Abstract
Both the medial temporal lobe and dorsolateral prefrontal cortex have been implicated in autism. In the present study, performance on two neuropsychological tasks—one tapping the medial temporal lobe and related limbic structures, and another tapping the dorsolateral prefrontal cortex—was examined in relation to performance on tasks assessing autistic symptoms in young children with autism, and developmentally matched groups of children with Down syndrome or typical development. Autistic symptoms included orienting to social stimuli, immediate and deferred motor imitation, shared attention, responses to emotional stimuli, and symbolic play. Compared with children with Down syndrome and typically developing children, children with autism performed significantly worse on both the medial temporal lobe and dorsolateral prefrontal tasks, and on tasks assessing symptoms domains. For children with autism, the severity of autistic symptoms was strongly and consistently correlated with performance on the medial temporal lobe task, but not the dorsolateral prefrontal task. The hypothesis that autism is related to dysfunction of the medial temporal lobe and related limbic structures, such as the orbital prefrontal cortex, is discussed.
INTRODUCTION
Advances in neurobiology, brain imaging, and neuropsychology have allowed new insights into the possible brain basis of autism (Bailey, Phillips, & Rutter, 1996). Several brain regions, ranging from the cerebellum and medial temporal lobe structures to the prefrontal cortex, have been suggested as possible core regions of abnormality in this disorder. Also, it is recognized that dysfunction in one brain region likely affects development and functioning of related brain regions (Dawson & Lewy, 1989). Indeed, autism most certainly involves dysfunction of brain circuits that support the functioning of a variety of brain regions.
Evidence for involvement in autism of the medial temporal lobe and related structures of the limbic system comes from a variety of sources, including behavioral/neuropsychological, animal lesion, and autopsy studies. A neuropsychological study conducted by Barth, Fein, and Waterhouse (1995) revealed that lower-functioning children with autism were impaired on a visual recognition memory task tapping medial temporal lobe functions. Furthermore, it is well established that individuals with autism have specific impairments in the processing of social and emotional stimuli, as evident on tasks such as face and emotion recognition, imitation of body movements, interpretation and use of gestures, and formation of a theory of mind (Baron-Cohen, Tager-Flusberg, & Cohen, 1993; Davies, Bishop, Manstead, & Tantam, 1994; Hobson, Ouston, & Lee, 1988a, 1988b; Mundy, Sigman, Ungerer, & Sherman, 1986; Smith & Bryson, 1994; Teunisse & DeGelder, 1994). This pattern of behavioral impairments suggests that autism is related to dysfunction of a brain system involved in social cognition. Animal and human lesion studies indicate that parts of the medial temporal lobe (amygdala, hippocampus, and entorhinal cortex) and the orbital frontal cortex are likely to comprise such a brain system, often referred to as the limbic system (Barbas, 1995; Brothers, 1990; Damasio, 1994; LeDoux, 1994).
A second line of evidence implicating the medial temporal lobe in autism is based on the results of early lesion studies of monkeys. Bachevalier (1994) has shown that monkeys with lesions of the hippocampus and amygdala made early in life exhibit persistent and severe cognitive and social impairments, as well as stereotyped and self-stimulatory behaviors. Monkeys with early damage only to the amygdaloid complex exhibit social disturbances similar to those found in animals with combined amygdalohippocampal lesions, although the disturbances are less severe. Finally, a third line of evidence supporting the role of the medial temporal lobe and related limbic regions in autism comes from autopsy studies (Bauman & Kemper, 1994) in which histoanatomic analysis revealed reduced neuronal cell size and increased cell-packing density in the hippocampus, amygdala, and adjacent limbic regions.
Other investigators have argued that autism is better characterized as a disorder of higher cortical functions, and specifically of the dorsolateral prefrontal cortex (Minshew & Goldstein, 1993; Ozonoff, Pennington, & Rogers, 1991; Rogers & Pennington, 1991). According to this view, core behavioral impairments in autism are related to impairment in executive functions, such as working memory. Evidence supporting this view comes from neuropsychological studies. Specifically, studies of high-functioning verbal individuals with autism have found impairments on tasks tapping executive functions, and intact functioning on memory tasks known to be mediated by the medial temporal lobe (Minshew & Goldstein, 1993). Also, research has shown an association between executive function skill and specific autistic symptom domains, including motor imitation ability and the ability to understand the mental states of others (theory-of-mind) (McEvoy, Rogers, & Pennington, 1993; Ozonoff et al., 1991). Rogers and Pennington (1991) have proposed a developmental model of autism in which a primary impairment in motor imitation, which they hypothesize to be linked to executive functioning, disrupts social-emotional development, particularly domains such as social and emotional reciprocity.
