. 90, 1612–1618. ( 10.3732/ajb.90.11.1612) [DOI] [PubMed] [Google Scholar]
- 35.Armbruster WS, Pélabon C, Hansen TF, Mulder CPH. 2004. Floral integration, modularity, and accuracy: distinguishing complex adaptations from genetic constraints. In Phenotypic integration: studying the ecology and evolution of complex phenotypes (eds Pigliucci M, Preston K.), pp. 23–49. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Armbruster WS, Hansen TF, Pélabon C, Pérez-Barrales R, Maad J. 2009. The adaptive accuracy of flowers: measurement and microevolutionary patterns. Ann. Bot. 103, 1529–1545. ( 10.1093/aob/mcp095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armbruster WS, Pélabon C, Hansen TF, Bolstad GH. 2009. Macroevolutionary patterns of pollination accuracy: a comparison of three genera. New Phytol. 183, 600–617. ( 10.1111/j.1469-8137.2009.02930.x) [DOI] [PubMed] [Google Scholar]
- 38.Rosas-Guerrero V, Quesada M, Armbruster WS, Pérez-Barrales R, DeWitt Smith S. 2011. Influence of pollination specialization and breeding system on floral integration and phenotypic variation in Ipomoea. Evolution 65, 350–364. ( 10.1111/j.1558-5646.2010.01140.x) [DOI] [PubMed] [Google Scholar]
- 39.Lande R. 1976. Natural selection and random genetic drift in phenotypic evolution. Evolution 30, 314–334. ( 10.2307/2407703) [DOI] [PubMed] [Google Scholar]
- 40.Goswami A. 2006. Morphological integration in the carnivoran skull. Evolution 60, 169–183. ( 10.1111/j.0014-3820.2006.tb01091.x) [DOI] [PubMed] [Google Scholar]
- 41.Pérez F, Arroyo MTK, Medel R. 2007. Phylogenetic analysis of floral integration in Schizanthus (Solanaceae): does pollination truly integrate corolla traits? J. Evol. Biol. 20, 1730–1738. ( 10.1111/j.1420-9101.2007.01393.x) [DOI] [PubMed] [Google Scholar]
- 42.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 43.Eckerrt CG, Samis KE, Lougheed SC. 2008. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol. Ecol. 17, 1170–1188. ( 10.1111/j.1365-294X.2007.03659.x) [DOI] [PubMed] [Google Scholar]
- 44.Eckert CG, Manicacci D, Barrett SCH. 1995. Genetic drift and founder effects in native versus introduced populations of an invading plant, Lythrum salicaria (Lythraceae). Evolution 50, 1512–1519. ( 10.2307/2410888) [DOI] [PubMed] [Google Scholar]
- 45.Tremblay RL, Ackerman JD. 2001. Gene flow and effective population size in Lepanthes (Orchidaceae): a case of genetic drift. Biol. J. Linn. Soc. 72, 47–62. ( 10.1111/j.1095-8312.2001.tb01300.x) [DOI] [Google Scholar]
- 46.Manica A, Amos W, Balloux F, Hanihara T. 2007. The effect of ancient population bottlenecks on human phenotypic variation. Nature 448, 346–348. ( 10.1038/nature05951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meeus S, Honnay O, Jacquemyn H. 2012. Strong differences in genetic structure across disjunct edge, and core populations of the dystylous forest herb Pulmonaria officinalis (Boraginaceae). Am. J. Bot. 99, 1809–1818. ( 10.3732/ajb.1200223) [DOI] [PubMed] [Google Scholar]
- 48.Caley MJ, Cripps E, Game ET. 2013. Phenotypic covariance at species’ borders. BMC Biol. 13, 105 ( 10.1186/1471-2148-13-105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos-Gally R, Gonzalez-Voyer R, Arroyo J. 2013. Deconstructing heterostyly: the evolutionary role of incompatibility system, pollinators, and floral architecture. Evolution 67, 2072–2082. ( 10.1111/evo.12087) [DOI] [PubMed] [Google Scholar]
