Abstract
The direct effects of expressing hypertrophic cardiomyopathy–associated (HCM-associated) mutant troponin T (TnT) proteins on the force generation of single adult cardiac myocytes have not been established. Replication-defective recombinant adenovirus vectors were generated for gene transfer of HCM-associated I79N and R92Q mutant cardiac TnT cDNAs into fully differentiated adult cardiac myocytes in primary culture. We tested the hypothesis that the mutant TnT proteins would be expressed and incorporated into the cardiac sarcomere and would behave as dominant-negative proteins to directly alter calcium-activated force generation at the level of the single cardiac myocyte. Interestingly, under identical experimental conditions, the ectopic expression of the mutant TnTs was significantly less (∼8% of total) than that obtained with expression of wild-type TnT (∼35%) in the myocytes. Confocal imaging of immunolabeled TnT showed a regular periodic pattern of localization of ectopic mutant TnT that was not different than that in normal controls, suggesting that mutant TnT incorporation had no deleterious effects on sarcomeric architecture. Direct measurements of isometric force production in single cardiac myocytes demonstrated marked desensitization of submaximal calcium-activated tension, with unchanged maximum tension generation in mutant TnT–expressing myocytes compared with control myocytes. Collectively, these results demonstrate an impaired expression of the mutant protein and a disabling of cardiac contraction in the submaximal range of myoplasmic calcium concentrations. Our functional results suggest that development of new pharmacological, chemical, or genetic approaches to sensitize the thin-filament regulatory protein system could ameliorate force deficits associated with expression of I79N and R92Q in adult cardiac myocytes.
Introduction
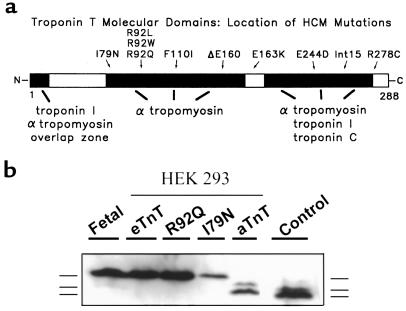
Hypertrophic cardiomyopathy (HCM) is a gene-based disease in humans inherited in an autosomal dominant manner and with an estimated prevalence of 0.2% in the general population (1). The disease penetrance is recognized to be highly variable, with the characteristic clinical features of HCM including hypertrophy of the ventricles and interventricular septum in the absence of systemic disease, syncope, myocardial arrhythmias, and, in its most dramatic form, increased risk for premature sudden death (2–4). An interesting feature of HCM is its genetic heterogeneity. To date, at least 7 different genetic loci have been identified as the cause of the disease (1). Thus far, the known disease genes share one common trait: they all encode key contractile or regulatory myofilament proteins. HCM is, therefore, characterized as a disease of the cardiac sarcomere. One of the HCM disease genes encodes the myofilament protein cardiac troponin T (cTnT) (5). Troponin T (TnT) is an essential component of the troponin regulatory complex and therefore plays a central role in calcium regulation of contraction in striated muscle (6, 7). Eleven mutations of cTnT in humans have been linked to HCM (1). Of these mutations, 9 are point mutations, 1 is a codon deletion that does not result in a frameshift, and 1 is a splice site mutation in intron 16 that is predicted to lead to production of a truncated protein (Figure 1a).
Figure 1.
(a) Schematic of the functional domains of the TnT protein and the location of the I79N and R92Q mutations in the protein. (b) Western blot showing expression of the eTnT, R92Q, I79N, and aTnT protein products in HEK 293 cells infected with the recombinant adenoviral constructs AdCMVeTnT, AdCMVR92Q, AdCMVI79N, and AdCMVaTnT. The migration patterns (from top to bottom) of the embryonic isoform, a minor adult isoform, and the major adult isoform are shown next to the blot. Control is from adult rat heart.
There are 2 predominant hypotheses for the mechanism by which the various mutations lead to HCM. The first is that the mutant protein acts as a poison peptide, meaning that the protein is expressed and incorporated into the sarcomere, thereby exerting a dominant-negative effect on the structure and/or function of the myocyte. The second hypothesis is that the mutant protein acts as a null allele and potentially leads to haplotype insufficiency. In this case, production of insufficient quantities of the normal protein would lead to altered stoichiometry of thick- or thin-filament components, thereby altering the structure and/or function of the sarcomere (1).
Several recent studies have used various techniques including in vitro motility assays, protein exchange into rabbit trabeculae, protein expression in quail myotubes, and transgenic animal models (8–13) to study the effects of mutations in TnT on contractile structure and function. However, these recent studies gave conflicting results on the effect of the mutant TnT proteins on shortening velocity (8, 10, 12) and on calcium sensitivity of contraction (10, 13). One key experiment not yet performed is to directly test the effects of mutant TnT expression in the context of an adult single cardiac myocyte.
We have developed an experimental system that uses genetically manipulated adult single cardiac myocytes in short-term primary cultures. The system’s key features include (a) stable adult cardiac myocyte cultures with unaltered contractile apparatus structure and function during the 1-week culture period; (b) highly efficient adenovirus-mediated gene delivery; and (c) direct measurement of single cardiac myocyte contractile function (14). This experimental approach is ideally suited to test directly the effects of TnT mutant proteins on myofilament structure and function in the context of the adult cardiac myocyte, making it possible to elucidate the primary effect of the mutant TnT on contractile structure and function.
In the present study, 2 adenoviral vectors containing HCM-associated I79N and R92Q mutant TnTs were generated for gene transfer into adult cardiac myocytes. We focused on these 2 missense mutations because of their malignant clinical outcomes (15), their putative shared structural domain for tropomyosin interaction (7), and the finding that codon 92 is an apparent mutational hot spot (16). The hypothesis tested was that the I79N and R92Q mutants would be expressed and incorporated into the sarcomere and would directly alter calcium-activated force generation of the adult single cardiac myocyte. Interestingly, the level of ectopic expression for the mutant TnTs was significantly less than that obtained with the wild-type cTnT. In single cardiac myocyte force measurements, the calcium required for one-half maximal tension development was significantly increased, indicating marked calcium desensitization of the contractile apparatus. Maximum tension was unaltered in these myocytes. These findings suggest that the I79N and R92Q mutations cause a reduced affinity/stability of mutant TnT for assembly into sarcomeres and an altered force generation by the myocytes over the submaximal concentration range of activating myoplasmic calcium, thereby supporting a dominant-negative mechanism of action.