In the present study, we examined autistic children’s performance on two neuropsychological tasks—one known to be mediated by limbic structures, including the medial temporal lobe and orbital prefrontal cortex, and one known to be mediated by the dorsolateral prefrontal cortex—and their relation to degree of impairment in domains reflecting early emerging core symptoms of autism. Symptom domains included orienting to social stimuli, immediate and deferred motor imitation, responses to emotional stimuli, shared attention, and symbolic play. We predicted that early core symptoms of autism would be more closely correlated with performance on tasks tapping the limbic system than with those tapping the dorsolateral prefrontal cortex. This prediction was based on the hypothesis that early emerging symptoms of autism reflect core affective and social impairments that can be linked to dysfunction of the limbic system, particularly the amygdala and hippocampus and closely related brain regions, such as the orbital frontal region (see Dawson, 1996, for more elaborate discussion of this hypothesis). The animal and brain damage literatures suggest that the limbic system, particularly the amygdala, is critical for social perception, such as recognition of faces and facial expressions (Aggleton, 1992; Jacobson, 1986; Nelson & deHaan, 1996), the recognition of the affective significance of stimuli (LeDoux, 1987), and the perception of body movements and gaze direction (Brothers, Ring, & Kling, 1990), and for certain cognitive abilities that are likely to be important for social perception, such as cross-modal association (Murray & Mishkin, 1985) and recall of event sequences (McDonough, Mandlers, McKee, & Squire, 1994). Furthermore, we theorized that such early dysfunction of the limbic system has “downstream” consequences for the development of higher-order prefrontal functions, including those associated with the dorsolateral prefrontal cortex. Thus, although we predicted that performance on dorsolateral prefrontal tasks would be less closely linked to core autistic symptoms, we nevertheless expected that children with autism would exhibit impaired performance on both the limbic and dorsolateral prefrontal tasks, relative to matched control subjects. Importantly, we chose medial temporal lobe and dorsolateral prefrontal tasks that tap abilities in the same developmental range (toddler-preschool) and that require no verbal abilities, making them suitable for use with young children with autism.
METHOD
Participants
Three groups of children participated in the study: 20 children with autism (N = 13) or Pervasive Developmental Disorder—Not Otherwise Specified (PDD.NOS) (N = 7), 19 children with Down syndrome, and 20 children with typical development. Descriptive information for the three groups of children regarding chronological age, ethnicity, sex, and language and cognitive ability is shown in Table 1.
Table 1.
Participant Characteristics
| Group | N, Male: Female | Ethnicity | CA (Months) | Vinelanda MA (Months) | Vineland Scale IQ | PLSb MA (Months) | PLS IQ | Nonverbal MA (Months) |
|---|---|---|---|---|---|---|---|---|
| Autism | 20, 19:1 | 18 Caucasian 2 Biracial |
64.6 (15.1) | 30.4 (13.4) | 62.0 (16.4) | 28.1 (14.9) | 58.9 (14.3) | 51.0 (26.2) |
| Down | 19, 16:3 | 17 Caucasian 1 African American 1 Native American |
65.3 (16.5) | 27.3 (10.2) | 57.2 (8.2) | 29.9 (12.3) | 56.7 (9.4) | 34.1 (11.8) |
| Typical | 20, 19:1 | 17 Caucasian 3 Biracial |
30.9 (14.4) | 32.4 (14.6) | 103.4 (44) | 31.8 (14.8) | 105.9 (12.6) | 33.2 (13.4) |
| F | .00 | .78 | .70 | .35 | .31 | 5.89 | ||
| P | nsc | ns | nsc | ns | nsc | .005 |
Note: Numbers represent means and standard deviations (in parentheses).
Vineland Scale refers to Communication Subscale.
Preschool Language Scale.
Comparison is between autism and Down syndrome groups only.
Diagnosis of autism or PDD.NOS was based on parent interview and a structured play session specifically designed to assess autistic symptoms listed in the Diagnostic and Statistical Manual, Third Edition—Revised (American Psychiatric Association, 1987). Diagnosis of each child was made independently by the first and third authors to insure reliability. In addition, each child was administered the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1986), and all children in the autism group scored above the clinical cutoff (30) on the CARS.
The three groups of children were matched in terms of their receptive language mental age as assessed by the Preschool Language Scale—3 (PLS; Zimmerman et al., 1991) and the communication sub-scale of the Vineland Adaptive Behavior Scales (Sparrow, Balla, & Cicchetti, 1984). In addition, children with autism were matched to children with Down syndrome in terms of chronological age and verbal IQ. Children with autism had significantly higher nonverbal ability as compared to the children with Down syndrome and typically developing children-Nonverbal ability was assessed by administration of a battery of developmentally graded visual-spatial tasks derived from the Bayley Scales of Infant Development, Second Edition, and the Stanford Binet IV. Nonverbal ability therefore was used as a covariate in analyses.
