Abstract
The aim of the present study was to evaluate the association of dopaminergic gene variants with emotion dysregulation (EMD) and attention-deficit/hyperactivity disorder (ADHD) symptoms in children with autism spectrum disorder (ASD). Three dopamine transporter gene (SLC6A3/DAT1) polymorphisms (intron8 5/6 VNTR, 3’-UTR 9/10 VNTR, rs27072 in the 3’-UTR) and one dopamine D2 receptor gene (DRD2) variant (rs2283265) were selected for genotyping based on à priori evidence of regulatory activity or, in the case of DAT1 9/10 VNTR, commonly reported associations with ADHD. A sample of 110 children with ASD was assessed with a rigorously validated DSM-IV-referenced rating scale. Global EMD severity (parents’ ratings) was associated with DAT1 intron8 (ηp2=0.063) and rs2283265 (ηp2=0.044). Findings for DAT1 intron8 were also significant for two EMD subscales, generalized anxiety (ηp2=0.065) and depression (ηp2=0.059), and for DRD2 rs2283265, depression (ηp2=0.053). DRD2 rs2283265 was associated with teachers’ global ratings of ADHD (ηp2=0.052). DAT1 intron8 was associated with parent-rated hyperactivity (ηp2=0.045) and both DAT1 9/10 VNTR (ηp2=0.105) and DRD2 rs2283265 (ηp2=0.069) were associated with teacher-rated inattention. These findings suggest that dopaminergic gene polymorphisms may modulate EMD and ADHD symptoms in children with ASD but require replication with larger independent samples.
Keywords: autism, autism spectrum disorder, depression, emotion dysregulation, ADHD, DAT1, DRD2
1. Introduction
Children with autism spectrum disorder (ASD) experience a wide range of psychiatric symptoms consistent with emotion dysregulation (EMD), such as depression, anxiety, anger, and irritability, as well as attention-deficit hyperactivity disorder (ADHD; Gadow, DeVincent, Pomeroy, & Azizian, 2005, 2006). These symptoms are associated with high rates of social and academic impairment (Kaat, Gadow, & Lecavalier, 2013), but little is known about pathogenic variables that may contribute to their co-occurrence with ASD. EMD and ADHD in children with ASD are phenomenologically similar in many ways to comparable symptoms and disorders in non-ASD youth (Gadow, DeVincent, & Drabick, 2008; Gadow, DeVincent, & Pomeroy, 2006; Gadow, Guttmann-Steinmetz, Rieffe, & DeVincent, 2012; Guttmann-Steinmetz, Gadow, DeVincent, & Crowell, 2010), and this suggests that research about the latter may inform the former (e.g., Cohen et al., 2011; Gadow et al., 2013; Guerini et al., 2011).
Dopamine, brain regions rich in dopamine receptors, and genes involved in dopamine metabolism and signaling are all shown to be involved in emotion regulation and dysregulation (Alcaro, Huber, & Panksepp, 2007; Badgaiyan, Fischman, & Alpert, 2009; Beiderbeck et al., 2012; Garcia-Garcia, Clemente, Domínguez-Borràs, & Escera, 2010; Levita, Dalley, & Robbins, 2002; Opmeer, Kortekaas, & Aleman, 2010; Salgado-Pineda, Delaveau, Blin, & Nieoullon, 2005), including depression (Klimek, Schenck, Han, Stockmeier, & Ordway, 2002; Meyer et al., 2001; Roy, Karoum, & Pollack, 1992), as well as ADHD (Levy, 1991; Wender, 1971). For example, the 9/10 variable number tandem repeat (VNTR) in the 3’-untranslated region (UTR) of the dopamine transporter (DAT) gene (DAT1/SLC6A3) was found to be associated with depression (Felten, Montag, Markett, Walter, & Reuter, 2011), anxiety (Gadow, Roohi, DeVincent, & Hatchwell, 2008), and processing negative emotional stimuli (Garcia-Garcia et al., 2010). It also evidences modest association with ADHD (Gizer, Ficks, & Waldman, 2009) as do other DAT1 variants including a VNTR on intron8 and the single nucleotide polymorphism (SNP) rs27072, but effect sizes across studies are generally heterogeneous. Researchers have recently established regulatory functions for the DAT1 intron8 5/6 repeat VNTR and rs27072 variants (Pinsonneault et al., 2011) as well as SNP (rs2283265) in the dopamine D2 receptor gene (DRD2) (Zhang et al., 2007), which enables a focused approach to gene-behavior associations founded on strong evidence for genetic influence in dopaminergic signaling.
Although research findings suggest that (a) dysregulation of dopamine metabolism or signaling is likely implicated in the etiology of EMD and ADHD, and (b) specific functional dopaminergic gene variants may modulate symptom severity, little is known of their relation with comparable symptoms in children with ASD. The primary objective of the present study was to explore potential relations of the aforementioned dopamine gene variants with severity of EMD and ADHD symptoms in children with ASD. This seemed reasonable because both spectra are common in children with ASD and often co-occur in ASD samples (suggesting shared pathogenic mechanisms). We also considered whether DAT1 intron8 and DRD2 rs2283265 jointly led to a non-additive association with symptom severity because prior work has documented a functional epistatic interaction (Sullivan et al., 2013).
