Abstract
Background
Altered nitric oxide (NO) signaling has been associated with the pathophysiology of Bipolar Disorder (BD), directly affecting neurotransmitter release and synaptic plasticity cascades. Lithium has shown to regulate NO levels in preclinical models. However, no study has addressed peripheral NO levels in unmedicated BD. Also, lithium's effects on NO levels have not been studied in humans.
Methods
Plasma NO was evaluated in subjects with BD I and II during a depressive episode (n = 26). Subjects had a score of ≥18 in the 21-item Hamilton Depression Rating Scale and were followed-up during a 6-week trial with lithium. Plasma NO levels were also compared to matched healthy controls (n = 28). NO was determined by chemiluminescence method.
Results
Lithium treatment significantly increased plasma NO levels after 6 weeks of treatment in comparison to baseline levels in bipolar depression (p = 0.016). Baseline NO levels during depressive episodes showed no difference when matching up to healthy controls (p = 0.66).
Conclusion
The present findings suggest that lithium upregulates NO signaling in unmedicated BD with short illness duration. Further studies with larger samples are needed to confirm the effects of lithium on NO pathway and its association with synaptic plasticity and therapeutics of BD.
Keywords: Bipolar disorder, Nitric oxide, Lithium, Treatment, Depression, Plasticity
1. Introduction
Recent studies have suggested that nitric oxide (NO) signaling may represent a potential therapeutic target in mood disorders (Ghasemi and Dehpour, 2011). Post-mortem studies in MDD showed a decrease in neuronal nitric oxide synthase levels in the locus coeruleus and lower number and density of nitric oxide synthase-immunoreactive neurons in the hypothalamic nuclei compared to healthy controls (Bernstein et al., 1998, 2002, 2005; Karolewicz et al., 2004). Regarding peripheral NO levels in MDD, Suzuki et al. (2001) found increased levels, whereas other study found no alteration (Kim et al., 2006). In medication-free depression, decreased NO was found in different studies (Chrapko et al., 2004; Selley, 2004; Herken et al., 2007; García et al., 2011). Regarding investigations in BD, increased NO levels were observed during different mood states (Andreazza et al., 2008), and selectively in depressive episodes (Selek et al., 2008). However, all previous studies in BD measured NO metabolites as an index of NO activity, rather than NO levels per se. Moreover, in the only study evaluating NO in bipolar depression, patients were selected in a naturalistic setting and using diverse psychotropic drugs (Selek et al., 2008).
The NO pathway is especially relevant in neuropsychiatric disorders. NO modulation was shown to affect the release of neuro-transmitters (Prast and Philippu, 2001) and synaptic plasticity (Bon and Garthwaite, 2003). NO has dose-dependent effects; at high concentrations it has neurotoxic properties and when at physiological concentrations it has a neuromodulator and a neuroprotective role (Calabrese et al., 2007). Its role in neuroprotection has been associated with decreased Ca2+ influx and consequent inhibition of cell death (Liu and Stamler, 1999). Also, NO increases the neuroprotective proteins Akt (Ciani et al., 2002) and cyclic-AMP-responsive-element-binding protein (CREB) (Riccio et al., 2006), also inducing the production of bilirubin, a potent antioxidant (Sergent et al., 1997).
NO effects are consistent with the neuroprotective and neurotrophic actions of lithium (Machado-Vieira et al., 2009; de Sousa et al., 2011). Lithium is a standard treatment for BD and used as first option treatment for bipolar depression (Bschor and Bauer, 2006). Its mechanism of action is complex, affecting multiple intracellular signaling pathways. Several animal models showed that lithium regulates central and peripheral NO levels (Ghasemi and Dehpour, 2011). Studies in rodents have shown that lithium regulates the expression of the enzyme NO synthase Bagetta et al. (1993); (Feinstein, 1998; Anai et al., 2001; Bhalla et al., 2010) and NO activity (Harvey et al., 1994; Maruta et al., 2005), but results are mixed.