Neuropsychological Tasks
Delayed Non-Matching to Sample
Delayed Non-Matching to Sample (DNMS) assesses rule-learning ability (specifically, the ability to abstract the quality of novelty and associate it with reinforcement) and visual recognition memory. It has been linked to the amygdala and hippocampus, and closely related cortical structures, including the entorhinal cortex and orbital prefrontal cortex, in monkeys1 (Bachevalier & Mishkin, 1986; Kowalska, Bachevalier, & Mishkin, 1991; Meunier, Bachevalier, & Mishkin, 1997; Zola-Morgan, Squire, & Amaral, 1989; Zola-Morgan & Squire, 1993) and in human amnesic patients, (Squire, Zola-Morgan, & Chen, 1988). The child was shown a novel object (the sample). The child then reached for and displaced it to retrieve a reward (dry food snack, such as cheerios) underneath. The sample was then removed and a delay of 5 s was imposed. Following the delay, the child was shown the sample again paired with something new (the non-matching object) and rewarded for reaching to the non-matching object. New stimuli were used on each trial. Trials were administered until the child had reached criterion performance (defined as reaching for the novel object on five consecutive trials), or a maximum of 15 trials had been administered. The dependent variables were (1) the number of errors (i.e., chose familiar rather than novel item) made until the child reached criterion performance and (2) the number of trials required until reaching criterion performance. Note that in the present study, the DNMS was primarily a task of rule-learning, because only a short delay period was imposed. Animal studies have suggested that the rule-learning aspect of the DNMS may be specifically linked to the orbital prefrontal cortex (Meunier et al., 1997).
Delayed response
Delayed response requires both working memory and response inhibition. It has been linked to the dorsolateral prefrontal cortex based on both human infant studies and animal lesion studies (Diamond & Goldman-Rakic, 1986, 1989; Goldman, Rosvold, & Mishkin, 1970). Lesions to the medial temporal lobe and parietal cortex in the adult animal do not disrupt performance on this task (Diamond & Goldman-Rakic, 1989; Diamond, Zola-Morgan, & Squire, 1989). The child watched as the experimenter (1) hid a small toy in a container at the midline and then (2) moved that container to the right or left. A screen was lowered during the 5 s delay that followed, during which time an identical container was placed on the other side of the table. When the screen was raised the child was allowed to reach for the container. The side of hiding was reversed after the child searched correctly for the toy on two consecutive trials. Trials continued until the child had searched on three reversal trials or had been administered 14 search trials. The dependent measure was the percent correct searches on reversal trials. The groups did not differ in terms of the average number of reversal trials administered.
Assessment of Core Autistic Symptoms
Social orienting
A more detailed description of the orienting task is described in Dawson, Meltzoff, Osterling, Rinaldi, and Brown (in press). Stimuli consisted of two social stimuli (clapping hands and calling child’s name) and two nonsocial stimuli (playing a musical jack-in-the-box and shaking a rattle). Stimuli were matched for duration (6 s), delivered by the same experimenter, and of similar loudness. Each stimulus was presented two times, once in the child’s visual field and once behind the child (30° to right or left). Order and location of each stimulus were counterbalanced across participants. The presentation of stimuli was interspersed between tasks designed to assess shared attention (described below). This maximized stimulus novelty and minimized children’s habituation to the orienting task. Children’s head turns to social and nonsocial stimuli were reliably recorded live by two independent coders (kappa >.70). The dependent measure was the total number of errors (failed to visually orient to when the stimulus was presented) made in response to the social stimulus.
Immediate and deferred motor imitation
The imitation tasks were adapted from previous work done by Meltzoff (1988a, 1988b; Rast & Meltzoff, 1995). The tasks were chosen to be sensitive to imitation abilities in the toddler–preschool developmental range. The battery consisted of 15 items, 10 immediate imitation tasks and 5 deferred imitation tasks. A range of tasks was used, including gestures that the participants could see themselves perform (hand opening/closing), those that they could not see themselves perform (eye-blinking, mouth opening/closing), novel acts (touching elbow to a panel), and familiar acts (banging wooden blocks). The tasks were administered while the experimenter was seated across a small table from the participant. After ensuring that the child was paying attention, the experimenter demonstrated each particular target act three times. There was no verbal description of the tasks, and no physical prompting of the child to try to elicit a response. The tasks were administered in two blocks, one for the immediate imitation test (10 items) and the other for the deferred imitation task (5 items), with block order counterbalanced within each population, and task order within each block randomly determined. For the deferred imitation test, the adult demonstrated each of the target acts, and then a 10 min memory interval was interposed. After the memory interval, the participants returned to the test room and were presented with the test objects one at a time in their original order. For the immediate imitation test, the same general procedure was followed, save that the response periods occurred directly after the demonstrated target acts. Correct or incorrect response to each imitation task was coded (kappa >.85) from videotapes by two independent coders. The dependent measure was the total number of acts imitated.