2. Material and Methods
2.1 Participants
Participants were recruited from referrals to a university hospital developmental disabilities specialty clinic located on Long Island, New York. All youth (N=110) between 4 and 14 years old with the prerequisite measures and a diagnosis of ASD were included in the present study. Demographic characteristics were as follows: age (M=7.5±2.6), gender (86% male), ethnicity (91% Caucasian), socioeconomic status assessed with Hollingshead’s (1975) index of occupational and educational social status (M=42.0±11.2), single-parent household (10%), and current psychotropic medication use (25%). Most children (73%) had IQs ≥70 based primarily on WISC or Standford-Binet test scores obtained from the school. A subsample of youth (n=67) participated in prior studies of other gene variants (e.g., Gadow et al., 2008; Roohi, DeVincent, Hatchwell, & Gadow, 2009) as did the entire (Gadow et al., 2013; Gadow, Smith, & Pinsonneault, in press). Both referred (Gadow et al., 2005) and epidemiologic (Simonoff et al., 2008) samples of children with ASD indicate high levels of co-occurring psychopathology. In the present study, the percentage of youth with T scores>65 for parent/teacher ratings (Gadow & Sprafkin, 1997, 2002, 2008) were as follows: ADHD (63%/46%), oppositional defiant disorder (22%/29%), generalized anxiety disorder (21%/25%), major depressive episode (36%/39%) and separation anxiety disorder (7%, parents’ ratings only).This study was approved by a university Institutional Review Board; informed consent was obtained; and appropriate measures were taken to protect patient (and rater) confidentiality.
2.2 Procedure
Prior to scheduling their initial clinic evaluation, the parents of potential participants were mailed a packet of materials including behavior rating scales, background information questionnaire, and permission for release of school evaluation records. Ratings of child behavior were obtained from parents (primarily the mother) and teachers for 105 and 97 children, respectively. Diagnoses of ASD were confirmed by an expert diagnostician and based on five sources of information about ASD symptoms to verify DSM-IV criteria: (a) comprehensive developmental history, (b) clinician interview with child and caregiver(s), (c) prior evaluations, (d) informal observations of the child in the clinic setting, and (d) review of validated ASD rating scales including the Child Symptom Inventory-4 (CSI-4) (Gadow & Sprafkin, 2002), which evidenced high sensitivity and specificity in identifying 5-12-year-old children with ASD in two independent studies (DeVincent & Gadow, 2009; Gadow, Schwartz, DeVincent, Strong, & Cuva, 2008). Most youth (81%) were also evaluated with the Autism Diagnostic Observation Schedule (Lord et al, 2000) and/or Autism Diagnostic Interview-Revised (Rutter, LeCouteur, & Lord, 2003). Exceptions were children who had previously received an ASD diagnosis from a qualified clinician.
2.3 Genotyping
Standard methods were employed to determine genotypes, and these have been described elsewhere for the DAT1 variants (Pinsonneault et al., 2011) and for DRD2 rs2283265 (Moyer et al., 2011).
2.4 Child Genotypes
The distribution (frequencies/percents) of genotypes were as follows: DAT1 intron8 VNTR 5-5 (7/6), 5-6 (38/35), 5-11 (1/1), 6-6 (63/57), and 6-12 (1/1); DAT1 3’-UTR VNTR were 9-6 (1/1), 9-9 (11/10), 9-10 (38/34), 10-10 (54/49), 10-11 (1/1); DAT1 rs27072 were C-C (76/69), T-C (32/29), and T-T (1/1); and DRD2 rs2283265 were G-G (73/66), T-G (29/26), and T-T (1/1). None of the variants deviated from Hardy-Weinberg equilibrium (p>.05). Genotype groups were not significantly different in terms of age, gender IQ, ethnicity, SES, maternal education, single parent household, current special education or current psychotropic medication. Owing to infrequency and uncertainties about the functionality of the rare variants and consistent with prior research, genotype groups were constructed as follows: DAT1 intron8 VNTR (5-6 versus 6-6 repeats), DAT1 3’-UTR VNTR (9-9, 9-10, 10-10 repeats), DAT1 rs27072 (C-C vs. T-C), and DRD2 rs2283265 (G-G vs. T-G).