Methods
Primary cultures of adult ventricular myocytes.
This method has been previously described in detail (14, 17). Briefly, the heart was removed from an adult female Sprague-Dawley rat and mounted on a modified Langendorff perfusion apparatus. The heart was perfused with Krebs-Henseleit buffer (KHB) (118 mmol/L NaCl, 4.8 mmol/L KCl, 25 mmol/L HEPES, 1.25 mmol/L K2HPO4, 1.25 mmol/L MgSO4, and 11 mmol/L glucose) containing 1 mM Ca2+ for 5 minutes, and then the perfusion was switched to Ca2+-free KHB (no added Ca2+). After 5 minutes, collagenase (0.5 mg/mL) and hyaluronidase (0.2 mg/mL) were added to the Ca2+-free KHB, and the heart was perfused for 15 minutes. After 15 minutes, the Ca2+ concentration in the perfusing solution was raised to 1 mM, and perfusion was continued for another 15 minutes. The heart was removed to a sterile beaker containing enzyme solution (1 mM Ca2+-KHB + 0.5 mg/mL collagenase + 0.2 mg/mL hyaluronidase). After removing the atria, the ventricles were minced gently and incubated in a 37°C water bath for three 10-minute incubations with gentle swirling. Digests were collected and centrifuged for 15 seconds, and the cells were resuspended in 1 mM Ca2+-KHB + 20 mg/mL BSA. After combining the cells from the digests, the solution [Ca2+] was increased to 1.75 mM. The cells were centrifuged briefly, the supernatant was removed, and the cells were resuspended in DMEM + 5% FBS + penicillin/streptomycin(P/S), counted, and plated on laminin-coated glass coverslips (18 mm2) at 2 × 104 myocytes per coverslip. After 2 hours, the medium was removed, and cells were either infected with adenovirus as described below or the medium was replaced with serum-free DMEM + P/S.
Site-directed mutagenesis.
The I79N and R92Q mutant proteins were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, California, USA). The primers used to generate the I79N mutation were (sense) 5′-GCCACCCAAGAACCCTGACGGAGAG-3′ and (antisense) 5′-CTCTCCGTCAGGGTTCTTGGGTGGC-3′. The primers used to generate the R92Q mutation were (sense) 5′-GATGACATCCACCAGAAGCGCATGG-3′ and (antisense) 5′-CCATGCGCTTCTGGTGGATGTCATC-3′. The nucleotide changes that create the mutations are underlined. The protocol for site-directed mutagenesis was followed according to the QuikChange kit instructions. The template DNA used was the rat embryonic cDNA obtained from Jim Lin at the University of Iowa (Iowa City, Iowa, USA)(18). DNA sequencing was performed to verify the introduction of the mutation.
Production of adenovirus.
This method has been described in detail elsewhere (17). Briefly, pJM17, a 0–100 map-unit derivative of adenovirus serotype 5 containing a partial deletion in the E3 region and a 4.3-kb pBRX insert at 3.7 map units (19, 20), and a shuttle plasmid containing the cDNA of interest (with a CMV promoter and SV40 polyadenylation signal), were cotransfected into HEK 293 cells using calcium phosphate precipitation. Homologous recombination occurred, and plaques began appearing within 7–10 days of cotransfection. Cells were collected after full cytopathic effect became evident, 3–5 days after plaque appearance. Virus was released by 3 cycles of freezing and thawing, and virus was collected by centrifuging at 2,500 rpm for 10 minutes and collecting the supernatant that contained the viral stock.
Plaque assays were performed to obtain clonal recombinant adenovirus stocks, as described previously (17). Briefly, serial dilutions of the viral stock were prepared and used to infect HEK 293 cells. After infection, the dishes were overlaid with MEM/agar and incubated for 7–10 days. Individual plaques were collected by transferring agar plugs containing well-isolated plaques to separate cryotubes containing DMEM + 10% FBS + P/S. Plaques were expanded by infecting HEK 293 cells and collecting the cell lysate after full cytopathic effect was evident, as described above. Plaques were screened by Southern blot analysis to identify recombinant adenovirus containing the cDNA of interest.
Plaque-purified recombinant adenovirus stocks were amplified and then purified by CsCl. Thirty 150-mm dishes of HEK 293 cells were infected with the clonal stock of recombinant adenovirus. After cytopathic effect was evident, cells were collected, centrifuged to pellet the cells, resuspended in 10 mM Tris-Cl (pH 8.0), lysed by freezing and thawing, and centrifuged to pellet the cellular debris. The supernatant was treated with RNase A and DNase I (50 mg/mL solutions), centrifuged, and the supernatant was transferred to a clean tube. The CsCl gradient was poured in Ultra-Clear centrifuge tubes and centrifuged using an SW28 rotor for 4 hours at 20,000 rpm at 5°C. The lowest band, which contains infectious recombinant adenovirus, was recovered and transferred to a Quick-Seal tube. The tube was filled with 1.34 g/mL CsCl and centrifuged using an NVTi65 rotor overnight at 63,000 rpm at 5°C. The band was recovered and dialyzed against PBS for 1–2 hours and then against PBS + 10% glycerol overnight. The purified recombinant virus stock was then diluted, and aliquots were placed in storage at –80°C.
Adenovirus-mediated gene transfer.