- 50.Arroyo J, Dafni A. 1995. Variations in habitat, season, flower traits and pollinators in dimorphic Narcissus tazetta L (Amaryllidaceae) in Israel. New Phytol. 129, 135–145. ( 10.1111/j.1469-8137.1995.tb03017.x) [DOI] [PubMed] [Google Scholar]
- 51.Barrett SCH, Lloyd DG, Arroyo J. 1996. Stylar polymorphisms and the evolution of heterostyly in Narcissus (Amaryllidaceae). In Floral biology: studies on floral evolution in animal-pollinated plants (eds Lloyd DG, Barrett SCH.), pp. 339–376. New York, NY: Chapman and Hall. [Google Scholar]
- 52.Arroyo J. 2002. Narcissus (Amaryllidaceae), la evolución de los polimorfismos florales y la conservación más allá de las ‘listas rojas’. Rev. Chil. Hist. Nat. 75, 39–55. [Google Scholar]
- 53.Arroyo J, Barrett SCH, Hidalgo R, Cole WW. 2002. Evolutionary maintenance of stigma-height dimorphism in Narcissus papyraceus (Amaryllidaceae). Am. J. Bot. 89, 1242–1249. ( 10.3732/ajb.89.8.1242) [DOI] [PubMed] [Google Scholar]
- 54.Pérez-Barrales R, Pino R, Albaladejo RG, Arroyo J. 2009. Geographic variation of flower traits in Narcissus papyraceus (Amaryllidaceae): do pollinators matter? J. Biogeogr. 36, 1411–1422. ( 10.1111/j.1365-2699.2008.01964.x) [DOI] [Google Scholar]
- 55.Santos-Gally R, Pérez-Barrales R, Simón VI, Arroyo J. 2013. The role of short-tongued insects in floral variation across the range of a style dimorphic plant. Ann. Bot. 111, 317–328. ( 10.1093/aob/mcs261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dulberger R. 1964. Flower dimorphism and self-incompatibility in Narcissus tazetta L. Evolution 18, 361–363. ( 10.2307/2406347) [DOI] [Google Scholar]
- 57.Thompson JD, Barrett SCH, Baker AM. 2003. Frequency dependent variation in reproductive success in Narcissus: implications for the maintenance of stigma-height dimorphism. Proc. R. Soc. B 270, 949–953. ( 10.1098/rspb.2002.2318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cesaro AC, Thompson JD. 2004. Darwin's cross-promotion hypothesis and the evolution of stylar polymorphism. Ecol. Lett. 7, 1209–1215. ( 10.1111/j.1461-0248.2004.00683.x) [DOI] [Google Scholar]
- 59.Pérez-Barrales R, Arroyo J. 2010. Pollinator shifts and the loss of style polymorphism in Narcissus papyraceus (Amaryllidaceae). J. Evol. Biol. 23, 1117–1128. ( 10.1111/j.1420-9101.2010.01988.x) [DOI] [PubMed] [Google Scholar]
- 60.Simon-Portcar VI, Santos-Gally R, Arroyo J. 2014. Long-tongued insects promote pollen transfer in style dimorphic Narcissus papyraceus (Amaryllidaceae). J. Ecol. 102, 116–125. ( 10.1111/1365-2745.12179) [DOI] [Google Scholar]
- 61.Barrett SCH, Harder DH. 2005. The evolution of polymorphic sexual systems in daffodils (Narcissus). New Phytol. 165, 45–53. ( 10.1111/j.1469-8137.2004.01183.x) [DOI] [PubMed] [Google Scholar]
- 62.Hodgins KA, Barrett SCH. 2007. Population structure and genetic diversity in tristylous Narcissus triandrus: insights from microsatellite and chloroplast DNA variation. Mol. Ecol. 16, 2317–2332. ( 10.1111/j.1365-294X.2007.03314.x) [DOI] [PubMed] [Google Scholar]