2.5 Measures
The CSI-4 (Gadow & Sprafkin, 2002) is a behavior rating scale that assesses the behavioral symptoms of a broad range of psychiatric syndromes and has both parent and teacher versions. The number of items in the parent and teacher versions is 97 and 78 items, respectively. The teacher version excludes symptoms from the parent version (e.g., separation anxiety) that teachers are unlikely to have direct knowledge. Individual items bear one-to-one correspondence with DSM-IV symptoms (i.e., high content validity). To assess symptom severity, items are scored (never=0, sometimes=1, often=2, and very often=3) and summed separately for each symptom dimension. Confirmatory factor analysis of parents’ and teachers’ ratings of children with diagnosed ASD supports the internal construct validity of DSM-IV syndromes in this clinical population (Lecavalier, Gadow, DeVincent, & Edwards, 2009). Numerous studies indicate that both parent and teacher CSI-4 symptom subscales demonstrate satisfactory psychometric properties in community-based normative, clinic-referred non-ASD, and ASD samples (Gadow & Sprafkin, 2011). CSI-4 scores show little relation to age, IQ, or SES. For the present study, symptom severity scores for oppositional defiant disorder (ODD) (anger, irritability), generalized anxiety disorder (GAD), separation anxiety disorder (SAD), and major depressive episode (MDE) were examined separately but were also transformed into z scores and then summed to create a global Emotion Dysregulation (EMD) score. The internal consistency (Cronbach’s alpha) of the EMD subscale based on untransformed scores was high (α=.89). Prior research has demonstrated that each of the ADHD subscales (inattention, hyperactivity, impulsivity) is associated with different clinical features in children with ASD (Gadow et al., 2006).
2.6 Statistical Analyses
Chi-square tests and ANOVAs were used to examine potential differences in demographic characteristics between genotype groups, and correlation analyses were conducted to determine associations between these characteristics and dependent variables. Both sets of analyses were used to identify potential covariates. Primary analyses pertained to two dependent variables: severity of EMD (transformed scores) and ADHD (all symptoms). One-way ANOVAs (and in some cases ANCOVAs) were conducted to examine main effects of genotype groups. Severity of ADHD and ASD were considered potential covariates in EMD and ADHD analyses, respectively, if they were not related to genotype (Miller & Chapman, 2001). Secondary analyses examined severity of component phenotypes. In the case of EMD, these were ODD, GAD, SAD, and MDE. For ADHD, these were inattention, hyperactivity, and impulsivity. We report partial eta-squared (ηp2) to gauge the magnitude of group differences (i.e., proportion of variance in dependent variables accounted for by independent variables). A rule of thumb for determining the magnitude of ηp2 suggests the following: 0.01-0.06=small, 0.06-0.14=moderate, and >0.14=large (Cohen, 1988).
3. Results
3.1 Emotion Dysregulation
DAT1 intron8 polymorphism was associated with parents’ ratings of EMD, with youth homozygous for the 6-repeat allele exhibiting more severe symptoms than 5/6 heterozygotes (Table 1). The association was somewhat stronger co-varying severity of ADHD symptoms, F=7.81, p=0.006, ηp2=0.077. Secondary analyses indicated that intron8 genotype was significant for subdomains of EMD (untransformed scores): depression (F=5.81, p=.018; ηp2=0.059) and generalized anxiety (F=6.47, p=.013; ηp2=0.065). Anger/irritability was marginally significant (F=3.74, p=.056; ηp2=0.038), but when co-varying severity of ADHD, was significant (p=0.032). In all cases, the 6/6 group obtained more severe ratings than the 5/6 group (Figure 1).
Table 1.
Informants’ Global Severity Ratings of Emotion Dysregulation and Attention-Deficit Hyperactivity Disorder
| Emotion Dysregulationa | ADHD Combined | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Genotypes | Mean (SD) | F | p= | ηp2 | Mean (SD) | F | p= | ηp2 |
| PARENTS’ RATINGS | ||||||||
| DAT1 intron8 VNTR | 6.28 | .014 | .063 | 0.33 | .856 | .000 | ||
| 5-6 repeat (n=36/36) | 14.2 (8.2) | 29.7 (10.8) | ||||||
| 6-6 repeat (n=60/59) | 18.4 (10.4) | 29.3 (10.6) | ||||||
| DAT1 3’ UTR VNTR | 0.85 | .430 | .018 | 1.40 | .252 | .028 | ||
| 10-10 (n=50/50) | 17.3 (10.6) | 28.8 (10.4) | ||||||
| 9-10 (n=37/36) | 17.0 (9.1) | 30.6 (11.2) | ||||||
| 9-9 (n=11/11) | 13.4 (8.6) | 24.2 (14.0) | ||||||
| DAT1 rs27072 | 0.27 | .603 | .003 | 1.13 | .290 | .011 | ||
| C-C (n=72/72) | 15.1 (10.6) | 28.5 (11.4) | ||||||
| T-C (n=30/29) | 15.4 (9.1) | 30.9 (9.7) | ||||||
| DRD2 rs2283265 | 4.37 | .039 | .044 | 0.39 | .534 | .004 | ||
| G-G (n=68/67) | 17.6 (10.7) | 29.9 (10.3) | ||||||
| T-G (n=28/28) | 14.0 (7.70) | 28.4 (11.8) | ||||||
| TEACHERS’ RATINGS | ||||||||
| DAT1 intron8 VNTR | 0.64 | .425 | .007 | 0.38 | .537 | .004 | ||
| 5-6 repeat (n=33/33) | 14.9 (11.9) | 28.8 (14.6) | ||||||
| 6-6 repeat (n=56/56) | 16.6 (9.4) | 27.0 (12.0) | ||||||
| DAT1 3’ UTR VNTR | 1.28 | .283 | .028 | 2.93 | .058 | .061 | ||
| 10-10 (n=46/46) | 14.7 (8.9) | 27.2 (11.9) | ||||||
| 9-10 (n=35/35) | 16.9 (11.1) | 28.2 (13.3) | ||||||
| 9-9 (n=12/12) | 11.3 (10.6) | 18.0 (16.0) | ||||||
| DAT1 rs27072 | 0.07 | .798 | .001 | 0.15 | .703 | .002 | ||
| C-C (n=66/66) | 15.1 (10.6) | 26.5 (14.1) | ||||||
| T-C (n=31/30) | 15.4 (9.1) | 27.6 (11.3) | ||||||
| DRD2 rs2283265 | 3.81 | .054 | .041 | 4.81 | .031 | .052 | ||
| G-G (n=64/63) | 15.8 (9.6) | 28.2 (12.6) | ||||||
| T-G (n=26/26) | 11.4 (9.6) | 21.6 (13.6) | ||||||
Untransformed scores
Figure 1.