The present study evaluates plasma NO levels in unmedicated subjects with BD patients during depressive episodes in comparison to healthy controls. To date, NO levels have not been studied in drug-free patients with bipolar depression. In addition, although many studies were performed in animal models or in vitro, lithium's effects on NO have not been studied in humans. Thus, lithium's effects on NO levels were evaluated in bipolar depression in this 6-week trial.
2. Methods
2.1. Subjects
Between August 2010 and June 2012, 26 outpatients, 7 (26.9%) men and 19 (73.1%) women, with a mean age of 27.7 (±4.8) years and a diagnosis of BD, experiencing a major depressive episode, diagnosed by Structured Clinical Interview for Axis I DSM-IV-TR Disorders (SCID) (First et al., 1995), were enrolled in the study. Patients were recruited through media advertisement and evaluated at the Institute of Psychiatry, University of Sao Paulo, Brazil.
Patients were required to have a score ≥18 on the 21-item Hamilton Depression Scale (HAM-D) (Hamilton, 1960) when enrolled. Assessment of symptoms was also made with Young Mania Rating Scale (YMRS), and Clinical Global Impression – Severity (CGI-S) (Petkova et al., 2000). Diagnosis and psychometric assessments were performed by experienced psychiatrists. Patients were excluded if they had any medical disorder that could affect the central nervous system, substance abuse in the last year or dependence or mental retardation.
For comparison with patients, 28 age-matched (±3 years) healthy controls, 16 (57.1%) men and 12 (42.9%) women, with a mean age of 28.0 years (±7.2) were studied. Controls were excluded from the present investigation if there was a lifetime history of any mental disorder (as assessed with SCID), including substance disorders, any disease affecting central nervous system or any first-degree relative with mood or psychotic disorder.
This study had approval by the local institutional review board, and all participants provided written informed consent prior to entering the study.
2.2. Study design
Patients had blood samples collected before (at baseline) and after treatment (at endpoint), and were matched with healthy controls. At baseline, patients received lithium carbonate at 450 mg/day, with flexible increases in doses according to clinical improvement. Most patients were taking lithium monotherapy, although the use of hypnotics as needed was allowed and 2 patients who were also using antipsychotics/mood stabilizers. Psychometric assessments were made at week 0 (baseline) and then at weeks 1, 2, 4 and 6 (endpoint). Criterion for clinical response was a decrease of 50% or more in the HAM-D at endpoint and for remission a HAM-D < 8 and YMRS < 8 at endpoint.
2.3. Assays
Blood samples were collected from 8:00 to 10:00 AM in vacutainer tubes from patients and controls in 8-h fasting. Samples were centrifuged at 20 °C and 1620 × g for 15 min to obtain plasma. Plasma samples were frozen and stored at −80 °C. To avoid plasma foaming caused by proteins, we deproteinized plasma by adding ZnSO4 and NaOH, and then centrifuging at 12,000 rpm for 5 min at room temperature.
Since Griess reaction has been shown not to be accurate for NO measures (Hunter et al., 2013), NO plasma samples were determined by chemiluminescence using Model 280 Nitric Oxide Analyzer (NOA™) from Sievers Instruments, Inc. (Boulder, CO, USA), which is a high-sensitive detector for measuring nitric oxide (Hunter et al., 2013), based on gas-phase chemiluminescent reaction between nitric oxide and ozone: and . The photon emission from electrically excited nitrogen dioxide was detected by a thermoelectrically cooled photomultiplier tube. Measurement of NO and its reaction products in liquid samples has a sensitivity around 1 pmol (Hampl et al., 1996) and is validated for plasma measurement (Yang et al., 1997). Three comparisons of standards and internal controls achieved high coefficients of correlation (r > 0.99).
2.4. Statistics
Chi-square test was used to compare gender in patients and controls. For samples with normal distribution Student's t test was used for comparisons and when samples had non-normal distribution, comparisons were performed with Mann–Whitney and Wilcoxon Signed Ranks tests. Correlations were evaluated with Spearman test. Statistical analysis was performed using SPSS 14.0. Significance level was set at <0.05 (two-tailed).