Shared attention
A more detailed description of the shared attention task is provided in Dawson et al. (in press). This assessment was based on an experimental method developed by Butterworth and Jarrett (1991) to assess shared attention skills in infants and toddlers. The child was seated at a table on which there was a toy. An experimenter sat opposite the child at the table. Four yellow crosses were mounted on the wall at the child’s eye level, and placed 30° in front of the child on the right and left, and 30° behind the child on the right and left. At a time when the child was attending to the experimenter’s face, one of four shared attention probes was delivered. These consisted of (1) pointing to the cross that was in front of child, (2) pointing to the cross that was behind child, (3) looking at the cross that was in front of child, and (4) looking at the cross that was behind child. The dependent measure was the total number of errors (child failed to look at the cross in response to probe) made across trials. Close to 60% of the videotapes were coded by two independent raters who were found to be reliable (kappa >.70).
Response to distress
Responses to emotional cues were assessed using a paradigm previously used by Sigman, Kasari, Kwon, and Yirmiya (1992) to measure children’s responses to another person’s distress. The child was seated at a table directly across from the experimenter. The experimenter showed the child how to use a wooden toy and hammer. During this demonstration, the experimenter pretended to hurt herself, and for 30 s displayed facial and vocal expressions of distress without using verbal descriptions. Then, the experimenter displayed 10 s of neutral affect, and subsequently showed the child that her finger did not hurt anymore. Degree of concern/comforting was coded on a 4 point scale ranging from no interest to active comforting behavior. Twenty-five percent of tapes were double-coded for purposes of assessing interrater reliability, which was .66 (kappa).
Symbolic play
Symbolic play was assessed during one spontaneous and two prompted conditions. For each of three trials, a Mickey Mouse doll and one of the following representational stimuli were placed in front of the child: a block to represent a cookie, a box top to represent a bed, or a cylindrical shaped block to represent a brush. The child was first allowed to play spontaneously with the play materials for 3 min. At this point, the experimenter provided a simple verbal prompt for spontaneous play (e.g., “What can you do with this?”) and allowed the child to play with the toys for another 3 min. Then, the experimenter provided a second, more direct prompt for symbolic play (e.g., “Give Mickey a cookie”). The child was allowed to play with the toys for another 3 min. Sessions were videotaped for later coding of the number of object-directed and self-directed symbolic play acts. Twenty-five percent of tapes were coded by two independent coders who were found to be reliable (kappa >.70).
Wing Social Subgroup Classification
The quality of social behavior for the autistic group was evaluated using the Wing social subgroup classification system. This classification system, which was originally proposed by Wing and Gould (1979), emphasizes differences in the quality of children’s social initiations and responses. Three subgroups are defined: (1) “Aloof” children are characterized by a failure to approach others socially and a tendency to ignore or withdraw from others when approached. (2) “Passive” children, on the other hand, are responsive when approached and will remain socially engaged (albeit in a limited manner) as long as the other person maintains the interaction. (3) Finally, “active-but-odd” children are those that actively seek interaction with others but do so in an odd, awkward, and often overly persistent manner. Independent research has demonstrated that children with autism can be reliably classified into these three subgroups based on symptoms reported by parents and clinicians, that parents and clinicians show adequate interrater reliability, and that the subgroup classification is predictive of other types of behavior, including communication, toy play, and perseverative behavior (Castelloe & Dawson, 1993).
To classify children, during the structured play session used to diagnosis the autism group, children with autism were observed in a playroom equipped with a one-way mirror. A range of developmentally appropriate toys was in the room. During the play session, several probes that pertained to social behavior were utilized. At the start of the play session, the experimenter sat passively and did not initiate inter-action with the child to observe whether the child would spontaneously initiate interaction. After 3 min, the experimenter approached the child and initiated interaction by engaging in parallel play with an object to observe whether the child withdrew from the approach or interacted with the examiner. Later in the play session, the experimenter again approached the child and entered cooperatively into the child’s activity. Finally, the experimenter introduced a developmentally appropriate game that involved reciprocal interaction to observe whether the child would engage in sustained interaction when such interaction was structured by the experimenter. For verbally capable children, the experimenter also attempted to engage the child in sustained conversation about a topic in which the child was interested. Based on the child’s response to these probes, children with autism were rated by two independent raters (the experimenter and a second rater who observed the play session from behind a one-way mirror) as falling into one of the three social subgroups, “active-but-odd,” “passive,” or “aloof.” Interreliability was above .70 (kappa).