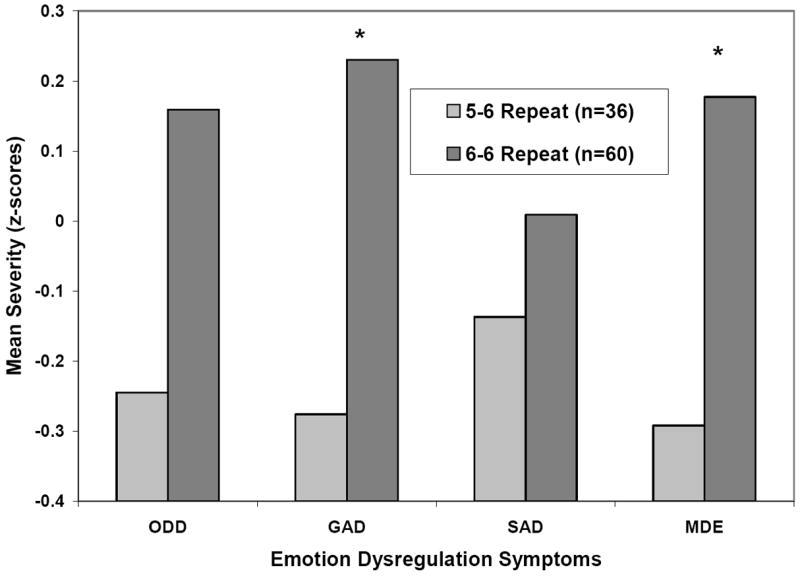
Association of DAT1 intron8 VNTR 5-6 and 6-6 repeat genotypes and severity of oppositional defiant disorder (ODD), generalized anxiety disorder (GAD), separation anxiety disorder (SAD), and major depressive episode (MDE) symptoms controlling for severity of ADHD symptoms. Group differences were significant (p<0.05) for GAD and MDE and marginally significant (p=0.056) for ODD.
Because the DAT1 intron8 variant was also associated with severity of parent-rated ASD (6/6 > 5/6), co-varying severity of ADHD (F=4.75, p=0.032; ηp2=0.049), we constructed a composite phenotype comprised of a summary of z-scores for EMD and total ASD symptoms. Children homozygous for the 6-repeat allele had more severe EMD+ASD symptoms than the 5/6 group (F=8.08; p=.005; ηp2=0.080), which was also the case when co-varying ADHD symptom severity (F=11.41, p=.001, ηp2=0.110).
DRD2 rs2283265 was associated with parents’ ratings of EMD, and youth homozygous for the main G allele had more severe symptoms than the T-G group (Table 1). Secondary analyses indicated that the G/G group (M=5.9±3.7) compared with heterozygotes (M=4.1±=2.7) had more severe depression symptoms (F=5.21, p=0.025, ηp2=0.053). Teachers’ ratings were marginally significant (p=.054) for EMD, and consistent with parents’ ratings, the G-G group received higher symptom scores than the T-G group (Table 1).
3.2 Attention-Deficit Hyperactivity Disorder
There was one significant (DRD2 rs2283265) and one marginally significant (DAT1 3’-UTR VNTR) effect of genotype for global severity of ADHD, and both pertained to teachers’ ratings (Table 1). There was a marginally significant (p=0.048) effect of DAT1 rs27072 for parents’ global ratings of ADHD co-varying ASD severity.
