Abstract
Background/aim
Peptidoglycan (PGN) recognition proteins (PGLYRPs) are innate immune molecules that recognise bacterial cell wall PGN, and participate in several inflammatory diseases such as arthritis. We sought to elucidate the contribution of PGLYRPs in murine uveitis (intraocular inflammatory disease) elicited by PGN, and the extent to which systemically administered PGN alters uveitis compared with arthritis versus locally triggered ocular responses.
Methods
Mice deficient for PGLYRP-2, PGLYRP-3 or PGLYRP-4 were administered PGN by an intraperitoneal or intraocular injection. Arthritis was assessed by near-infrared imaging and histopathology, while uveitis was measured by intravital videomicroscopy and histopathology.
Results
Systemic PGN exposure predisposed to arthritis through a PGLYRP-2 dependent mechanism. By contrast, systemic PGN exposure did not predispose to uveitis, and PGLYRP-2 deficiency had no impact on the development the uveitis. When PGN was administered locally, a robust uveitis ensued, which occurred independently of PGLYRP-2. Regardless of whether PGN was administered systemically or locally, neither PGLYRP-3 nor PGLYRP-4 deficiency significantly altered ocular inflammation compared with wild-type control animals.
Conclusions
Our findings highlight the complexity of PGLYRPs and how PGLYRP-2 may use different molecular pathways in the joints versus eyes. Collectively, our results support a non-essential or redundant role for PGLYRPs-2, -3, -4 in uveitis.
INTRODUCTION
The innate immune system serves as a first line of defence against invading microbes. This rapid process is facilitated by pattern recognition receptors (PRRs) that have evolved to respond to conserved microbial structures. Toll-like receptors (TLR) and nucleotide-binding domain and leucine-rich repeat domain-containing receptors (NLR) constitute two well-described PRR families. The NLR member, NOD2, (NLRC2 or CARD15) has been of particular interest to eye researchers because mutations in NOD2 are the cause of Blau syndrome,1 an inherited syndrome wherein intraocular inflammation develops along with inflammation of the joints and skin.2,3 NOD2 responds to muramyl dipeptide (MDP), a fragment of peptidoglycan (PGN),4,5 which is present in the cell walls of nearly all bacteria. In mammals, several PGN recognition molecules exist aside from NOD2, including NOD1, TLR2, CD14, PGN recognition proteins (PGLYRPs), mannose-binding lectin, RegIIIg C-type lectin and amidases.6,7 PGN elicits a potent host immunostimulatory response, but the extent to which any of these molecules mediates ocular inflammatory responses to PGN is unclear.
Intraocular inflammation, or uveitis, is one of the leading causes of visual impairment that has both infectious and non-infectious aetiologies.8 Uveitis is often associated with multisystemic disorders, and is one of the most clinically important extra-articular manifestations of several types of arthritic diseases, including Blau syndrome, ankylosing spondylitis (AS), sarcoidosis, or Behçet’s disease. The underlying mechanisms of uveitis are poorly understood, but one might hypothesise that common pathways are shared among the eyes and joints. Increasing compelling data support the role for innate immune activation in uveitis. Even in ‘non-infectious’ uveitis, bacterial products, such as PGN, have been implicated in its pathogenesis.9 Indeed, IgG antibodies to Streptococcus pyogenes group A PGN have been detected in patients with juvenile onset AS; and antibody cross-reactivity between HLA-B27 and Gram-negative bacteria is known to occur. Thus, investigation of the eye’s responsiveness to bacterial products, such as PGN may be important for our understanding of uveitis.
A recent study demonstrated that PGLYRP-2 promotes local inflammatory responses to PGN in joints.10 The role of PGLYRP-2 in development of uveitis has not been tested, but we hypothesised that PGLYRP-2 plays a similar role in promoting uveitis as it does in arthritis. PGLYRPs are evolutionarily conserved proteins; and in mammals there are four identified PGLYRPs encoded by three genes, as PGLYRP-3 and -4 are splice products of the same gene.11 PGLYRP-1, 2, 3, and 4 are responsible for recognising breakdown products of PGN but have immunomodulatory functions as well.12 Differential expression among the PGLYRPs in mammals has been described, with PGLYRP-2 being widely expressed in bone marrow, liver, spleen, skin and kidney, is secreted from the liver into the bloodstream wherein it hydrolyses PGN.12, 13
Given the immense relevance of the PGLYRPs in PGN recognition, we examined whether PGLYRP-2 plays an essential role in ocular inflammation elicited by PGN. Using mice deficient in PGLYRP-2, we investigated the extent to which systemically administered PGN altered uveitis compared with arthritis. We further explored the potential of PGLYRP-2 expression to modulate uveitis triggered by locally administered PGN. Uveitis onset and severity in PGLYRP-2 knockout (KO) mice was compared with mice lacking additional PGN recognition proteins, such as PGLYRP-3 or PGLYRP-4.