3. Results
3.1. Demographic and clinical data
Demographic and clinical data of BD patients and controls are summarized in Table 1. From the 26 patients enrolled, 9 (34.6%) had diagnosis of type I BD and 17 (65.4%) of type II BD; 24 (92.3%) patients were medication-free for at least 6 weeks before enrollment in the study and among those, 20 (76.9%) were drug-naïve. Patients had illness duration of mean 36.6 months (±19.4) and history of previous psychotic mood episode was present in only 3 subjects (11.5%) patients. A significant decrease in depressive symptoms measured by HAM-D was observed from baseline (22.6 ± 2.9) to endpoint (7.0 ± 6.2) (z = −4.35, p < 0.001), 22 (84.6%) patients responded to treatment and 16 (61.5%) patients achieved remission.
Table 1.
Demographic and clinical characteristics of bipolar depression patients and healthy controls.
| Controls (n = 28) | Bipolar(n = 26) | p | |
|---|---|---|---|
| Gender | |||
| Male/Female, n (%) | 16 (57.1)/12 (42.9) | 7 (26.9)/19 (73.1) | 0.03*a |
| Age, years | 28.0 (±7.2) | 27.7 (±4.8) | 0.86b |
| Bipolar Disorder Type | |||
| Type I/Type II, n (%) | 9 (34.6)/17 (65.4) | ||
| Duration of illness, months | 36.6 (±19.4) | ||
| Drug-naïve, n (%) | 20 (76.9) | ||
| Medication-free, n (%) | 24 (92.3) | ||
| History of Psychosis, n (%) | 3 (11.5) | ||
| HAM-D | |||
| Baseline/Endpoint | 22.6 (±2.9)/7.0 (±6.2) | ||
| YMRS | |||
| Baseline/Endpoint | 5.8 (±5.3)/3.8 (±9.2) | ||
| Response, n (%) | 22 (84.6) | ||
| Remission, n (%) | 16 (61.5) | ||
| Dropout, n (%) | 1 (3.8) | ||
| Endpoint serum lithium, mEq/L | 0.52 (±0.21) | ||
HAM-D – Hamilton Depression Scale, YMRS – Young Mania Rating Scale.
Significantly different.
– Chi-square,
– Student's t test.
3.2. Lithium treatment increased NO plasma levels
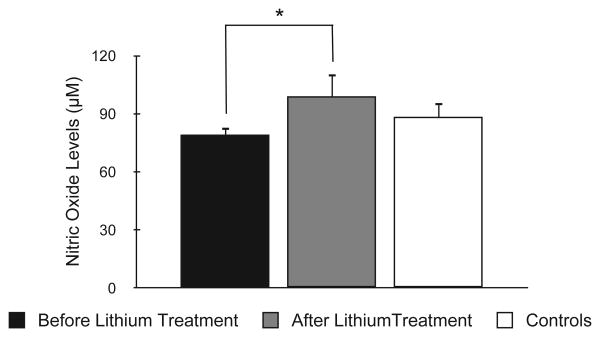
Lithium treatment significantly increased NO levels from baseline (78.8 ± 20.4 μM) to endpoint (99.1 ± 53.6 μM) (z = −2.42, p = 0.016) (Fig. 1). NO at endpoint was not different in responders compared to non-responders (z = −0.79, p = 0.43) and in remitters versus non-remitters (t = −0.70, p = 0.48). Also, NO changes over time were not associated with depressive symptoms improvement (measured by HAM-D) (ρ = −0.10, p = 0.63). NO levels at endpoint did not correlate to age (p = 0.28), plasma lithium (p = 0.25), illness duration (p = 0.60), or any other demographic/clinical variable (data not shown).
Fig. 1.
Comparison of nitric oxide levels in bipolar depression patients before treatment, after treatment, and in healthy controls. Bars display standard error mean. *p = 0.016.