Secondary analyses examining the three subdomains of ADHD revealed that the DAT1 intron8 5-6 repeat genotype was associated with more severe parent-rated hyperactivity symptoms than 6-6 repeat genotype co-varying ASD severity (Table 2). There was a significant effect of DAT1 3’-UTR VNTR for teachers’ ADHD inattention symptoms (Table 2), and post hoc analyses indicated significant differences between 9-9 and 9-10 (p=0.010) and 9-9 and 10-10 (p=0.007). The DRD2 rs2283265 G-G group had more severe teacher-rated inattention symptoms than the T-G group.
Table 2.
Informants’ Severity Ratings of Attention-Deficit Hyperactivity Disorder Subdomain Symptoms
| Inattention | Hyperactivity | Impulsivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Genotypes | M (SD) | F | p= | ηp2 | M (SD) | F | p= | ηp2 | M (SD) | F | p= | ηp2 |
| PARENTS’ RATINGS | ||||||||||||
| DAT1 intron8 VNTRa | 0.31 | .577 | .003 | 4.34 | .045 | .045 | 0.18 | .674 | .002 | |||
| 5-6 repeat (n=36) | 16.0 (6.2) | 8.9 (4.3) | 4.9 (2.9) | |||||||||
| 6-6 repeat (n=59) | 16.5 (5.8) | 8.1 (4.5) | 4.8 (3.1) | |||||||||
| DAT1 3’ UTR VNTRa | 2.15 | .122 | .044 | 0.16 | .851 | .003 | 0.14 | .866 | .003 | |||
| 10-10 (n=50) | 15.7 (5.8) | 8.1 (4.2) | 4.9 (3.2) | |||||||||
| 9-10 (n=36) | 17.5 (6.3) | 8.6 (4.5) | 4.9 (2.8) | |||||||||
| 9-9 (n=11) | 12.9 (6.9) | 7.1 (5.2) | 4.2 (3.8) | |||||||||
| DAT1 rs27072a | 2.67 | .105 | .027 | 0.90 | .344 | .009 | 0.43 | .512 | .004 | |||
| C-C (n=72) | 15.5 (6.4) | 8.2 (4.6) | 4.7 (3.1) | |||||||||
| T-C (n=29) | 17.5 (5.1) | 9.0 (3.7) | 5.1 (3.1) | |||||||||
| DRD2 rs2283265a | 0.00 | .982 | .000 | 0.22 | .642 | .002 | 0.06 | .801 | .001 | |||
| G-G (n=67) | 16.5 (6.1) | 8.5 (4.2) | 5.0 (3.0) | |||||||||
| T-G (n=28) | 16.0 (6.1) | 7.9 (4.7) | 4.7 (3.4) | |||||||||
| TEACHERS’ RATINGS | ||||||||||||
| DAT1 intron8 VNTR | 0.10 | .752 | .001 | 2.26 | .137 | .026 | 0.35 | .554 | .004 | |||
| 5-6 repeat (n=33) | 16.3 (8.1) | 8.3 (5.0) | 4.1 (3.7) | |||||||||
| 6-6 repeat (n=56) | 15.8 (6.6) | 6.6 (5.2) | 4.6 (3.6) | |||||||||
| DAT1 3’ UTR VNTR | 5.30 | .007 | .105 | 0.95 | .391 | .021 | 0.37 | .690 | .008 | |||
| 10-10 (n=46) | 16.3 (7.1) | 6.6 (4.8) | 4.3 (3.8) | |||||||||
| 9-10 (n=35) | 16.3 (6.4) | 7.3 (5.4) | 4.6 (3.6) | |||||||||
| 9-9 (n=12) | 9.1 (9.1) | 4.9 (5.2) | 3.5 (3.1) | |||||||||
| DAT1 rs27072 | 0.65 | .423 | .007 | 0.77 | .782 | .001 | 0.57 | .454 | .006 | |||
| C-C (n=66) | 15.2 (7.9) | 6.9 (5.4) | 4.5 (3.8) | |||||||||
| T-C (n=30) | 16.5 (6.0) | 7.2 (4.7) | 3.9 (3.2) | |||||||||
| DRD2 rs2283265 | 6.53 | .012 | .069 | 0.61 | .437 | .007 | 3.30 | .073 | .037 | |||
| G-G (n=64) | 16.8 (7.2) | 6.9 (5.1) | 4.5 (3.5) | |||||||||
| T-G (n=26) | 12.4 (7.6) | 6.0 (4.9) | 3.0 (3.2) | |||||||||
Controlling for severity of autism spectrum disorder symptoms.
3.3 Gene-Gene Interaction
A 2 × 2 ANOVA examining a statistical interaction between DAT1 intron8 5/6 repeat VNTR and DRD2 rs2283265 found that the interaction term was not significant for EMD (p>0.10); however, consistent with our prior analyses for individual variants, the 6-6 repeat/G-G group did evidence the most severe EMD whereas the 5-6/TG group had the least severe symptoms (Figure 2).
Figure 2.