MATERIALS AND METHODS
Mice
Female mice deficient in PGLYRP-2, PGLYRP-3 or PGLYRP-4 on the BALB/c background were kindly provided by Dr Roman Dziarski’s laboratory (Department of Microbiology & Immunology, Indiana University School of Medicine, Indiana, USA) and have been described.10 Wild-type BALB/c (WT) mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Experiments were carried out in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International and Oregon Health and Science University and Portland VA Medical Center guidelines. For systemic administration of PGN (purified from Staphylococcus aureus, Sigma), mice were administered an intravenous injection of 500 μg PGN or saline into the tail vein (150 μl). For intraocular administration of PGN, anaesthetised mice (1.7% isoflurane in oxygen) were administered a 2 μl intravitreal injection of 1 μg PGN or saline with a 30.5 G needle under guidance of a stereomicroscope.
Assessment of joint inflammation
At the time of sacrifice, near-infrared (NIR)-imaging was performed using described methodology.14 The NIR-fluorescent probe ProSense 680 (VisEn Medical, Woburn, Massachusetts, USA), a protease substrate that emits NIR-fluorescence when cleaved, was intravenously injected (2 nmol/150 μl) 24 h prior to assessment. Images of ankles were analysed with LI-COR software (Lincoln, Nebraska), and mean differences in fluorescence intensity were normalised to WT mice injected with saline.
Intravital videomicroscopy
The in vivo cellular trafficking response within the iris vasculature and tissue was assessed using intravital videomicroscopy as described.15 To visualise leukocytes, mice were intraperitoneally injected with rhodamine 6G (35 mg/kg, Sigma–Aldrich) at the time of microscopy. Digital videos (10 s each) were captured in three independent regions of the iris using a monochrome camera (Kappa, Gleichen, Germany). Measurements of iris tissue area and vessel diameter and length, and quantification of rolling, adherent and infiltrating leukocytes were performed off-line using Image J.
Histology
Histological assessment was performed as previously described.15 Tissue sections stained with haematoxylin and eosin were assessed for inflammatory changes, and the number of infiltrated leukocytes present in the aqueous humour of the anterior segment and vitreous body of the posterior segment were quantified in masked fashion.
Statistical analysis
All values are presented as mean±SEM. Data were compared by analysis of variance (ANOVA) followed by Student’s t test. Statistical analysis was performed using Prism (GraphPad Software, La Jolla, California, USA) and significance was considered when p<0.05.
RESULTS
Systemic PGN exposure predisposes to arthritis through a PGLYRP-2-dependent mechanism
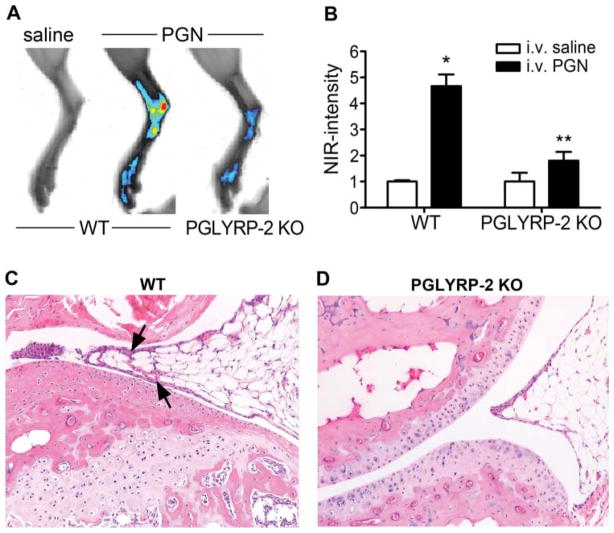
Given the previously reported role for PGLYRP-2 in inflammatory arthritis, wherein mice systemically exposed to PGN develop joint inflammation,10 we sought to elucidate the extent to which PGN affects ocular inflammation and the contribution of PGLYRP-2. PGLYRP-2 KO mice and WT-BALB/c controls were intravenously administered PGN and examined for the onset and severity of inflammation within the ankle joints and eyes. WT mice develop mild inflammation within the ankles at 72 h post-PGN injection, as detected by increased NIR-fluorescence intensity (figure 1A,B), indicative of protease activation. We have previously demonstrated that protease activation, an early event in the inflammatory cascade that promotes arthritis, closely predicts histopathological changes.14 The NIR intensity coincided histopathologically with mild synovitis in the joint, as indicated by arrows (figure 1C). Consistent with a prior report10 we found that PGLYRP-2 deficiency significantly impairs PGN-triggered joint inflammation (figure 1D).