RESULTS
Group Comparisons of Performance on Neuropsychological Tasks
Figure 1 shows the mean performance levels of the autism, Down syndrome, and typically developing groups on the Delayed Non-Matching to Sample (DNMS), based on two measures: (1) the number of trials required before reaching criterion performance, and (2) the number of errors committed before reaching criterion performance. Note that whereas 73% of Down syndrome participants and 100% of typically developing children reached criterion performance (which reflected rule-learning ability), only 61% of children with autism did so, χ2(2, N = 59) = 8.89, p < .02. One-way analyses of variance (ANOVA) with nonverbal mental age entered as a covariate indicated that the autism group performed worse than the Down syndrome and typically developing groups on this task, both in terms of the errors committed, F(2, 55) = 7.87, p = .001, and the number of trials required, F(2, 55) = 5.43, p = .007. T test comparisons of the number of errors committed revealed that the participants with autism were significantly impaired on the DNMS relative to the Down syndrome and typically developing children, ts = −1.79 and −3.93, respectively, ps < .05.
Figure 1.
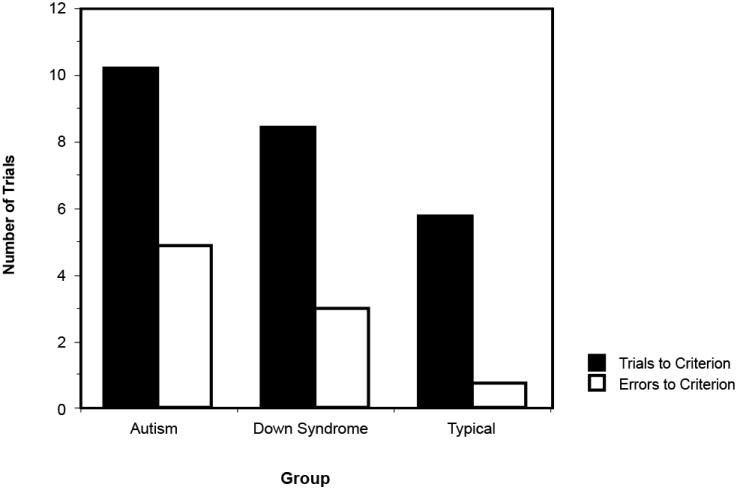
Performance levels of children with autism, Down syndrome, and typical development on the Delayed Non-Matching to Sample, as measured by the number of trials required before achieving criterion performance, and the number of errors committed before achieving criterion performance.
Figure 2 shows the mean performance levels of the autism, Down syndrome, and typically developing groups on the delayed response task. A one-way ANOVA with nonverbal mental age entered as a covariate indicated that the autism group also performed significantly worse than the Down syndrome and typically developing groups on this task in terms of the percentage correct searches on reversal trials, F(2, 55) = 3.68, p = .03. T test comparisons of the percent correct searches revealed that the participants with autism were significantly impaired on the delayed response relative to the Down syndrome and typically developing children, ts = 2.66 and 1.89, respectively, ps < .05.
Figure 2.
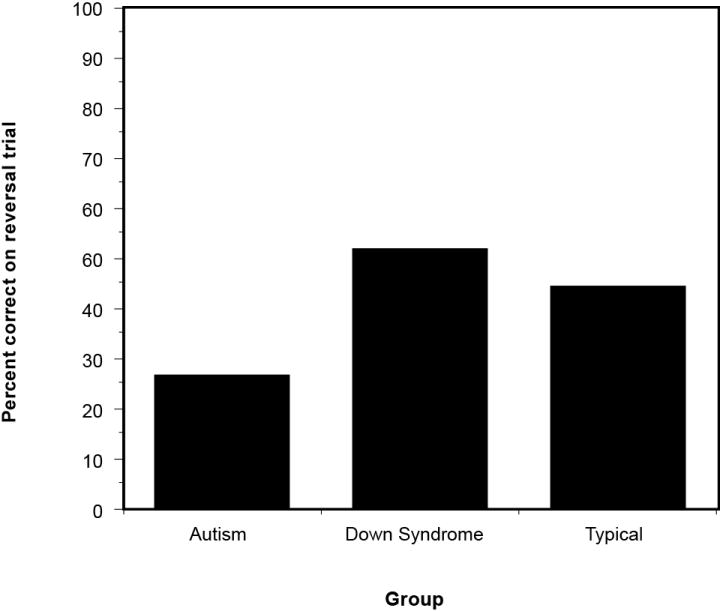
Performance levels of children with autism, Down syndrome, and typical development on the delayed response, as measured by the percentage of correct searches on reversal trials.