3.3. Similar plasma NO levels in bipolar depression and controls
Plasma NO levels in bipolar depression at baseline (78.6 ± 19.8 μM) were not significantly different from NO levels in healthy controls (88.4 ± 39.9 μM) (z = −0.48, p = 0.63) (Fig. 1). Although BD and control groups showed difference for gender, no significant differences were observed between males and females with regard to NO levels (z = −0.45, p = 0.66). Baseline depressive symptoms did not show a significant correlation with NO levels (ρ = −0.35, p = 0.08). Also, baseline NO was not correlated with age (ρ = 0.03, p = 0.90), illness duration (ρ = 0.06, p = 0.79) or any other variable (data not shown).
4. Discussion
To our best knowledge, this is the first study reporting the effects of lithium at NO pathway in humans. It was shown that lithium significantly increased NO levels in BD depression after 6 weeks of treatment. No significant difference in NO levels was observed in unmedicated bipolar depression compared to matched healthy controls. For the first time, NO levels were evaluated in a sample which most of the BD patients were drug-naive. Also, no association between lithium-induced NO increase and clinical improvement was observed.
The present findings suggest that NO may be regulated by lithium in humans. Similar to our findings, a growing body of preclinical evidence suggests that lithium has direct effects targeting at NO signaling (reviewed in Ghasemi and Dehpour, 2011). Lithium has been shown to increase NO synthase (NOs) mRNA expression in glial cultured cells (Feinstein, 1998), hypothalamus (Anai et al., 2001), hippocampus (Bagetta et al., 1993), as well as enhanced cortical NO metabolites (Harvey et al., 1994) in rodents. Other preclinical studies, however, found lithium decreasing NO metabolites in rat neural tissue (Maruta et al., 2005; Bhalla et al., 2010). The increased NO levels were not associated with clinical improvement, raising the possibility that lithium effects on NO may represent an epiphenomenon or an intermediate pathway for antidepressant efficacy.
Although there is no consensus about lithium mechanisms influencing NO levels, lithium is associated with increases in beta-catenin levels (Wada, 2009), which triggers a cascade of events, leading to induction of NO synthase and increase in NO levels (Du et al., 2006; Bandino et al., 2008).
Reinforcing the validity of the present findings in peripheral blood, a modest but significant correlation (r = 0.5) between serum and cerebrospinal fluid NO levels has been demonstrated in humans after acute brain injury (Rejdak et al., 2003). Moreover, NO alterations in peripheral blood and cerebrospinal fluid have shown the same direction when associated with clinical findings in multiple sclerosis (Yuceyar et al., 2001; Acar et al., 2003), which may lead to a wider use of peripheral NO in neurological diseases (Ibragic et al., 2012).
In the present investigation, plasma NO levels in bipolar depression were not different from healthy controls. In mood disorders, studies on NO showed mixed results. In MDD, NO was decreased when evaluating drug-free patients during depressive episodes (Chrapko et al., 2004; Selley, 2004; Herken et al., 2007; García et al., 2011), while increased (Suzuki et al., 2001) or unaltered (Kim et al., 2006) NO was also observed in other studies.
Meanwhile, in BD, increased NO was observed in most of the investigations (Andreazza et al., 2008). Only one study, however, evaluated NO during a depressive episode in BD (Selek et al., 2008). This study showed elevated NO metabolites in subjects with bipolar depression under multiple medications, which can influence NO (Lara et al., 2003; Ikenouchi-Sugita et al., 2009; Maia-de-Oliveira et al., 2012). The fact that our sample comprised drug-free patients with short duration of illness is a possible explanation for the discordance with the other study in bipolar depression. It is also possible that NO may have a more important role in bipolar depression later in the course of the illness, after patients have been exposed to chronic insults such as recurrent episodes, medications effects, comorbidities and others. For instance, the number of previous mood episodes was positively correlated with NO levels in BD (Savas et al., 2006). Also, more than half of the patients enrolled in this study were BD II subjects, while other studies evaluating NO included predominantly individuals with BD I.