Figure 1.
Systemic peptidoglycan (PGN) exposure predisposes to inflammatory arthritis. Severity of ankle inflammation was assessed in wild-type (WT) and PGLYRP-2 knockout (KO) mice 72 h after systemic PGN or saline exposure. (A) Representative NIR-images of the ankle. (B) Quantification of NIR-fluorescence intensity; *p<0.05 comparison with saline controls within a genotype, **p<0.05 comparison between PGN-treated WT and PGLYRP-2 KO mice (n=10 mice/treatment/genotype). (C) and (D) are representative histological images of ankle joints from WT and PGLYRP-2 KO mice, respectively (H&E stain, original magnification at 200 ×). Arrows indicate proliferation of synovial membrane that coincides with a minor cellular infiltrate in the WT mice, which is absent in the PGLYRP-2 KO mice. H&E, haematoxylin and eosin.
Systemic PGN exposure does not predispose to uveitis, which occurs independently of PGLYRP-2
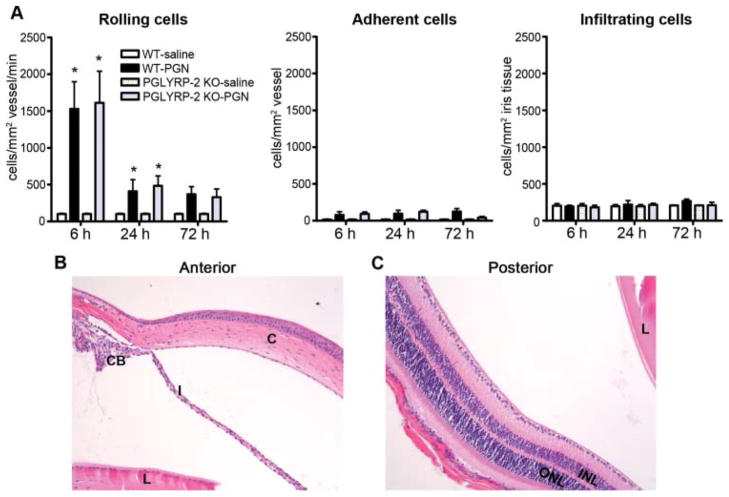
The extent to which the eye is influenced by systemically administered PGN has not been investigated in mice. Anterior uveitis is the most frequently diagnosed type of uveitis with the iris being a target tissue. From intravital videomicroscopy images, cellular responses in the iris were quantified as a function of time in mice intravenously administered PGN (figure 2). An initial increase in the number of rolling cells was observed in WT mice exposed to PGN with the first 24 h period, but this did not correspond to increases in cellular adherence or infiltration into the iris tissue (figure 2A). PGLRYP-2 deficiency did not significantly alter cell trafficking responses. Histological assessment of the eyes, performed at 72 h to relate to the coincidence of arthritis (figure 2B,C), did not support the development of uveitis as a consequence of systemically administered PGN or PGLYRP-2 deficiency. These data indicate that the mouse eye is not especially susceptible to disease after systemic exposure to PGN, and that PGLYRP-2 plays a non-essential role in regulation of ocular inflammation.
Figure 2.
Systemic peptidoglycan (PGN) exposure does not predispose to uveitis. Onset and severity of ocular inflammation were monitored over a 72 h period after systemic exposure to PGN or saline as described above in figure 1. (A) The leukocyte trafficking response within the iris was measured by intravital videomicroscopy, and the number of rolling (left panel), adherent (middle panel) and infiltrating (right panel) cells was quantified, *p<0.05 comparison to saline controls within a genotype (n=10 mice/treatment/genotype/time). (B) and (C) are histological images of the anterior and posterior eye segments from a PGLYRP-2 knockout mouse obtained at 72 h post PGN injection (H&E stain, original magnification at 200 ×). C: cornea; I: iris; CB: ciliary body; L: lens; INL: inner nuclear layer; ONL: outer nuclear layer; H&E, haematoxylin and eosin.