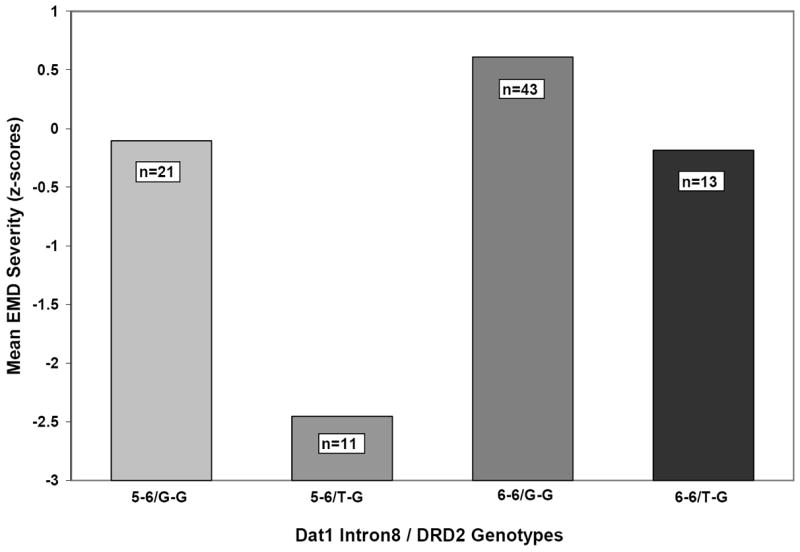
Mean severity of parent-rated emotion dysregulation symptoms (EMD) for DAT1 intron8 5/6 repeat VNTR (5-6, 6-6) and DRD2 rs2283265 (G-G, G-T) genotype groups. The interaction was not significant (p=0.41).
4. Discussion
Findings of this study suggest that dopaminergic gene variants may be modulators of EMD and ADHD symptoms in children with ASD. Specifically, there was a moderate-strength (ηp2=.063) association of the DAT1 intron8 VNTR with parent-rated EMD. Children with lower activity 6-6 repeat genotype had more severe symptoms than 5-6 heterozygotes. Of particular interest is the breadth of associations to include generalized anxiety, depression, and anger/irritability, and a moderate-strength (ηp2=0.110) association with a composite EMD+ASD phenotype. The DRD2 rs2283265 variant was also associated with parent-rated EMD (ηp2=0.041) where children homozygous for the G allele had more severe symptoms than heterozygotes (T-G), and follow-up analyses indicated a significant association for depression (ηp2=0.053).
Children homozygous for the DAT1 intron8 6-repeat allele were rated by their parents as having more severe ADHD hyperactivity than 5/6 heterozygotes (co-varying ASD severity), but the effect size was modest (ηp2=0.045). A meta-analysis of five studies of the DAT1 intron8 variant and ADHD indicated a modest association with the 6-repeat being the risk allele, but there was significant heterogeneity in effect sizes across studies (Gizer et al., 2009). In the present study, there was a significant effect of DAT1 3’-UTR VNTR genotype for teachers’ ratings of ADHD inattention (ηp2=0.105) where children homozygous for the 9-repeat allele had less severe symptoms that the 9-10 and 10-10 groups. A meta-analysis of 34 studies of children with ADHD found the10-repeat allele to be a risk variant, but the magnitude of the association was modest and effect sizes across studies were heterogeneous (Gizer et al., 2009). Research indicates the DAT1 3’-UTR VNTR does not appear to be a functional variant (Pinsonneault et al., 2011), but may be a marker for other causative DAT1 haplotypes. There was a significant association for DRD2 rs2283265 and teacher-rated inattention (ηp2=0.069), but there is little prior research about this SNP and ADHD.
Some differences were observed in genotype-phenotype associations between teacher and parent report, and this is consistent with an extensive literature that supports contextual variation in child behavior (behavioral plasticity), which appears to vary as a function of genotype (Belsky, et al., 2009; Gadow & Drabick, 2012; Gadow et al., 2013; Kuepper et al., 2012). In other words, children may behave differently in a classroom compared with the home because different types of demands are placed on them in each setting, and this likely contributes to discrepancies between informant reports of symptoms. Importantly, it is only by examining both parent and teacher evaluations that we can begin to understand the implications of contextual variation for gene-behavior relations.
5. Study Strengths, Limitations, and Future Directions
The strengths of this study include limiting the sample to children within a relatively restricted age range, assessment of symptom phenotypes with a psychometrically sound and validated measure, and focus on a small number of gene variants previously shown to (a) regulate expression of DAT1 and DRD2 (Pinsonneault et al., 2011; Zhang et al., 2007), key players in dopaminergic signaling, and (b) be associated with EMD or ADHD in prior research with non-ASD individuals. However, generalization of results is bounded by several qualifications. The size of the study sample is relatively small, and this may increase the probability of Type 1 (false positives) and Type 2 (false negatives) errors. With regard to the former, our results are strengthened by the facts that risk genotypes were relatively common, and targeted variants and symptoms were informed by à priori research findings. Conversely, some marginally significant results (e.g., DRD2 rs2283265 and teachers’ ratings of EMD) warrant consideration in future studies of youth with ASD as does the potential epistatic effect in DAT1 intron8 5/6 repeat VNTR and DRD2 rs2283265. Results were unchanged when analyses were limited to Caucasians, but findings may not generalize to other ethnicities. It is widely acknowledged that a range of biopsychosocial variables impact psychiatric symptom expression, and the present study did not examine other aspects of the child’s genetic environment or their interactions with environmental variables. Although some of our results are consistent with the extant literature involving non-ASD samples, they are presented here primarily as guides for future research and should not be considered as hypothesis confirming.