- 63.Pérez-Alquicira J, Molina-Freaner FE, Piñero D, Weller SG, Martínez-Meyer E, Rozas J, Domínguez CA. 2010. The role of historical factors and natural selection in the evolution of breeding systems of Oxalis alpina in the Sonoran desert ‘Sky Islands’. J. Evol. Biol. 23, 2163–2175. ( 10.1111/j.1420-9101.2010.02075.x) [DOI] [PubMed] [Google Scholar]
- 64.Zhou W, Barrett SCH, Wang H, Li DZ. 2012. Loss of floral polymorphism in heterostylous Luculia pinceana (Rubiaceae): a molecular phylogeographic perspective. Mol. Ecol. 21, 4631–4645. ( 10.1111/j.1365-294X.2012.05707.x) [DOI] [PubMed] [Google Scholar]
- 65.Kissling J, Barrett SCH. 2013. Variation and evolution of herkogamy in Exochaenium (Gentianaceae): implications for the evolution of distyly. Ann. Bot. 112, 95–102. ( 10.1093/aob/mct097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lloyd DG, Webb CJ. 1992. The evolution of heterostyly. In Evolution and function of heterostyly. (ed. Barrett SCH.), pp. 151–178. Berlin, Germany: Springer. [Google Scholar]
- 67.Lloyd DG, Webb CJ. 1992. The selection of heterostyly. In Evolution and function of heterostyly. (ed. Barrett SCH.), pp. 179–207. Berlin, Germany: Springer. [Google Scholar]
- 68.Stone JL, Thompson JD. 1994. The evolution of distyly: pollen transfer in artificial flowers. Evolution 48, 1595–1606. ( 10.2307/2410250) [DOI] [PubMed] [Google Scholar]
- 69.Hodgins KA, Barrett SCH. 2008. Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution. 62, 1751–1763. ( 10.1111/j.1558-5646.2008.00404.x) [DOI] [PubMed] [Google Scholar]
- 70.Ferrero V, Chapela I, Arroyo J, Navarro L. 2011. Reciprocal style polymorphisms are not easily categorised: the case of heterostyly in Lithodora and Glandora (Boraginaceae). Plant Biol. 13, 7–18. ( 10.1111/j.1438-8677.2009.00307.x) [DOI] [PubMed] [Google Scholar]
- 71.Ferrero V, Castro S, Sánchez SM, Navarro L. 2011. Stigma–anther reciprocity, pollinators, and pollen transfer efficiency in populations of heterostylous species of Lithodora and Glandora (Boraginaceae). Plant. Syst. Evol. 291, 267–276. ( 10.1007/s00606-010-0387-x) [DOI] [Google Scholar]
- 72.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndroms and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403. ( 10.1146/annurev.ecolsys.34.011802.132347) [DOI] [Google Scholar]
- 73.Cosacov A, Cocucci AA, Sérsic AN. 2014. Geographical differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza: do pollinators matter? Ann. Bot. 113, 251–266. ( 10.1093/aob/mct239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner GP. 1984. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidences for a non-random organization of quantitative character variation. J. Math. Biol. 21, 77–95. ( 10.1007/BF00275224) [DOI] [Google Scholar]
- 75.Cheverud JM, Wagner GP, Dow MM. 1989. Methods for the comparative analysis of variation patterns. Syst. Zool. 38, 201–213. ( 10.2307/2992282) [DOI] [Google Scholar]
- 76.Manly FJ. 1998. Randomization, bootstrap and Monte Carlo methods in biology, 2nd edn London, UK: Chapman and Hall. [Google Scholar]
- 77.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing. [Google Scholar]
- 78.Bernartzky R, Tanksley S. 1986. Genetics of acting-related sequences in tomato. Theor. Appl. Genet. 72, 314–324. ( 10.1007/BF00288567) [DOI] [PubMed] [Google Scholar]