Local PGN administration elicits a robust uveitis, which occurs independently of PGLYRP-2
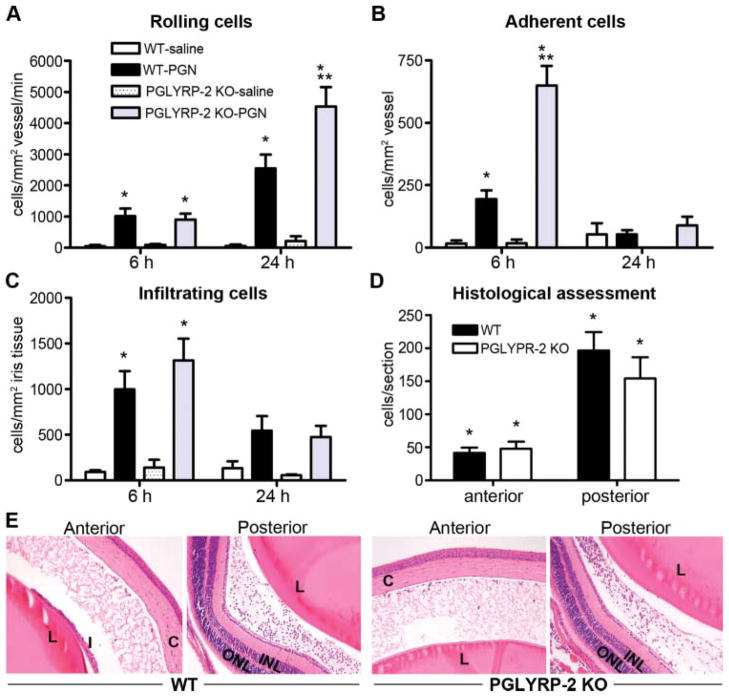
In the case of lipopolysaccharide (LPS), the mouse eye is much more responsive to locally administered LPS compared with systemic LPS exposure,16 and the ensuing uveitis is well documented. We found that in contrast with systemic PGN exposure, a robust uveitis ensues in response to locally administered PGN (figure 3). We therefore explored the potential of PGLYRP-2 to influence uveitis triggered by locally administered PGN. Mice were administered an intraocular injection of PGN, and the cellular trafficking response within the iris was quantified by intra-vital videomicroscopy (figure 3A–C). PGN elicited a marked increase in the number of rolling, adherent and infiltrating leukocytes. In contrast with our hypothesis, however, PGLYRP-2 deficient mice did not demonstrate alterations in cell infiltration (figure 3C–E); albeit a significant genotypic effect was observed for cellular rolling and adherence (figure 1A,B). Histological assessment further revealed the extent to which cells accumulate within the aqueous or vitreous was comparable between the two genotypes (figure 3D). Locally administered PGN-triggered uveitis involved increased fibrin deposition that was accompanied by leukocyte infiltration into the aqueous humour and vitreous (figure 3E), in both WT and PGLYRP-2 KO mice. These data indicate that the eye is extremely sensitive to locally administered PGN, yet the contribution of PGLYRP-2 is restricted to intravascular effects and it plays a non-essential role in uveitis as defined by cellular infiltration.
Figure 3.
Local peptidoglycan (PGN) exposure leads to uveitis independently of PGLYRP-2. Onset and severity of ocular inflammation were monitored over a 24 h period after administration of an intravitreal injection of PGN or saline. The leukocyte trafficking response within the iris was measured by intravital videomicroscopy and the number of rolling (A), adherent (B) and infiltrating (C) cells was quantified. At 24 h following PGN injection uveitis was assessed histologically, wherein the number of infiltrating cells within the anterior or posterior chambers was enumerated (D). (E) Depicts representative images of the anterior and posterior eye segments of PGN-injected wild type (WT) and PGLYRP-2 knockout (KO) mice (H&E stains, original magnification at 200 ×). *p<0.05 comparison to saline controls within a genotype, **p<0.05 comparison between PGN-injected WT and PGLYRP-2 KO mice (n=14–18 mice/treatment/genotype/time). C: cornea; I: iris; L: lens; INL: inner nuclear layer; ONL: outer nuclear layer; H&E, haematoxylin and eosin.