For these studies, 4 recombinant adenovirus vectors were made that contained eukaryotic expression cassettes with the following cDNAs: (a) the embryonic isoform of rat troponin T (eTnT); (b) the adult isoform of rat troponin T (aTnT); (c) the I79N mutation in eTnT; and (d) the R92Q mutation in eTnT. The replication-defective adenoviral constructs were named AdCMVeTnT, AdCMVaTnT, AdCMVI79N, and AdCMVR92Q, respectively. For cardiac myocyte infection, adenovirus was diluted in DMEM + P/S to the desired dose. Two hundred microliters of the virus dilution was placed in aliquot parts onto each coverslip (the moi ranged from 20 plaque-forming units [PFU] per myocyte to 50,000 PFU per myocyte), and the cells were incubated for 1 hour before addition of 2 mL of DMEM + P/S. Most data were collected from myocytes infected with an moi of ∼200–2,000 PFU. Twenty-four hours later, the medium was replaced with fresh DMEM + P/S, and the myocytes were incubated for 1–7 days before analysis.
SDS-PAGE and immunoblotting.
Glass micropipettes were used to scrape cultured ventricular myocytes from coverslips into 10 μL of sample buffer in a microfuge tube for subsequent gel analysis. SDS-PAGE was run at a constant current of 20 mA for 4–5 hours and then transblotted onto a 0.45-μm polyvinylidene difluoride membrane (Millipore Corp., Bedford, Massachusetts, USA) for 2,000 V/h and fixed in PBS containing 0.25% glutaraldehyde. Western blots were carried out by initially blocking nonspecific binding sites with Tris-buffered saline containing 5% nonfat dry milk for 2 hours. Blots were then incubated with an anti-cTnT mAb (MAB 1695, 1:500; Chemicon International, Temecula, California, USA) or an anti–striated muscle TnT mAb (T-6277, clone JLT-12, 1:200; Sigma Chemical Co., St. Louis, Missouri, USA) for 2 hours, and then washed in Tris-buffered saline with 0.5% milk. Alternatively, blots were incubated with an anti-tropomyosin mAb (T-2780, clone TM311, 1:1 × 106; Sigma Chemical Co.) or an anti–troponin I antibody (MAB 1691, 1:500; Chemicon International). Primary antibody was detected by peroxidase-conjugated goat anti-mouse IgG (1:1,000) and an enhanced chemiluminescence detection assay (Amersham Pharmacia Biotech, Piscataway, New Jersey,USA).
Indirect immunofluorescence.
Myocytes on coverslips were fixed in 3% paraformaldehyde, washed with PBS, and treated with 50 mM ammonium chloride to remove the aldehyde. Cells were blocked with 20% normal goat serum in 0.5% Triton X-100 (TX-100), and primary antibody (anti-TnT mAb, clone JLT-12; Sigma Chemical Co.) was added for 1.5 hours. Cells were washed with PBS + 0.5% TX-100 and blocked with 2% normal goat serum in 0.5% TX-100, and secondary antibody conjugated to Texas red (TR) (T-862; Molecular Probes, Eugene, Oregon, USA) was added for 1 hour. Cells were washed as above and incubated with 1:20 dilution of goat anti-mouse IgG antibody (M-8645; Sigma Chemical Co.) overnight at 4°C. Coverslips were then washed as above and incubated with a 1:20 dilution of goat anti-mouse Fab fragments (code no. 115-007-003; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) for 1.5 hours. A second primary antibody against α-actinin (A-7811, clone EA-53; Sigma Chemical Co.) was applied after blocking with 20% normal goat serum in 0.5% TX-100 as above. Coverslips were washed, blocked, and incubated with a secondary antibody conjugated to FITC (F-2012; Sigma Chemical Co.) as described above. Coverslips were washed in PBS 4 times for 5 minutes each, mounted onto slides in glycerol/PBS/p-phenyldiamine solution, and viewed on a Nikon Diaphot 200 microscope fitted with a Noran confocal laser imaging system and Silicon Graphics Indy workstation (Noran Instruments, Middleton, Wisconsin, USA). Coverslips were viewed, and rod-shaped myocytes were scored as TnT+ if they showed a striated pattern of staining under rhodamine/TR fluorescence, and as α-actinin+ if they showed a striated pattern of staining under FITC fluorescence. Four data groups were generated: TnT+/α-actinin+, TnT+/α-actinin–, TnT–/α-actinin+, and TnT–/α-actinin–. The average number of cells counted per coverslip was 40 (minimum, 17; maximum, 94).
Experimental chamber and apparatus for mechanical studies.
The chamber used for myocyte force measurements was 6 in. × 6 in. × 0.75 in. and had 4 troughs (volume per trough, ∼200 μL) that provided wells for attaching, relaxing, and activating the myocyte. A force transducer (Model 403A; sensitivity, 5 mg; 1–99% response time, 1 millisecond; Cambridge Technology, Watertown, Massachusetts, USA) and moving coil galvanometer (6350 optical scanner; Cambridge Technology) were attached to the chamber using three-way positioners to allow their exact positioning. The temperature of the experimental chamber was controlled (15°C) using thermoelectric modules (Melcor Inc., Trenton, New Jersey, USA) that were coupled to a recirculating water bath heat sink. The chamber was positioned on an antivibration table.
Attachment procedure for cardiac myocytes in primary culture.
After 1–6 days in primary culture, the coverslips were removed from the dishes and the DMEM was replaced using several washes of relaxing solution (see below). Single, rod-shaped cardiac myocytes were then attached to the glass micropipettes (21). For all cardiac myocytes used in the mechanical studies, sarcomere length was set at 2.20 μm by viewing the myocyte using light microscopy and then adjusting the overall length of the preparation by way of three-way translators.
Activating and relaxing solutions.
Relaxing and activating solutions contained 7 mmol/L EGTA, 1 mmol/L free Mg2+, 4 mmol/L MgATP, 14.5 mmol/L creatine phosphate, 20 mmol/L imidazole, and sufficient KCl to yield a total ionic strength of 180 mmol/L. Solution pH was adjusted to 7.00 with KOH. The pCa (i.e., –log [Ca2+]) of the relaxing solution was set at 9.0, whereas the pCa of the solution for maximal activation was 4.0.