A strength of our study was that we used a chemiluminescence analyzer for measurement of NO levels. The Griess reaction, which was used to evaluate NO in some of previous studies in MDD (Kim et al., 2006; Herken et al., 2007; García et al., 2011) and all studies in BD (Andreazza et al., 2008), has less accuracy than the chemiluminescence method employed in the present study (Hunter et al., 2013). Moreover, our sample comprised young patients, with short duration of illness, and mostly drug-free, increasing the specificity of findings. The relative small sample size of the study may represent a limitation.
Overall, the present findings suggest that lithium modulates NO signaling in subjects with BD during depressive episode, with no direct association with the pathophysiology of the illness. These findings may support a potential role for NO signaling in the neurotrophic and neuroprotective effects of lithium in BD and other neuropsychiatric disorders. Further studies with larger samples are needed to confirm these preliminary findings.
Acknowledgments
The Laboratory of Neuroscience is supported by the Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
Role of the funding source: This study was sponsored by Sao Paulo Research Foundation (Fapesp, Brazil 2009/14891-9). The Laboratory of Neuroscience is supported by the Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
Abbreviations
- BD
Bipolar Disorder
- CGI
Clinical Global Impression
- CREB
cyclic-AMP-responsive-element-binding protein
- HAM-D
21-item Hamilton Depression Scale
- MDD
Major Depressive Disorder
- NO
Nitric Oxide
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- SCID
Structured Clinical Interview for Axis I DSM-IV-TR Disorders
- YMRS
Young Mania Rating Scale
Footnotes
Contributors: Dr. Machado-Vieira designed the study and collected clinical data. Dr Zanetti and de Sousa collected clinical data. Margaret G. Mouro and Elisa M. Higa performed the laboratorial essays. Dr Gattaz and Zarate provided invaluable scientific input. All authors contributed to and have approved the final manuscript.
Conflict of interest: CAZ is listed as co-inventor on a patent for the use of ketamine in major depression and has assigned their patent rights on ketamine to the US government. The other authors report no conflict of interest.
References
- Acar G, Idiman F, Idiman E, Kirkali G, Cakmakçi H, Ozakbaş S. Nitric oxide as an activity marker in multiple sclerosis. J Neurol. 2003;250:588–92. doi: 10.1007/s00415-003-1041-0. [DOI] [PubMed] [Google Scholar]
- Anai H, Ueta Y, Serino R, Nomura M, Nakashima Y, Yamashita H. Activation of hypothalamic neuronal nitric oxide synthase in lithium-induced diabetes insipidus rats. Psychoneuroendocrinology. 2001;26:109–20. doi: 10.1016/s0306-4530(00)00030-5. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kauer–Sant'anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Melino G, Paoletti AM, Finazzi-Agrò A, Nisticò G. Lithium and tacrine increase the expression of nitric oxide synthase mRNA in the hippocampus of rat. Biochem Biophys Res Commun. 1993;197:1132–9. doi: 10.1006/bbrc.1993.2595. [DOI] [PubMed] [Google Scholar]
- Bandino A, Compagnone A, Bravoco V, Cravanzola C, Lomartire A, Rossetto C, et al. Beta-catenin triggers nuclear factor kappaB-dependent up-regulation of hepatocyte inducible nitric oxide synthase. Int J Biochem Cell Biol. 2008;40:1861–71. doi: 10.1016/j.biocel.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Heinemann A, Krell D, Dobrowolny H, Bielau H, Keilhoff G, et al. Hypothalamic nitric oxide synthase in affective disorder: focus on the suprachiasmatic nucleus. Cell Mol Biol (Noisy-le-grand) 2005;51:279–84. [PubMed] [Google Scholar]