Group Comparisons of Performance on Tasks Assessing Autistic Symptoms
Table 2 displays the mean performance levels of the three groups of children on the behavioral tasks assessing early emerging core symptoms of autism: social orienting, immediate and deferred imitation, shared attention, response to distress, and symbolic play. In all domains, children with autism performed significantly worse than children with Down syndrome and typical development.
Table 2.
Performance on Tasks Assessing Autistic Symptom Domains
| Task | Autism | Down Syndrome | Typical Development | |
|---|---|---|---|---|
| Social orienting (# errors) | 1.86 (1.15) | .31 (.59) | .36 (.82) | F(2, 54) = 15.84, p < .001 |
| Immediate imitation (# imitative acts) | 5.1 (3.5) | 8.7 (2.6) | 7.6 (3.1) | F(2, 55) = 8.63, p = .001 |
| Deferred imitation (# imitative acts) | 2.7 (1.6) | 4.2 (1.4) | 4.5 (.8) | F(2, 55) = 12.95, p < .001 |
| Shared attention (# errors) | 1.48 (1.25) | .90 (.91) | .47 (.82) | F(2, 54) = 4.81, p = .01 |
| Response to distress (rating of concern) | 2.30 (.9) | 3.04 (.3) | 2.85 (.4) | F(2, 54) = 7.01, p = .002 |
| Symbolic play (# symbolic play acts) | 1.64 (1.15) | 2.50 (1.16) | 2.72 (.83) | F(2, 55) = 7.73, p = .001 |
Note: Numbers represent adjusted means and standard deviations (in parentheses).
Relations between Neuropsychological Performance and Severity of Autistic Symptoms and Social Subgroup Classification
For the group with autism, Table 3 displays the correlations between performance on the DNMS and delayed response tasks and the degree of impairment shown in the symptom domains and Wing social subgroup classification. Whereas strong and consistent correlations between DNMS performance and severity of autistic symptoms were found, only immediate imitation ability was found to be correlated with performance on the delayed response task. In addition, Wing subgroup classification, a classification that captures degree of social impairment, was correlated with DNMS performance, but not with delayed response performance.
Table 3.
Correlations between Severity of Autistic Symptoms and Neuropsychological Performance in Children with Autism (N = 20)
| Symptom Domain | Neuropsychological Task
|
|
|---|---|---|
| Delayed Non-Matching to Samplea | Delayed Responseb | |
| Social orienting | .58** | .07 |
| Immediate imitation | −.58** | .54* |
| Deferred imitation | −.81*** | .25 |
| Shared attention | .46* | .05 |
| Response to distress | −.46* | .39 |
| Symbolic play | −.72*** | .28 |
| Wing classification | .52* | −.30 |
Number of errors committed before achieving correct performance.
Percentage of correct searches on reversal trials.
p < .05;
p < .01;
p < .001.
The children with Down syndrome and typical development did not show consistent patterns of correlations between performance on the neuropsychological tasks and the tasks assessing autistic symptoms. For children with Down syndrome, no significant correlations were found between DNMS task performance and performance on tasks assessing autistic symptoms, and delayed response task performance was found to be correlated only with symbolic play ability, r(18) = .57, p < .05. For children with typical development, again, no significant correlations were found between DNMS task performance and performance on tasks assessing autistic symptoms, and delayed response task performance again was found to be correlated only with symbolic play ability, r(19) = .59, p < .01.
DISCUSSION
It was found that children with autism were impaired on a task tapping the medial temporal lobe and related limbic structures (DNMS) and on a task tapping the dorsolateral prefrontal cortex (delayed response), as compared with children with Down syndrome and typical development. As would be expected, children with autism also showed significant impairments in all autistic symptom domains compared with Down syndrome and typically developing children. These symptom domains included social orienting, immediate and deferred imitation, shared attention, responses to emotional cues, and symbolic play. Of most interest, however, was the finding that, for children with autism, degree of impairment in these symptom domains was strongly and consistently correlated with performance on the DNMS, but not consistently so with the delayed response. Moreover, DNMS performance, but not delayed response performance, was found to be significantly related to degree of social impairment as measured by the Wing social subgroup classification.