The potential of PGLYRP-3 or PGLYRP-4 to influence ocular inflammation triggered by systemically administered PGN
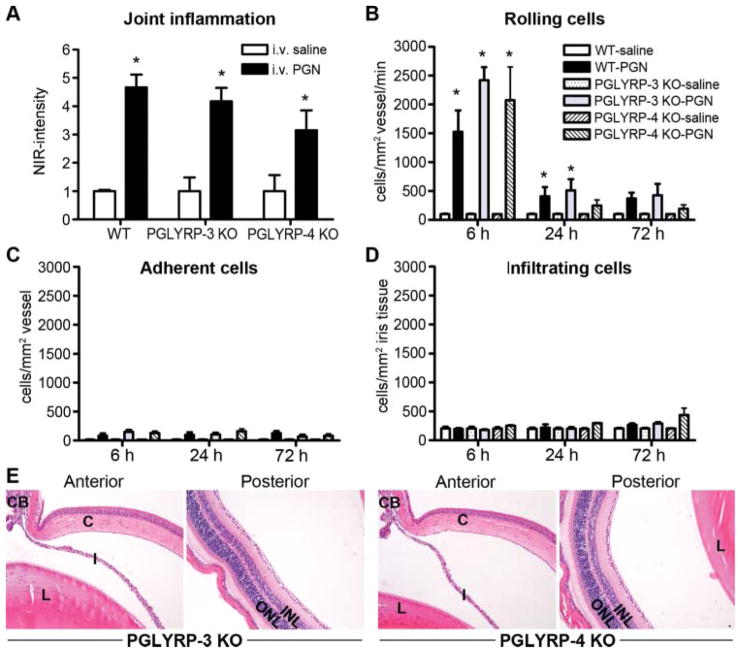
Differential and, in some cases opposing effects of PGLYRPs, have been reported in murine experimental models of dermatitis17 and colitis,18 versus arthritis.10 Thus, we considered the potential of additional PGLYRPs, such as PGLYRP-3 or PGLYRP-4 to influence uveitis. As previously described (figure 1), mice were systemically administered PGN, and inflammation within the ankles and eyes was assessed. Consistent with a prior report,10 PGLYRP-3 or PGLYRP-4 deficiency did not alter the severity of inflammation within the ankle (figure 4A), which was corroborated histologically (data not shown). Moreover, the cellular trafficking responses, as assessed by intravital videomicroscopy, were not altered as a consequence of PGLYRP-3 or PGLYRP-4 deficiency (figure 4B–D). Histological assessment of the eyes revealed negligible cellular influx into the aqueous or vitreous (all genotypes exhibited <3 cells/eye section; figure 4E). These data indicate a non-essential regulatory role for PGLRYP-3 or PGLYRP-4 in ocular inflammation triggered by systemic PGN.
Figure 4.
Assessment for a contribution of PGLYRP-3 or PGLYRP-4 in arthritis and uveitis triggered by systemic peptidoglycan (PGN) exposure. Mice were systemically administered PGN or saline. Severity of ankle inflammation was assessed by near-infrared-imaging at 72 h postinjection (A). The leukocyte trafficking response within the iris was documented by intravital microscopy, and the number of rolling (B), adherent (C) and infiltrating (D) cells was quantified. At 72 h following PGN exposure, uveitis was assessed histologically and panel E depicts representative histological images of anterior and posterior eye segments of PGLYRP-3 and PGLYRP-4 knockout mice (H&E stain, original magnification at 200 ×). *p<0.05 comparison to saline controls within a genotype (n=10 mice/treatment/genotype). H&E, haematoxylin and eosin.
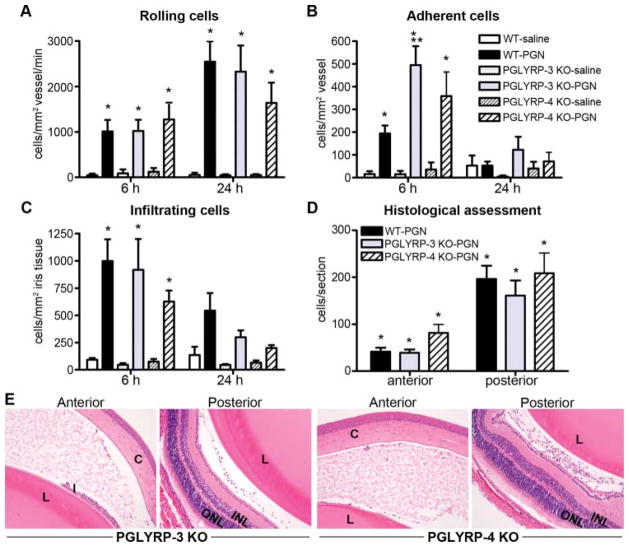
The contribution of PGLYRP-3 or PGLYRP-4 in uveitis triggered by local PGN exposure
We further examined the potential of PGLYRP-3 or PGLYRP-4 to modulate uveitis in response to local PGN exposure. Overall, cellular trafficking responses in PGLYRP-3 KO or PGLYRP-4 KO mice in response to intraocular injection of PGN did not significantly differ from those in WT mice (figure 5A–C) as well as the extent to which leukocytes infiltrated the aqueous or vitreous (figure 5D, E). However, a significant genotypic effect was observed for PGLYRP-3 KO mice, but only at 6 h post-PGN injection, and only for adherent cells. Collectively, these data indicate non-essential or possibly redundant roles for PGLYRPs in ocular responsiveness to PGN.