- Bernstein HG, Heinemann A, Krell D, Mawrin C, Bielau H, Danos P, et al. Further immunohistochemical evidence for impaired NO signaling in the hypothalamus of depressed patients. Ann N Y Acad Sci. 2002;973:91–3. doi: 10.1111/j.1749-6632.2002.tb04613.x. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, et al. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83:867–75. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- Bhalla P, Singla N, Dhawan DK. Potential of lithium to reduce aluminium-induced cytotoxic effects in rat brain. Biometals. 2010;23:197–206. doi: 10.1007/s10534-009-9278-4. [DOI] [PubMed] [Google Scholar]
- Bon CL, Garthwaite J. On the role of nitric oxide in hippocampal long-term potentiation. J Neurosci. 2003;23:1941–8. doi: 10.1523/JNEUROSCI.23-05-01941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bschor T, Bauer M. Efficacy and mechanisms of action of lithium augmentation in refractory major depression. Curr Pharm Des. 2006;12:2985–92. doi: 10.2174/138161206777947650. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–75. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Mellédo JM. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129–34. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Ciani E, Virgili M, Contestabile A. Akt pathway mediates a cGMP-dependent survival role of nitric oxide in cerebellar granule neurones. J Neurochem. 2002;81:218–28. doi: 10.1046/j.1471-4159.2002.00857.x. [DOI] [PubMed] [Google Scholar]
- de Sousa RT, van de Bilt MT, Diniz BS, Ladeira RB, Portela LV, Souza DO, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett. 2011;494:54–6. doi: 10.1016/j.neulet.2011.02.054. [DOI] [PubMed] [Google Scholar]
- Du Q, Park KS, Guo Z, He P, Nagashima M, Shao L, et al. Regulation of human nitric oxide synthase 2 expression by Wnt beta-catenin signaling. Cancer Res. 2006;66:7024–31. doi: 10.1158/0008-5472.CAN-05-4110. [DOI] [PubMed] [Google Scholar]
- Feinstein DL. Potentiation of astroglial nitric oxide synthase type-2 expression by lithium chloride. J Neurochem. 1998;71:883–6. doi: 10.1046/j.1471-4159.1998.71020883.x. [DOI] [PubMed] [Google Scholar]
- First M, Riswan S, Mike G, Jbw W. Structured clinical interview for DSM-IV Axis I disorders. Patient ed. New York: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- García RG, Zarruk JG, Barrera C, Pinzón A, Trillos E, Arenas WD, et al. Plasma nitrate levels and flow-mediated vasodilation in untreated major depression. Psychosom Med. 2011;73:344–9. doi: 10.1097/PSY.0b013e31821566cf. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Dehpour AR. The NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of lithium. Trends Pharmacol Sci. 2011;32:420–34. doi: 10.1016/j.tips.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl V, Waters C, Ascher S. Determination of nitric oxide by the chemiluminescence reaction with ozone. In: Feelisch M, Stamler JS, editors. Methods in nitric oxide. John Wiley & Sons Ltd; 1996. pp. 309–18. [Google Scholar]
- Harvey BH, Carstens ME, Taljaard JJ. Evidence that lithium induces a glutamatergic: nitric oxide-mediated response in rat brain. Neurochem Res. 1994;19:469–74. doi: 10.1007/BF00967326. [DOI] [PubMed] [Google Scholar]
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–52. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hunter RA, Storm WL, Coneski PN, Schoenfisch MH. Inaccuracies of nitric oxide measurement methods in biological media. Anal Chem. 2013;85:1957–63. doi: 10.1021/ac303787p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragic S, Sofic E, Suljic E, Avdagic N, Bajraktarevic A, Tahirovic I. Serum nitric oxide concentrations in patients with multiple sclerosis and patients with epilepsy. J Neural Transm. 2012;119:7–11. doi: 10.1007/s00702-011-0686-6. [DOI] [PubMed] [Google Scholar]