A significant relation between the delayed response and the severity of autistic symptoms was found only for one domain: immediate imitation. The dorsolateral prefrontal cortex is important for motor planning and therefore may contribute to impairments in immediate motor imitation ability. Interestingly, however, the ability to later recall and reproduce events, as reflected in deferred motor imitation ability, was highly correlated with DNMS, r(19) = .81, p < .000, but not delayed response, r(19) = .25, ns, performance. This pattern of results fits well with theories regarding the type of memory system tapped by deferred imitation (Meltzoff, 1988b) and with work by McDonough et al. (1994) showing severely impaired performance on deferred imitation tasks in adult patients with lesions of the medial temporal lobe including the hippocampus. In general, our results suggest that severity of early behavioral impairments in autism may be linked to functioning of the medial temporal lobe and related limbic structures, specifically, the orbital prefrontal cortex. It is important to point out that, because the present study used a short delay period with the DNMS, the rule-learning aspect of the DNMS was emphasized. A recent study has suggested that the orbital frontal cortex may be responsible for acquisition of the DNMS rule that a novel stimulus is associated with reward (Meunier et al., 1997).
It is noteworthy that DNMS performance was highly correlated with all symptom domains for children with autism, but not so for the two comparison groups of children. One possible explanation for this pattern of results is a developmental one. According to this view, it may be that the integrity of the medial temporal lobe and related brain structures is important for certain developmental prerequisites for the symptom domains assessed (Dawson, 1996). Such developmental prerequisites might include, for example, face recognition, recognition of the affective significance of stimuli, perception of body movement, and cross-modal associations. For children with autism, failure to achieve these prerequisites may interfere with the development of motor imitation, joint attention, and so on. In contrast, for the Down syndrome and typically developing children, because the contribution of limbic structures was critical for an ability that was acquired earlier than the domains assessed (i.e., for the prerequisites rather than for the skills actually assessed), no relation between DNMS performance and behavioral functioning would be expected at this later point in development. In other words, the functional integrity of the medial temporal lobe and related limbic structures may be critical for the very early development of social perception and cognition, and may thus help to explain the core symptoms of autism that are apparent by early childhood. Two specific examples serve to further illustrate this idea. Impairments in the ability to recognize the affective significance of stimuli, which has been associated with the amygdala (LeDoux, 1987), may help to explain why children with autism fail to orient to social stimuli, such as a person’s face or speech. Shared attention skills, such as alternating gaze between a toy and another’s face or visually referencing another’s face while pointing to an object, would presumably require that the child be interested in attending to another person. Impairments in the ability to recall event sequences, an ability that has been related to the hippocampus (McDonough et al., 1994), may help to explain why children with autism have difficulty imitating the motor actions of others, especially deferred imitation. A failure to participate in social imitative interactions may preclude the development of other social skills, such as social reciprocity and empathy (Dawson & Lewy, 1989; Meltzoff & Gopnik, 1993).
In future research, it will be important to explore in more detail the exact nature of early neuropsychological impairments in autism. Although the clinical and experimental literature indicates that performance on the DNMS is severely affected by damage to the amygdala and hippocampus,2 data in monkeys have also demonstrated that performance on this task is significantly affected by damage to other brain regions, most importantly, the orbital frontal cortex, as mentioned above (Bachevalier & Mishkin, 1986; Kowalska et al., 1991; Meunier et al., 1997). Thus, poor performance on this task may reflect medial temporal lobe dysfunction, orbital frontal dysfunction, or both. These brain regions are part of the limbic system and are both intimately involved in social behavior, including social cognition, the regulation of emotions, and social interactions (Barbas, 1995). An imaging study using SPECT associated theory-of-mind ability with the orbital frontal cortex (Baron-Cohen et al., 1994). A limitation of the present study is that only one measure each of limbic and dorsolateral prefrontal functioning was used. Nevertheless, the weight of the evidence suggests that these tasks are indeed reflective of these distinct brain regions. A question for future research will be to determine the specificity and variability of neuropsychological impairments in autism by using a wider range of neuropsychological tasks.
Another question that remains to be addressed is whether or not early dysfunction of the limbic system, if found to be present in autism, disrupts the normal development of higher cortical functions. Engagement in early social experiences, which may be supported by the medial temporal lobe, may facilitate the acquisition of higher cortical functions that are experience-dependent (Dawson, 1994). Given that subcortical dysfunction may result in such secondary effects on higher cortical development, and the natural tendency of the brain to reorganize and compensate for early dysfunction, studies of brain function in very young children with autism may be critical for shedding light on the primary nature of brain dysfunction in autism, and on issues of brain plasticity in autism.
The possibility that there may exist neuropsychological subgroups in autism should also be considered (Dawson, 1996). It is unclear from the present study whether the variability in performance on the DNMS found in the children with autism reflects a continuum of severity in limbic dysfunction (Bachevalier, 1994) or distinct subgroups of children, some of whom may have little or no involvement of subcortical structures, such as the amygdala and hippocampus (see Dawson, 1996, and Waterhouse, Fein, & Modahl, 1996, for discussion of the subgroup hypothesis). Knowledge about the nature of early brain dysfunction, its variability across children, and the plasticity of the dysfunctional brain ultimately will allow us to develop more individualized and effective methods of early intervention for children with autism.