Measurement of steady-state isometric tension-Ca2+ relationship.
At each pCa, steady-state isometric tension was allowed to develop, after which the fiber was rapidly (<0.5 milliseconds) slackened to obtain the tension baseline. The myocyte was then relaxed. The difference between developed tension and the tension baseline following the slack step was measured as total tension. To obtain active tension, resting tension measured at pCa 9.0 was subtracted from total tension. The myocyte was transferred to relaxing solution after each activation at a given pCa. Tension-pCa relationships were constructed by expressing tensions (P) at various submaximal Ca2+ concentrations as fractions of the tension at maximal activation (Po; isometric tension at pCa 4.0) that bracketed the submaximal activations.
Data acquisition, curve fitting, and statistics.
Data were acquired using a Nicolet storage oscilloscope (model 310). To derive values for the Hill coefficient (nH) and midpoint (termed pCa50, or K) from the tension-pCa relationship, data were fit using the Marquardt-Levenberg nonlinear least squares fitting algorithm using the Hill equation in the form P = [Ca2+]nH/(KnH + [Ca2+]nH), where P is tension as a fraction of maximum tension (Po), K is the [Ca2+] that yields one-half maximum tension, and nH is the Hill coefficient. ANOVA was used to examine whether there were significant differences in pCa50, nH, or Po. A probability level of P < 0.05 indicated significance.
Results
Recombinant adenovirus vectors.
The HCM-associated TnT mutations were introduced in eTnT because a critical feature of these experiments was to be able to distinguish the ectopic TnT expression from the endogenous TnT expression. Adult rat ventricular myocytes exclusively expressed the aTnT isoform, whereas the eTnT is expressed in early cardiac development and is identical to aTnT except for inclusion of an alternatively spliced exon encoding 10 amino acids not found in aTnT (18). The mutations were placed in eTnT to detect and quantitate the amount of ectopic TnT expressed, which is possible because eTnT migrates more slowly than aTnT in one-dimensional SDS-PAGE. Importantly, we found eTnT overexpression to have no detectable effects on calcium-activated tension in our system (see Figure 9).
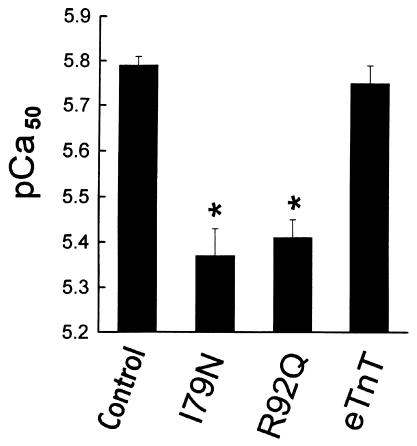
Figure 9.
Summary of the effects of wild-type and mutant TnT expression on the pCa50 of adult single cardiac myocytes. n = 14 (control); n = 6 (I79N); n = 7 (R92Q); and n = 13 (eTnT). *Significant difference of mutant-expressing myocytes compared with controls or wild-type (eTnT). Values are mean ± SE.
Gene transfer and expression of the normal and mutant TnTs.
The first studies were designed to demonstrate production of the encoded protein isoforms in HEK 293 cells 24 hours after infection with each adenoviral construct. Western blot analysis showed that each of the proteins was of the appropriate size, as compared with the positive control lanes loaded with fetal (eTnT) and adult (aTnT) cardiac myocyte samples (Figure 1b).
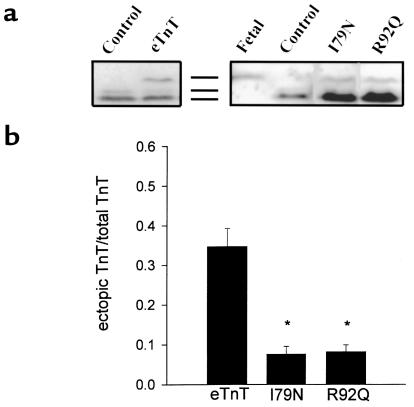
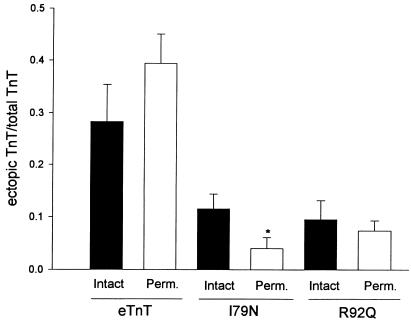
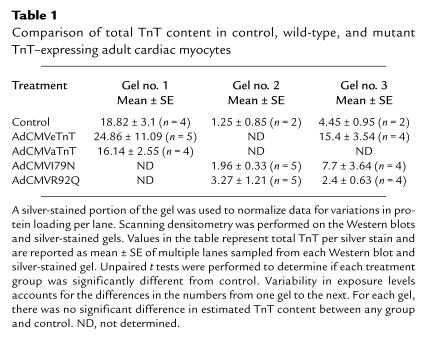
Western blot analysis was also used to follow ectopic eTnT, I79N, and R92Q expression in adult cardiac myocytes after gene transfer (Figure 2a). All of the introduced proteins are expressed in adult cardiac myocytes; however, it is important to note that the wild-type eTnT protein expression is much higher than levels obtained with either of the mutant eTnT proteins (Figure 2b). This difference in expression levels between wild-type and mutant eTnT proteins suggests that the I79N and R92Q mutations markedly affect the ability of the protein to be expressed and/or incorporated into the myofilaments. The relative expression patterns of eTnT, I79N, and R92Q were also examined in membrane-intact and membrane-permeabilized preparations to indirectly address whether these proteins were incorporated into myofilaments. Results showed similar values between intact and permeabilized samples for eTnT and R92Q, indicating that the ectopically expressed wild-type and mutant TnTs were bound to the myofilaments (Figure 3). The expression of the I79N protein was also clearly evident in permeabilized samples, providing evidence of its strong binding in the myofilaments. There was a small but statistically significant difference in I79N values between membrane-permeabilized samples and membrane-intact samples (Figure 3). Scanning densitometry was performed on the silver-stained gels and the Western blots to determine if there was a change in the total TnT protein (endogenous aTnT + ectopic TnT) between control and treated myocytes. Such a change would indicate a change in the stoichiometry of the myofilament proteins, which could, by itself, affect myocyte function (22, 23). Table 1 shows a comparison of densitometry for 3 sets of Western blot analyses and silver-stained gels. As shown in the table, none of the treatment groups were different from the control group, as determined by unpaired t tests, indicating that there was not a change in the stoichiometry of TnT compared with other proteins.