6. Conclusion
Research conducted in several different countries indicates that most children with ASD have EMD or ADHD symptoms that are severe enough to impair social or academic functioning. Our results suggest that at least some regulatory dopaminergic gene variants may modulate the severity of these co-occurring symptoms, but they are tentative pending replication in larger, independent samples.
Highlights.
Most children with ASD experience impairing emotion dysregulation (EMD) or ADHD symptoms.
Evidence suggests that dysregulation of dopamine metabolism or signaling is likely implicated in the etiology of EMD and ADHD
Results suggest some regulatory dopaminergic gene variants may modulate the severity of these co-occurring symptoms
Findings should be considered tentative pending replication in larger, independent samples.
Acknowledgments
This study was supported in part by the Matt and Debra Cody Center for Autism and Developmental Disorders, charitable contributions, and a grant from the National Institute of Health General Medical Sciences U01 GM092655 (WS). The authors wish to thank Dr. John Pomeroy, Stony Brook University (Department of Pediatrics) for supervising the clinical diagnoses and Dr. Carla DeVincent, Stony Brook University (Department of Radiology) for managing data collection.
Footnotes
Conflicts of Interest
Dr. Gadow is shareholder in Checkmate Plus, publisher of the Child Symptom Inventory-4. For the remaining authors, none were declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kenneth D. Gadow, Department of Psychiatry, Stony Brook University, Stony Brook, NY 11794-8790, Phone: (631) 632-8858, FAX: (631) 632-8953
Julia Pinsonneault, Department of Pharmacology, Program in Pharmacogenomics, Ohio State University Wexner Medical Center, 333 West 10th Ave, Columbus, 43210, Phone: 614-292-5165, FAX: 614-292-7232, pinsonneault.2@osu.edu.
Greg Perlman, Department of Psychiatry, Stony Brook University, Stony Brook, NY 11794-8790, Phone: (631), gperlman@gmail.com.
Wolfgang Sadee, Department of Pharmacology, Program in Pharmacogenomics, Ohio State University Wexner Medical Center, 333 West 10th Ave, Columbus, 43210, Phone: (614) 292-1597, Fax: (614) 292-7232, Wolfgang.Sadee@osumc.edu.
References
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Research Reviews. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. NeuroImage. 2009;47:2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID. High and abnormal forms of aggression in rats with extremes in trait anxiety – involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37:1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cohen IL, Liu X, Lewis MES, Chudley A, Foster-Gibson C, Gonzalez M, Jenkins EC, Brown WT, Holden JJA. Autism severity is associated with child and maternal MAOA genotypes. Clinical Genetics. 2011;79:355–362. doi: 10.1111/j.1399-0004.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- DeVincent CJ, Gadow KD. Relative clinical utility of three Child Symptom Inventory-4 scoring algorithms for differentiating children with autism spectrum disorder versus attention-deficit hyperactivity disorder. Autism Research. 2009;2:312–321. doi: 10.1002/aur.106. [DOI] [PubMed] [Google Scholar]
- Felten A, Montag C, Markett S, Walter N, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain and Behavior. 2011;1:109–118. doi: 10.1002/brb3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Drabick DAG. Oppositional defiant disorder as a clinical phenotype in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008;38:1302–1310. doi: 10.1007/s10803-007-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. Journal of Autism and Developmental Disorders. 2006;36:271–283. doi: 10.1007/s10803-005-0060-3. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-aged children with PDD versus clinic and community samples. Autism. 2005;9:392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Siegal VI, Olvet DM, Kibria S, Kirsch SF, Hatchwell E. Allele-specific associations of 5-HTTLPR/rs25531 with ADHD and autism spectrum disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40:292–297. doi: 10.1016/j.pnpbp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Drabick DAG. Anger and irritability symptoms among youth with ODD: Cross-informant versus source-exclusive syndromes. Journal of Abnormal Child Psychology. 2012;40:1073–1085. doi: 10.1007/s10802-012-9637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Drabick DAG, Loney J, Sprafkin J, Salisbury H, Azizian A, Schwartz J. Comparison of ADHD symptom subtypes as source-specific syndromes. Journal of Child Psychology and Psychiatry. 2004;45:1135–1149. doi: 10.1111/j.1469-7610.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Guttmann-Steinmetz S, Rieffe C, DeVincent CJ. Depression symptoms in boys with autism spectrum disorder and comparison samples. Journal of Autism and Developmental Disorders. 2012;42:1353–1363. doi: 10.1007/s10803-011-1367-x. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Roohi J, DeVincent CJ, Hatchwell E. Association of ADHD, tics, and anxiety with dopamine transporter (DAT1) genotype in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2008;49:1331–1338. doi: 10.1111/j.1469-7610.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Schwartz J, DeVincent C, Strong G, Cuva S. Clinical utility of autism spectrum disorder scoring algorithms for the Child Symptom Inventory. Journal of Autism and Developmental Disorders. 