- 79.Simón VI, Picó FX, Arroyo J. 2010. New microsatellite loci for Narcissus papyraceus (Amaryllidaceae) and cross-amplification in other congeneric species. Am. J. Bot. 97, e10–e13. ( 10.3732/ajb.1000023) [DOI] [PubMed] [Google Scholar]
- 80.Peakall R, Smouse P. 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295. ( 10.1111/j.1471-8286.2005.01155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalinowski ST. 2005. HP-Rare: a computer program for performing rarefaction on measures of allelic diversity. Mol. Ecol. Notes 5, 187–189. ( 10.1111/j.1471-8286.2004.00845.x) [DOI] [Google Scholar]
- 82.Santos-Gally R, Vargas P, Arroyo J. 2012. Insights into Neogene Mediterranean biogeography based on phylogenetic relationships of mountain and lowland lineages of Narcissus (Amaryllidaceae). J. Biogeogr. 39, 782–789. ( 10.1111/j.1365-2699.2011.02526.x) [DOI] [Google Scholar]
- 83.Pérez-Barrales R, Vargas P, Arroyo J. 2006. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytol. 171, 553–567. ( 10.1111/j.1469-8137.2006.01819.x) [DOI] [PubMed] [Google Scholar]
- 84.Simón-Portcar V. 2013. Evolutionary ecology of stylar polymorphism in Narcissus papyraceus Ker-Gawl. (Amaryllidaceae). PhD thesis, University of Seville, Sevilla, Spain. [Google Scholar]
- 85.Riska B. 1985. Group size factors and geographic variation of morphometric correlation. Evolution 39, 792–803. ( 10.2307/2408679) [DOI] [PubMed] [Google Scholar]
- 86.Bryant EH, Meffert LM. 1990. Multivariate phenotypic differentiation among bottleneck lines of the housefly. Evolution 44, 660–668. ( 10.2307/2409443) [DOI] [PubMed] [Google Scholar]
- 87.Cresswell JE. 1998. Stabilizing selection and the structural variability of flowers within species. Ann. Bot. 81, 463–473. ( 10.1006/anbo.1998.0594) [DOI] [Google Scholar]
- 88.Cresswell JE. 2000. Manipulation of female architecture in flowers reveals a narrow optimum for pollen deposition. Ecology 81, 3244–3249. ( 10.1890/0012-9658(2000)081[3244:MOFAIF]2.0.CO;2) [DOI] [Google Scholar]
- 89.Faivre AE. 2000. Ontogenetic differences in heterostylous plants and implications for development from herkogamous ancestor. Evolution 54, 847–858. ( 10.1111/j.0014-3820.2000.tb00085.x) [DOI] [PubMed] [Google Scholar]
- 90.Faivre AE, McDade LA. 2001. Population-level variation in the expression of heterostyly in three species of Rubiaceae: does reciprocal placement of anthers and stigmas characterize heterostyly? Am. J. Bot. 88, 841–853. ( 10.2307/2657036) [DOI] [PubMed] [Google Scholar]
- 91.Ashman TL, Majetic CJ. 2006. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96, 343–352. ( 10.1038/sj.hdy.6800815) [DOI] [PubMed] [Google Scholar]
- 92.Thompson JD, Cesaro AC, Arroyo J. 2012. Morph ratio variation and sex organ reciprocity in style-dimorphic Narcissus assoanus. Int. J. Plant Sci. 173, 885–893. ( 10.1086/667231) [DOI] [Google Scholar]
- 93.Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiospems II. Herkogamy. N. Z. J. Bot. 24, 163–178. ( 10.1080/0028825X.1986.10409726) [DOI] [Google Scholar]
- 94.Cesaro AC, Barrett SCH, Maurice S, Vaissiere BE, Thompson JD. 2004. An experimental evaluation of self-interference in Narcissus assoanus: functional and evolutionary implications. J. Evol. Biol. 17, 1367–1376. ( 10.1111/j.1420-9101.2004.00767.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