Figure 2.
Expression of wild-type and mutant TnT proteins in adult cardiac myocytes. (a) Western blots showing representative expression of control, eTnT-, I79N-,and R92Q-infected adult cardiac myocytes. The antibody used was either an anti-cTnT mAb (MAB 1695; left blot) or an anti–striated muscle TnT mAb (T6277, clone JLT-12; right blot). The migration pattern markers are shown between the 2 blots (from top to bottom: the embryonic isoform, a minor adult isoform, and the major adult isoform). (b) The fraction of the total TnT (endogenous aTnT + ectopic TnT) protein that is either eTnT (n = 19), I79N (n = 23), or R92Q (n = 20). Scanning densitometry was performed to determine the ectopic TnT/total TnT ratio. Bars represent the mean ± SE. *Significantly different than eTnT.
Figure 3.
Comparison of ectopic protein expression in membrane-intact and membrane-permeabilized myocyte samples. Scanning densitometry was performed to determine the ectopic TnT/total TnT ratio. eTnT: n = 9 (intact), n = 11 (permeabilized); I79N: n = 11 (intact), n = 12 (permeabilized); R92Q: n = 7 (intact), n = 13 (permeabilized). Bars represent the mean ± SE. *Significant difference between intact and permeabilized. For each ectopic protein, membrane-intact and membrane-permeabilized data were compared using an unpaired t test. P = 0.23 for eTnT; P = 0.04 for I79N; and P = 0.57 for R92Q.
Table 1.
Comparison of total TnT content in control, wild-type, and mutant TnT–expressing adult cardiac myocytes
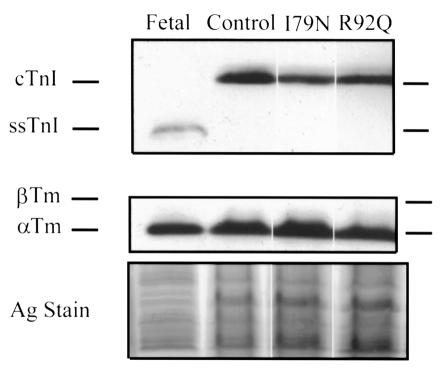
It was also important to test whether expression of the introduced proteins affected the expression of other protein isoforms, for example by inducing isoform switching from the adult to the embryonic isoform, which would indicate that the cardiac myocytes were undergoing dedifferentiation. Successive probing of Western blots with antibodies against tropomyosin and troponin I was carried out to test for changes in isoform expression of these 2 proteins. As is shown in Figure 4, the normal adult isoform expression pattern for troponin I and α-tropomyosin was not altered by mutant TnT gene transfer. This result provided support for the idea that the mutant TnT proteins were being expressed and incorporated into the myofilaments without causing other nonspecific changes in the cardiac myocytes.
Figure 4.
Tropomyosin and troponin I isoform expression pattern in adult myocytes following gene transfer. Western blots and silver-stained gel (to show loading) of control, AdCMVI79N-infected, and AdCMVR92Q-infected adult cardiac myocytes. The migration patterns of cardiac troponin I (cTnI), slow skeletal troponin I (ssTnI), β-tropomyosin (β-Tm), and α-tropomyosin (α-Tm) are shown by markers placed next to the figure.
Sarcomere incorporation and ultrastructure.
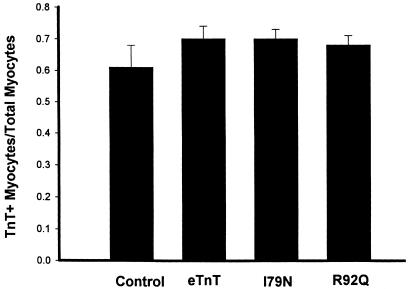
To further evaluate sarcomeric incorporation of ectopically expressed TnTs, the adult cardiac myocytes were examined using dual monoclonal immunofluorescence labeling. Rod-shaped myocytes dual labeled with an anti-TnT antibody and an anti–α-actinin antibody were scored as TnT+ if they showed a clear striation pattern under rhodamine/TR fluorescence. A diffuse labeling of TnT in treated myocytes compared with controls would be an indication that the introduced TnT protein was not being incorporated appropriately into the myofilament and was either nonspecifically binding or accumulating in the cytoplasm. Results showed, however, that the sarcomeric pattern of TnT labeling was unchanged between control and eTnT-, I79N-, and R92Q-expressing myocytes (Figure 5). There was no significant difference between the means of the groups, as determined by one-way ANOVA (Figure 5), nor was there any change in the pattern of α-actinin binding between the groups (data not shown), indicating that expression of the introduced TnT proteins in cardiac myocytes does not alter the gross sarcomeric structure compared with control myocytes.
Figure 5.
Summary of scoring of immunofluorescence data for cardiac myocytes following gene transfer. The graph shows the fraction of total rod-shaped myocytes counted that showed a striated pattern of TnT staining (TnT+). Refer to Methods for details on scoring coverslips. Numbers of coverslips examined were as follows: control = 3; eTnT = 7; I79 = 11; and R92Q = 11. Bars represent the mean ± SE.