Figure 5.
Assessment of PGLYRP-3 and PGLYRP-4 in uveitis triggered by intraocular peptidoglycan (PGN). Onset and severity of ocular inflammation was monitored over a 24 h period after administration of an intravitreal injection of PGN or saline. The leukocyte trafficking response within the iris was documented by intravital videomicroscopy, and the number of rolling (A), adherent (B) and infiltrating (C) cells was quantified. At 24 h following PGN injection, uveitis was assessed histologically, wherein the number of infiltrating cells within the anterior or posterior chambers was enumerated (D). Panel E depicts representative images of the anterior and posterior eye segments of PGLYRP-3 KO or PGLYRP-4 KO mice (H&E stain, original magnification at 200 ×). *p<0.05 comparison with saline controls within a genotype, **p<0.05 comparison between PGN-injected wild type and PGLYRP-3 KO mice (n=14–18 mice/treatment/genotype/time). C, cornea; I, iris; CB, ciliary body; L, lens; INL, inner nuclear layer; ONL, outer nuclear layer; H&E, haematoxylin and eosin.
DISCUSSION
PGLYRPs participate in host defence and have been demonstrated to play important roles in various inflammatory diseases. Here, we inquired whether PGLYRPs 2, 3, or 4 participate in ocular inflammatory responses to PGN in a murine model of uveitis. We demonstrate that systemic exposure to PGN results in mild arthritis that does not coincide with uveitis; albeit a significant induction of rolling cells within the iris microvasculature was observed. PGLYRP-2 deficiency impaired arthritis development; however, deficiency in PGLYRP-2, -3, or -4 did not alter the onset or susceptibility to uveitis. In contrast with systemically administered PGN, locally administered PGN elicited a florid uveitis distinguished by increased numbers of cells that roll, adhere or infiltrate. The increased cellular trafficking responses coincided histopathologically with leukocyte accumulation within the aqueous and vitreous. However, we could find no obvious requirement for PGLYRP-2, -3, or -4 in uveitis triggered by locally administered PGN.
Very little work has investigated the inflammatory potential of PGLYRPs in the eye. Prior work has demonstrated the importance for PGLYRP-1 and -2 in corneal defence mechanisms. PGLYRP-1 is constitutively expressed in the corneal epithelium,19,20 wherein its antimicrobial effects are protective in infectious keratitis involving Pseudomonas aeruginosa.20 Although expressed at lower levels, PGLYRP-2 is inducible, and its production is likely important in antibacterial responses.21 Our own observations support the constitutively high expression of Pglyrp-1 in the cornea, which was in contrast with the relatively low expression of all Pglyrps in the iris (data not shown), which may be one explanation for the lack of phenotype observed in the KO animals.
Given the association of uveitis with arthritis in several inflammatory diseases wherein bacterial products such as PGN have been implicated, we hypothesised that the PGLYRP-2 pathway may be common to the eye and joints. We found that in contrast with the joint, wherein local inflammation ensues in response to systemically administered PGN, the eye was minimally affected. Increased number of rolling leukocytes was observed, which may be indicative of a systemic, vascular responsiveness to PGN that ensues independently of PGLYRPs; however, the lack of subsequent cellular adherence or infiltration would suggest minimal impact on the ocular barrier. Our data provide new insight into the differential responsiveness of the eyes versus joints to systemic exposure of PGN, which differs from reports of ocular disease in rabbits or rats systemically challenged with PGN and/or streptococcal cell wall components.22,23 PGN, in this way, displays similar properties as LPS, wherein the mouse eye is extremely susceptible to induction of uveitis to locally, but not systemically, administered LPS.16 In contrast with systemic PGN exposure, the murine eye is extremely sensitive to locally administered PGN, and we observed a florid uveitis. However, the data do not support our hypothesis that PGLYRP-2 deficiency impairs uveitis induction. Indeed, there was no difference in outcome (ie, worsened or reduced uveitis) in any of the KO mice we examined. Collectively, our data do not support a function for PGLYRPs in uveitis.