2008;38:419–427. doi: 10.1007/s10803-007-0408-y. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Smith RM, Pinsonneault JK. Possible association of serotonin 2A receptor gene (HTR2A) regulatory variants with severity of depression symptoms in children with autism spectrum disorder. Cognitive and Behavioral Neurology. doi: 10.1097/WNN.0000000000000028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Early Childhood Inventory-4 norms manual. Stony Brook, NY: Checkmate Plus; 1997. [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventory-4 screening and norms manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- Gadow KD, Sprafkin J. Adolescent Symptom Inventory-4 screening and norms manual. Stony Brook, NY: Checkmate Plus; 2008. [Google Scholar]
- Gadow KD, Sprafkin J. The Symptom Inventories: An Annotated Bibliography [On-line] Stony Brook, NY: Checkmate Plus; 2011. www.checkmateplus.com. [Google Scholar]
- Garcia-Garcia M, Clemente I, Domínguez-Borràs J, Escera C. Dopamine transporter regulates the enhancement of novelty processing by a negative emotional context. Neuropsychologia. 2010;48:1483–1488. doi: 10.1016/j.neuropsychologia.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Guerini FR, Bolognesi E, Chiappedi M, Manca S, Ghezzo A, Agliardi C, Sotgiu S, Usai S, Matteoli M, Clerici M. SNAP-25 single nucleotide polymorphisms are associated with hyperactivity in autism spectrum disorders. Pharmacological Research. 2011;64:83–288. doi: 10.1016/j.phrs.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Guttmann-Steinmetz S, Gadow KD, DeVincent CJ. Oppositional defiant and conduct disorder behaviors in boys with autism spectrum disorder with and without attention-deficit hyperactivity disorder versus several comparison samples. Journal of Autism and Developmental Disorders. 2009;39:976–985. doi: 10.1007/s10803-009-0706-7. [DOI] [PubMed] [Google Scholar]
- Guttmann-Steinmetz S, Gadow KD, DeVincent CJ, Crowell J. Anxiety symptoms in boys with autism spectrum disorder, attention-deficit hyperactivity disorder, or chronic multiple tic disorder and community controls. Journal of Autism and Developmental Disorders. 2010;40:1006–1016. doi: 10.1007/s10803-010-0950-x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven, CT: 1975. [Google Scholar]
- Kaat AJ, Gadow KD, Lecavalier L. Psychiatric symptom impairment in children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2013;41:959–969. doi: 10.1007/s10802-013-9739-7. [DOI] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression; a postmortem study. Biological Psychiatry. 2002;552:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Kuepper Y, Wielpuetz C, Alexander N, Mueller E, Grant P, Hennig J. 5-HTTLPR S-allele: a genetic plasticity factor regarding the effects of life events on personality? Genes, Brain and Behavior. 2012;11:643–650. doi: 10.1111/j.1601-183X.2012.00783.x. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Gadow KD, DeVincent CJ, Edwards MC. Validation of DSM-IV model of psychiatric syndromes in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:278–289. doi: 10.1007/s10803-008-0622-2. [DOI] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Nucleus accumbens dopamine and learned fear revisited: A review and some new findings. Behavioural Brain Research. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) Australian and New Zealand Journal of Psychiatry. 1991;25:277–283. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH. Lower dopamine transporter binding potential in striatum during depression. NeuroReport. 2001;12:4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, Sadee W. Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology. 2011;36:753–762. doi: 10.1038/npp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signaling. Progress in Neurobiology. 2010;92:112–133. doi: 10.1016/j.pneurobio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine transporter gene variant affecting expression of human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644–1655. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohi J, DeVincent CJ, Hatchwell E, Gadow KD. Association of a monoamine oxidase-A gene promoter polymorphism with ADHD and anxiety in boys with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:67–74. doi: 10.1007/s10803-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine metabolism among patients with depression who attempt suicide. Archives of General Psychiatry. 1992;49:447–450. doi: 10.1001/archpsyc.1992.01820060027004. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clinical Neuropharmacology. 2005;28:228–237. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Pinsonneault JK, Papp AC, Zhu H, Lemeshow S, Mash DC, Sadee S. Dopamine transporter DAT and receptor DRD2 variants affect risk of lethal cocaine abuse: A gene-gene-environment interaction. Translational Psychiatry. 2013;3:e222. doi: 10.1038/tp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PH. Minimal brain dysfunction. New York: Wiley-Interscience; 1971. [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee MT, Xiao T, Papp A, Wang D, Sadee W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Procedings of the National Academy of Scencesi USA. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]