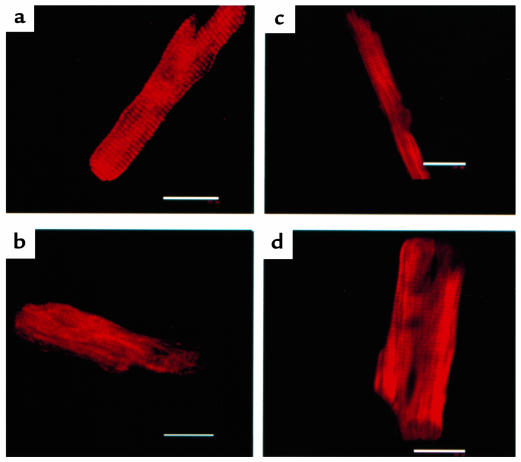
Confocal microscope image analysis also showed that the sarcomeric structure was identical between control myocytes and myocytes expressing eTnT, I79N, or R92Q (Figure 6). These results support the conclusion that the expressed TnT protein is incorporated into the myofilament and does not affect the characteristic sarcomeric periodicity across the entire length and cross-section of the myocyte.
Figure 6.
Representative confocal images of myocytes labeled by indirect immunofluorescence for TnT. (a) Control myocyte. (b) eTnT-expressing myocyte. (c) I79N-expressing myocyte. (d) R92Q-expressing myocyte. Scale bar: 20 μm.
Transmission electron microscopy was performed on control and treated myocytes for a more detailed assessment of the effects of mutant TnT expression on sarcomere ultrastructure. Sarcomere architecture is highly comparable in control myocytes and those expressing eTnT, I79N, or R92Q (Figure 7). Together with the Western blot and confocal imaging results, this observation supports the conclusion that expression and incorporation of the I79N and R92Q mutant TnT proteins into adult cardiac myocytes do not lead to an alteration in adult cardiac myocyte structure, at least in these conditions in primary culture.
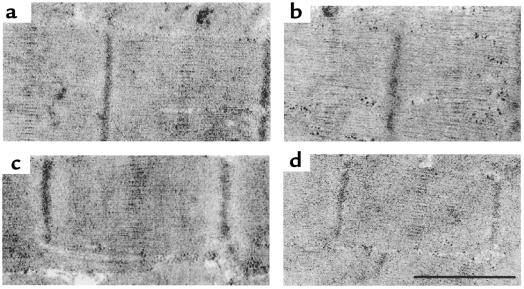
Figure 7.
Representative transmission electron micrographs comparing sarcomeric ultrastructure between control myocytes and myocytes after gene transfer. (a) Control. (b) eTnT. (c) R92Q. (d) I79N. Scale bar in d represents 1 μm for a, 0.8 μm for b, 1.1 μm for c, and 1 μm for d.
Single myocyte contractile function.
Calcium-activated tension was measured in control myocytes and aTnT-, eTnT-, I79N-, and R92Q-expressing myocytes at 5–7 days after gene transfer. Previous studies showed that the shape and position of the tension-pCa relationship were not altered in either control myocytes or myocytes infected with a β-galactosidase recombinant adenovirus vector over time in culture (14). In control experiments, there was no change in the shape and position of the tension-pCa curves, due to ectopic expression of aTnT (data not shown) or eTnT (see Figure 9). This indicates that neither overexpression of the aTnT protein nor expression of the wild-type eTnT protein at 35–45% of total TnT protein affects the calcium sensitivity of contraction in cardiac myocytes.
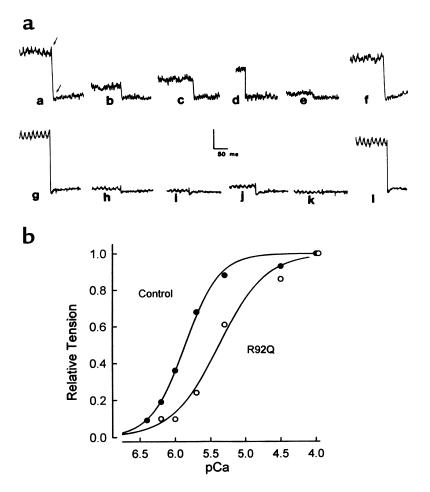
In contrast, there were marked effects on calcium-activated isometric tension in adult cardiac myocytes expressing either the I79N or R92Q mutant TnTs. Fast time-base records of isometric tension from a representative single cardiac myocyte after R92Q gene transfer are shown in Figure 8a. Two interesting outcomes are seen in these records. First, at each pCa tested in the low-to-intermediate range of calcium concentrations, isometric tension was markedly blunted in the mutant TnT–expressing myocyte compared with the control myocyte. Secondly, differences in tension generation are no longer apparent at saturating calcium concentrations. Analysis of the averaged data for all groups indicated that there was a significant rightward shift in the position of the tension-pCa relationship (Figures 8b and 9), indicating a marked desensitization of the contractile machinery to activation by calcium. These results are summarized in Figure 9, which shows that the pCa50 (pCa at which tension is one-half maximum) was unchanged for the wild-type eTnT group but was significantly depressed for the I79N and R92Q groups. The steepness of the tension-pCa relationship, which reflects molecular cooperativity within the myofilaments, and the maximum isometric tension were not significantly altered in any of the experimental groups.
Figure 8.
Direct determination of calcium-activated force generation in single adult cardiac myocytes. (a) Fast time-base records of isometric tension for a single control cardiac myocyte (records a–f) and a single cardiac myocyte after AdCMVR92Q gene transfer (records g–l). pCa values for records a–l are as follows: a and g = 4.0; b and h = 6.2; c and i = 6.0; d and j = 5.7; e and k = 9.0; f and l = 4.0. (b) Summary of the tension-pCa relationships for the control (filled circles) and R92Q (open circles) single cardiac myocytes shown in a. Tension values are normalized to the maximum tension value obtained in each myocyte at pCa 4.0.