Interestingly, despite the implications of PGLYRPs in diverse diseases, such as experimental dermatitis,17 colitis18 or arthritis,10 they appear to have distinct biological functions within each target organ. In the case of murine experimental arthritis, PGLYRP-2 contributes to joint inflammation and is required for arthritis induction, while PGLYRP-3 and -4 did not participate. In contrast with arthritis, wherein PGLYRP-2 promotes disease, in a murine model of colitis PGLYRP-2 negatively regulates inflammation and PGLYRP-2 deficiency worsens colitis.18 In experimental contact dermatitis, PGLYRP-2 deficiency had no effect on skin inflammation, whereas PGLYRP-3 and -4 deficiencies suppressed inflammation and worsened dermatitis.17 Thus, our understanding of the role of PGLYRPs in inflammatory processes within different organ systems is evolving. The tissue-dependency of PGLYRPs would be akin to our prior reports for NOD2 demonstrating that PGN triggers inflammation in the joint through a pathway involving both TLR2 and NOD2.14 However, this is different in the eye, wherein deficiency in TLR signalling in MyD88 KO mice abrogated PGN-induced uveitis, while NOD2 played a counter-regulatory role.24 This highlights the complexity of NOD2 as well as PGLYRPs, and how different molecular pathways may be used in the eyes versus joints in response to a single stimulant such as PGN. An additional consideration relevant to intraocular PGN-induced uveitis is the extent to which certain PGLYRPs such as PGLYRP-2, which are produced systemically from the liver, are able to respond and penetrate the blood-ocular barrier within the rapid time frame in which locally injected PGN causes uveitis. Nor is it known to what extent the local ocular microenvironment is amenable to activation of PGLYRPs or their antimicrobial function.
It is still possible that collectively, the PGLYRPs represent an important aspect of immune responsiveness to PGN within the eye. Our studies here in single KO mice preclude the identification of phenotypic differences in uveitis if a redundancy among the PGLYRPs exists. Unfortunately, we were unable to pursue the contribution of PGLYRP-1 in uveitis in these experiments so its importance remains to be determined. Additional caveats to our studies would be that we do not know the potential role for PGLYRPs in resolution of uveitis, which was not determined here because we did not examine later time points, nor did we assay for a potential dosage effect. The list of mediators responsible for immunostimulatory responses of PGN is continually expanding. Our observations here would support proteins aside from PGLYRPs that may play more dominant roles in modulation of ocular responses to PGN. Our prior data supports the importance of TLR signalling in PGN-induced uveitis.24 NOD1 plays a non-essential role in uveitis triggered by S aureus PGN,24 yet activation of NOD1 on its own by meso-diaminopimelic acid (iE-DAP), the minimal motif of PGN recognised by NOD1, sufficiently elicits cellular trafficking responses within the iris.25 Activation of NOD2 by MDP also elicits iris inflammation,26 yet it can also dampen uveitis, indicating a bi-modal role for NOD2 in promoting or tempering ocular inflammatory responses.
The eye’s responsiveness to PGN is an area of research that has not been actively pursued, and the interplay among the various PGN-recognition proteins remains to be determined. In conclusion, the studies here sought to elucidate the function of PGLYRPs in murine uveitis triggered by PGN. Our findings suggest that PGLYRPs 2, 3 and 4 play non-essential or redundant roles in ocular inflammatory responses elicited by PGN. These results highlight the complexity of PGLYRPs and how they may use different molecular pathways in the joints versus eyes, which may further impact our understanding of predisposing mechanisms of uveitis versus arthritis.
Acknowledgments
This work was made possible by funding from the National Institutes of Health (NEI-EY019020) and the Research to Prevent Blindness Foundation. We thank Drs Roman Dziarski and Shin Yong Park (Department of Microbiology & Immunology, Indiana University School of Medicine, IN) for gifting the PGLYRP KO mice and their helpful discussions relevant to this manuscript. The authors are grateful for technical support provided by Emily E Vance and Joe E Lewis.