- Ikenouchi-Sugita A, Yoshimura R, Hori H, Umene-Nakano W, Ueda N, Nakamura J. Effects of antidepressants on plasma metabolites of nitric oxide in major depressive disorder: comparison between milnacipran and paroxetine. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1451–3. doi: 10.1016/j.pnpbp.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Stockmeier CA, Konick L, Overholser JC, Jurjus G, et al. Low nNOS protein in the locus coeruleus in major depression. J Neurochem. 2004;91:1057–66. doi: 10.1111/j.1471-4159.2004.02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Paik JW, Lee SW, Yoon D, Han C, Lee BH. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1091–6. doi: 10.1016/j.pnpbp.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Lara N, Archer SL, Baker GB, Le Mellédo JM. Paroxetine-induced increase in metabolic end products of nitric oxide. J Clin Psychopharmacol. 2003;23:408–12. doi: 10.1097/01.jcp.0000085416.08426.1d. [DOI] [PubMed] [Google Scholar]
- Liu L, Stamler JS. NO: an inhibitor of cell death. Cell Death Differ. 1999;6:937–42. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl. 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia-de-Oliveira JP, Trzesniak C, Oliveira IR, Kempton MJ, Rezende TM, Iego S, et al. Nitric oxide plasma/serum levels in patients with schizophrenia: a systematic review and meta-analysis. Rev Bras Psiquiatr. 2012;34(Suppl. 2):S149–55. doi: 10.1016/j.rbp.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Maruta S, Suzuki E, Yokoyama M, Sato T, Inada K, Watanabe S, et al. Effects of intraperitoneally injected lithium, imipramine and diazepam on nitrate levels in rat amygdala. Psychiatry Clin Neurosci. 2005;59:358–61. doi: 10.1111/j.1440-1819.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- Petkova E, Quitkin FM, McGrath PJ, Stewart JW, Klein DF. A method to quantify rater bias in antidepressant trials. Neuropsychopharmacology. 2000;22:559–65. doi: 10.1016/S0893-133X(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Rejdak K, Petzold A, Sharpe MA, Smith M, Keir G, Stelmasiak Z, et al. Serum and urine nitrate and nitrite are not reliable indicators of intrathecal nitric oxide production in acute brain injury. J Neurol Sci. 2003;208:1–7. doi: 10.1016/s0022-510x(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, et al. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol Cell. 2006;21:283–94. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Savas HA, Gergerlioglu HS, Armutcu F, Herken H, Yilmaz HR, Kocoglu E, et al. Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes. World J Biol Psychiatry. 2006;7:51–5. doi: 10.1080/15622970510029993. [DOI] [PubMed] [Google Scholar]
- Selek S, Savas HA, Gergerlioglu HS, Bulbul F, Uz E, Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord. 2008;107:89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Selley ML. Increased (E)-4-hydroxy-2-nonenal and asymmetric dimethylarginine concentrations and decreased nitric oxide concentrations in the plasma of patients with major depression. J Affect Disord. 2004;80:249–56. doi: 10.1016/S0165-0327(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Sergent O, Griffon B, Morel I, Chevanne M, Dubos MP, Cillard P, et al. Effect of nitric oxide on iron-mediated oxidative stress in primary rat hepatocyte culture. Hepatology. 1997;25:122–7. doi: 10.1002/hep.510250123. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M. Elevated plasma nitrate levels in depressive states. J Affect Disord. 2001;63:221–4. doi: 10.1016/s0165-0327(00)00164-6. [DOI] [PubMed] [Google Scholar]
- Wada A. Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J Pharmacol Sci. 2009;110:14–28. doi: 10.1254/jphs.09r02cr. [DOI] [PubMed] [Google Scholar]
- Yang F, Troncy E, Francoeur M, Vinet B, Vinay P, Czaika G, et al. Effects of reducing reagents and temperature on conversion of nitrite and nitrate to nitric oxide and detection of NO by chemiluminescence. Clin Chem. 1997;43:657–62. [PubMed] [Google Scholar]
- Yuceyar N, Taşkiran D, Sağduyu A. Serum and cerebrospinal fluid nitrite and nitrate levels in relapsing-remitting and secondary progressive multiple sclerosis patients. Clin Neurol Neurosurg. 2001;103:206–11. doi: 10.1016/s0303-8467(01)00144-5. [DOI] [PubMed] [Google Scholar]