Discussion
To our knowledge, this is the first report providing direct evidence of altered submaximal force generation in adult single cardiac myocytes expressing HCM-associated I79N and R92Q mutant TnT proteins after direct gene transfer. Importantly, maximum calcium-activated force generation was unaltered in the myocytes. Western blot analysis of intact and permeabilized samples, together with localization of immunolabeled TnT by confocal image analysis, provided evidence that the I79N and R92Q mutant TnTs incorporated into the myofilaments. However, when studied under identical experimental conditions, relative expression of the mutant TnTs did not attain the values found with wild-type TnT (Figure 2b). The basis for this difference is not known but could reflect a reduced affinity of the mutant for available TnT binding sites on the thin filament. Alternatively, it could result from a reduced stability of the mutant once bound to the regulatory complex. Regardless of the actual mechanism, our findings show that a primary effect of the I79N and R92Q TnT mutations is to disable cardiac contraction in the submaximal range of myoplasmic calcium concentrations. These changes in calcium sensitivity are large on a physiological scale and support a dominant-negative effect of HCM mutant TnT proteins on cardiac function.
It is important that the changes in calcium sensitivity observed in the present study occur in the context of an intact sarcomere, with no apparent change in the stoichiometry of the myofilament proteins and without alteration of maximum tension development. Our results differ from a recent report showing marked reductions in maximum calcium-activated force production, as well as altered calcium sensitivity, in quail skeletal myotubes expressing I79N (10). This difference is important from a mechanistic standpoint, given that reduced maximum calcium-activated force indicates a change in the ability of the cross-bridges to interact with the thin filament, which, in itself, could account for changes in calcium sensitivity at submaximal calcium concentrations (24), as discussed below.
Another valuable gene-based approach to the study of mutant sarcomeric proteins is the generation of transgenic mice. A shared trait of the transgenic mouse and direct gene-transfer approaches is that the expression of the introduced protein is controlled by the cell’s own transcription and translation machinery. It is interesting, therefore, that recently published results using transgenic mice to study an HCM-associated mutant TnT report that the relative expression of the mutant is dramatically reduced, as compared with overexpression of control TnT (11), a result highly comparable to our findings in short-term primary cultures of adult cardiac myocytes. The feasibility of the direct gene-transfer approach relies, in part, on the turnover rate of myofilament proteins allowing incorporation of the introduced protein over the 1 week in which the adult myocytes are stable in primary culture. For wild-type eTnT, we achieved ∼35% incorporation over 5–7 days in primary culture, slightly less than predicted by the 3- to 4-day half-life of cTnT in rat hearts in vivo (25). Our values, however, agree favorably with those obtained in transgenic adult mice overexpressing an otherwise normal, epitope-tagged TnT (11). Mice expressing the truncated TnT mutant had significant alterations in cardiac morphology at both the cellular and organ levels, and isolated working heart preparations were shown to have altered relaxation properties leading to diastolic dysfunction (11). A challenge of whole-animal studies of heart function is that it cannot be known for certain that the phenotype observed is a direct result of the dysfunctional TnT or, rather, a secondary result of structural abnormalities resulting from mutant transgene expression. One strength of the direct gene-transfer approach is that it sheds light on the primary defect caused by assembly of the mutant TnT into an otherwise normal sarcomere. The challenge of this approach, of course, is to translate information obtained at the isolated myocyte level to the complex functioning of the intact heart in animals. Our findings suggest that a primary contractile deficit due to mutant TnT incorporation could cause the secondary, compensatory effects in whole-heart morphology seen in HCM. Clearly, a combination of experimental approaches will ultimately be required to fully understand the pathogenesis of mutant sarcomeric proteins in HCM from the cellular level to the organ level.
Potential mechanisms of contractile desensitization to calcium.
The precise mechanism by which the I79N and R92Q mutations cause desensitization of contractile filaments to calcium is not known. Nonetheless, substantial information has been accumulated about the function of normal troponin T in the contractile assembly, and this information is valuable in order to begin understanding potential mechanisms. Because of its binding interactions with tropomyosin and troponins I and C, TnT is viewed from a structural standpoint as the molecular glue of the thin-filament regulatory complex (Figure 1a). It plays an essential role in establishing the periodic spacing of troponin complex binding at every seventh actin monomer along the entire 1,000-nm length of the thin filament, thus defining structurally the functional regulatory unit of the sarcomere (6, 7).
There is a growing appreciation that activating ligands for this thin-filament regulatory system include both calcium and myosin (24). McKillop and Geeves (26) proposed a three-state model of thin-filament activation that incorporates (a) a blocked state where stereospecific binding of myosin is prohibited; (b) a closed state where non–force-producing interactions between actin and myosin are permitted; and (c) an open state where strong-binding, force-generating myosin cross-bridge formation occurs. The activating ligands calcium and myosin exert their primary thin-filament activating functions by controlling the blocked-to-closed– and closed-to-open–state transitions, respectively. Because the mutant TnTs affected the calcium sensitivity of tension development without any alterations in maximum tension, perhaps the most straightforward mechanism is that the mutants hinder the blocked-to-closed–state transition of the thin filament. Mechanistically, this likely would be mediated through tropomyosin. There is good evidence that tropomyosin can physically occupy the stereospecific and nonstereospecific myosin binding sites on actin, lending structural support for the block-closed-open thin-filament states (27). Because the HCM-associated mutant TnTs tested in the present study are located in a domain of TnT thought to be critical for tropomyosin interaction (Figure 1a and ref. 7), it is reasonable to propose that altered TnT-tropomyosin interactions cause a change in tropomyosin structure, making it more likely to conform to a blocked, rather than closed, configuration. An alternative, and perhaps less likely, possibility is that the TnT mutations directly or indirectly influence TnC to alter calcium binding to the calcium regulatory site on this molecule (7). Either of these mechanisms could result in the observed calcium desensitization of the contractile apparatus.
From myofilament desensitization to disease.