Footnotes
Contributors JSC and JJA were involved in study design, collection and analysis of data, and helped in manuscript preparation. EJL and HLR supervised collection and analysis of data and manuscript preparation.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 2.Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985;107:689–93. doi: 10.1016/s0022-3476(85)80394-2. [DOI] [PubMed] [Google Scholar]
- 3.Jabs DA, Houk JL, Bias WB, et al. Familial granulomatous synovitis, uveitis, and cranial neuropathies. Am J Med. 1985;78:801–4. doi: 10.1016/0002-9343(85)90286-4. [DOI] [PubMed] [Google Scholar]
- 4.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 5.Girardin SE, Travassos LH, Herve M, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–8. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 6.Sorbara MT, Philpott DJ. Peptidoglycan: a critical activator of the mammalian immune system during infection and homeostasis. Immunol Rev. 2011;243:40–60. doi: 10.1111/j.1600-065X.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 7.Dziarski R, Gupta D. Peptidoglycan recognition in innate immunity. J Endotoxin Res. 2005;11:304–10. doi: 10.1179/096805105X67256. [DOI] [PubMed] [Google Scholar]
- 8.Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm. 2002;10:263–79. doi: 10.1076/ocii.10.4.263.15592. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum JT, Rosenzweig HL, Smith JR, et al. Uveitis secondary to bacterial products. Ophthalmic Res. 2008;40:165–8. doi: 10.1159/000119870. [DOI] [PubMed] [Google Scholar]
- 10.Saha S, Qi J, Wang S, et al. PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe. 2009;5:137–50. doi: 10.1016/j.chom.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, Xu Z, Gupta D, et al. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem. 2001;276:34686–94. doi: 10.1074/jbc.M105566200. [DOI] [PubMed] [Google Scholar]
- 12.Dziarski R, Gupta D. Review: mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 2010;16:168–74. doi: 10.1177/1753425910366059. [DOI] [PubMed] [Google Scholar]
- 13.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenzweig HL, Jann MJ, Vance EE, et al. Nucleotide-binding oligomerization domain 2 and Toll-like receptor 2 function independently in a murine model of arthritis triggered by intraarticular peptidoglycan. Arthritis Rheum. 2010;62:1051–9. doi: 10.1002/art.27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allensworth JJ, Planck SR, Rosenbaum JT, et al. Investigation of the differential potentials of TLR agonists to elicit uveitis in mice. J Leukoc Biol. 2011;90:1159–66. doi: 10.1189/jlb.0511249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum JT, Woods A, Kezic J, et al. Contrasting ocular effects of local versus systemic endotoxin. Invest Ophthalmol Vis Sci. 2011;52:6472–7. doi: 10.1167/iovs.11-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SY, Gupta D, Kim CH, et al. Differential effects of peptidoglycan recognition proteins on experimental atopic and contact dermatitis mediated by Treg and Th17 cells. PloS One. 2011;6:e24961. doi: 10.1371/journal.pone.0024961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Jing X, Park SY, et al. Peptidoglycan recognition proteins protect mice from experimental colitis by promoting normal gut flora and preventing induction of interferon-gamma. Cell Host Microbe. 2010;8:147–62. doi: 10.1016/j.chom.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Lee S, Schumacher M, et al. Differential gene expression patterns of the developing and adult mouse cornea compared to the lens and tendon. Exp Eye Res. 2008;87:214–25. doi: 10.1016/j.exer.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, Lee S, Dziarski R, et al. A novel antimicrobial peptidoglycan recognition protein in the cornea. Invest Ophthalmol Vis Sci. 2009;50:4185–91. doi: 10.1167/iovs.08-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Gupta S, Li X, et al. Peptidoglycan recognition protein 2 (N-acetylmuramoyl-L-Ala amidase) is induced in keratinocytes by bacteria through the p38 kinase pathway. Infect Immun. 2005;73:7216–25. doi: 10.1128/IAI.73.11.7216-7225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells A, Pararajasegaram G, Baldwin M, et al. Uveitis and arthritis induced by systemic injection of streptococcal cell walls. Invest Ophthalmol Vis Sci. 1986;27:921–5. [PubMed] [Google Scholar]
- 23.Waksman BH, Bullington SJ. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. III. Lesions of the eye. Arch Ophthalmol. 1960;64:751–62. doi: 10.1001/archopht.1960.01840010753018. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig HL, Galster K, Vance EE, et al. NOD2 deficiency results in increased susceptibility to peptidoglycan-induced uveitis in mice. Invest Ophthalmol Vis Sci. 2011;52:4106–12. doi: 10.1167/iovs.10-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenzweig HL, Galster KT, Planck SR, et al. NOD1 expression in the eye and functional contribution to IL-1beta-dependent ocular inflammation in mice. Invest Ophthalmol Vis Sci. 2009;50:1746–53. doi: 10.1167/iovs.08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenzweig HL, Martin TM, Jann MM, et al. NOD2, the gene responsible for familial granulomatous uveitis, in a mouse model of uveitis. Invest Ophthalmol Vis Sci. 2008;49:1518–24. doi: 10.1167/iovs.07-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]