The observed reduced calcium sensitivity in permeabilized myocytes is expected to translate to reduced force at the normal physiological range of the calcium transient in intact myocytes. In this hypocontractile state, the heart may be expected to generate a compensatory hypertrophy. In general, however, patients with cTnT mutations do not show marked hypertrophy but do have a high incidence of sudden death (5). In the absence of compensation via myocardial hypertrophy, one mechanism by which the heart could compensate for the dysfunctional TnTs would be to increase calcium signaling to the myofilaments. Based on our findings that force production at maximal calcium was not altered by the mutant TnTs, a heightened calcium release intracellularly would largely overcome any force deficit. One way to accomplish this would be by increasing β-adrenergic stimulation to the heart, which would result in a heightened intracellular calcium transient (28) and tend to lessen force differences between mutant and normal TnT. Over time, however, this compensatory scenario would likely have negative consequences in that the altered calcium homeostasis may precipitate electrical disturbances at the cellular level and increase the probability of arrhythmias and sudden death at the organ and whole-animal levels. Our results, therefore, indicate the importance of investigating new experimental or therapeutic strategies that would reverse calcium desensitization of the myofilaments without resorting to modifications in intracellular calcium signaling.
Acknowledgments
We would like to express our gratitude to Jim Lin for the gift of the rat eTnT and aTnT cDNAs, to Krystyna Pasyk for expert technical assistance, and to Dan Michele and Margaret Westfall for helpful comments on earlier versions of this manuscript. This study was funded by grants from the National Institutes of Health and the American Heart Association (to J.M. Metzger). J.M. Metzger is an Established Investigator of the American Heart Association.
Footnotes
Portions of this work have appeared in abstract form (1998. Biophys. J. 74:A348; 1999. Biophys. J. 76:A283).
References
- 1.Bonne G, Carrier L, Richard P, Hainque B, Schwartz K. Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ Res. 1998;83:580–593. doi: 10.1161/01.res.83.6.580. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. I. Interrelations of clinical manifestations, pathophysiology, and therapy. N Engl J Med. 1987;316:780–789. doi: 10.1056/NEJM198703263161305. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. II. Interrelations of clinical manifestations, pathophysiology, and therapy. N Engl J Med. 1987;316:844–852. doi: 10.1056/NEJM198704023161405. [DOI] [PubMed] [Google Scholar]
- 4.Marian AJ. Sudden cardiac death in patients with hypertrophic cardiomyopathy: from bench to bedside with an emphasis on genetic markers. Clin Cardiol. 1995;18:189–198. doi: 10.1002/clc.4960180403. [DOI] [PubMed] [Google Scholar]
- 5.Thierfelder L, et al. α-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 6.Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- 7.Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- 8.Lin D, Bobkova A, Homsher E, Tobacman LS. Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy. J Clin Invest. 1996;97:2842–2848. doi: 10.1172/JCI118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins H, Seidman CE, Seidman JG, Feng HS, Sweeney HL. Expression and functional assessment of a truncated cardiac troponin T that causes hypertrophic cardiomyopathy: evidence for a dominant negative action. J Clin Invest. 1996;98:2456–2461. doi: 10.1172/JCI119063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney HL, Feng HS, Yang Z, Watkins H. Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proc Natl Acad Sci USA. 1998;95:14406–14410. doi: 10.1073/pnas.95.24.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardiff JC, et al. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101:2800–2811. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marian AJ, Zhao G, Seta Y, Roberts R, Yu Q-t. Expression of a mutant (Arg92Gln) human cardiac troponin T, known to cause hypertrophic cardiomyopathy, impairs adult cardiac myocyte contractility. Circ Res. 1997;81:76–85. doi: 10.1161/01.res.81.1.76. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto S, Yanaga F, Minakami R, Ohtsuki I. Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am J Physiol. 1998;275:C200–C207. doi: 10.1152/ajpcell.1998.275.1.C200. [DOI] [PubMed] [Google Scholar]
- 14.Rust EM, Westfall MV, Metzger JM. Stability of the contractile assembly and Ca2+-activated tension in adenovirus infected adult cardiac myocytes. Mol Cell Biochem. 1998;181:143–155. doi: 10.1023/a:1006802719136. [DOI] [PubMed] [Google Scholar]
- 15.Watkins H, et al. Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 16.Forissier JF, et al. Codon 102 of the cardiac troponin T gene is a putative hot spot for mutations in familial hypertrophic cardiomyopathy. Circulation. 1996;94:3069–3073. doi: 10.1161/01.cir.94.12.3069. [DOI] [PubMed] [Google Scholar]
- 17.Westfall MV, Rust EM, Albayya F, Metzger JM. Adenovirus-mediated myofilament gene transfer into adult cardiac myocytes. Methods Cell Biol. 1998;52:307–322. [PubMed] [Google Scholar]
- 18.Jin J-P, Lin JJ-C. Isolation and characterization of the cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem. 1989;264:14471–14477. [PubMed] [Google Scholar]
- 19.Graham FL, Prevec L. Manipulation of adenovirus vectors. Methods Mol Biol. 1991;7:109–128. doi: 10.1385/0-89603-178-0:109. [DOI] [PubMed] [Google Scholar]
- 20.Becker TC, et al. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 21.Metzger JM. Myosin binding-induced cooperative activation of the thin filament in cardiac myocytes and skeletal muscle fibers. Biophys J. 1995;68:1430–1442. doi: 10.1016/S0006-3495(95)80316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt PW, Diamond MS, Schachat FH. The thin filament of vertebrate skeletal muscle co-operatively operates as a unit. J Mol Biol. 1984;180:379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- 23.Moss RL. Ca2+ regulation of mechanical properties of striated muscle. Circ Res. 1992;70:865–884. doi: 10.1161/01.res.70.5.865. [DOI] [PubMed] [Google Scholar]
- 24.Hannon JD, Martyn DA, Gordon AM. Effects of cycling and rigor crossbridges on the conformation of cardiac troponin C. Circ Res. 1991;71:984–991. doi: 10.1161/01.res.71.4.984. [DOI] [PubMed] [Google Scholar]
- 25.Martin AF. Turnover of cardiac troponin subunits. J Biol Chem. 1981;236:964–968. [PubMed] [Google Scholar]
- 26.McKillop DFA, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 28.Blinks JR, Endoh M. Modification of myofibrillar responsiveness to Ca++ as an inotropic mechanism. Circulation. 1986;73:85–98. [PubMed] [Google Scholar]