Acknowledgments
This research was funded by grants from the National Institute of Neurological Disorders and Stroke (RO1NS26678) and the National Institute of Child Health and Human Development and the National Institute on Deafness and Communication Disorders (PO1HD34565). We gratefully acknowledge an anonymous reviewer whose comments were very helpful, the parents and their children who participated in this study, and several other people who made significant contributions to this research: Cathy Brock, Emily Brown, Craig Harris, Calle Fisher, and several undergraduate research assistants.
Footnotes
The central role of the amygdala and hippocampus in visual recognition memory has been disputed based on evidence that the adjacent rhinal and perirhinal cortices may be more directly responsible for this type of memory (see Zola-Morgan & Squire, 1993, for review).
See note 1 above.
Contributor Information
Geraldine Dawson, University of Washington.
Andrew N. Meltzoff, University of Washington
Julie Osterling, University of Washington.
Julie Rinaldi, University of Washington.
References
- Aggleton JP. The functional effects of amygdala lesions in humans: A comparison with findings from monkeys. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 485–503. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: APA; 1987. [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: A review of clinical and experimental findings. Neuropsychologia. 1994;32:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behavioral Brain Research. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: Toward an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Reviews. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition and mental state terms: Clinical findings in children with autism and a functional neuroimaging study of normal adults. British Journal of Psychiatry. 1994;165:640–649. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Tager-Flusberg H, Cohen D. Understanding other minds: Perspectives from autism. Oxford: Oxford University Press; 1993. [Google Scholar]
- Barth C, Fein D, Waterhouse L. Delayed match-to-sample performance in autistic children. Developmental Neuropsychology. 1995;11:53–69. [Google Scholar]
- Bauman M, Kemper AT. Neuroanatomic observations of the brain in autism. In: Bauman ML, Kemper TL, editors. The neurology of autism. Baltimore: Johns Hopkins University Press; 1994. pp. 119–145. [Google Scholar]
- Brothers LA. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Brothers LA, Ring BD, Kling AS. Response of temporal lobe neurons to social stimuli in Maraca arctoides. Society of Neuroscience Abstract. 1990;16:184. [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: Spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Castelloe P, Dawson G. Subclassification of children with autism and pervasive developmental disorder. Journal of Autism and Developmental Disorders. 1993;23:229–241. doi: 10.1007/BF01046217. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes error: Emotion, reason and the human brain. New York: G. P. Putnam’s Sons; 1994. [Google Scholar]
- Davies S, Bishop D, Manstead ASR, Tantam D. Face perception in children with autism and asperger’s syndrome. Journal of Child Psychology and Psychiatry. 1994;35:1033–1057. doi: 10.1111/j.1469-7610.1994.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Dawson G. Development of emotional expression and emotion regulation in infancy: Contributions of the frontal lobe. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 346–378. [Google Scholar]
- Dawson G. Neuropsychology of autism: A report on the state of the science. Journal of Autism and Development Disorders. 1996;26:179–184. doi: 10.1007/BF02172008. [DOI] [PubMed] [Google Scholar]
- Dawson G, Lewy A. Reciprocal subcortical-cortical influences in autism: The role of attentional mechanisms. In: Dawson G, editor. Autism: Nature, diagnosis and treatment. New York: Guilford; 1989. [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally-occurring social stimuli. Journal of Autism and Developmental Disorders. doi: 10.1023/a:1026043926488. in press. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparative development in human infants and infant rhesus monkeys of cognitive functions that depend on prefrontal cortex. Society for Neuroscience Abstracts. 1986;12:742. [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget’s AB task: Evidence for dependence on dorsolateral prefrontal cortex. Experimental Brain Research. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Diamond A, Zola-Morgan S, Squire LR. Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human in. Behavioral Neuroscience. 1989;103:526–537. doi: 10.1037//0735-7044.103.3.526. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Mishkin M. Evidence for behavioral impairment following prefrontal lobectomy in the infant monkey. Journal of Comparative Physiology and Psychology. 1970;70:454–463. doi: 10.1037/h0028701. [DOI] [PubMed] [Google Scholar]
- Hobson P, Ouston J, Lee A. What’s in a face? The case of autism. British Journal of Psychology. 1988a;79:441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hobson P, Ouston J, Lee A. Emotion recognition in autism: Coordinating faces and voices. Psychological Medicine. 1988b;18:911–923. doi: 10.1017/s0033291700009843. [DOI] [PubMed] [Google Scholar]
- Jacobson R. Case report: Disorders of facial recognition, social behaviour and affect after combined bilateral amygdalotomy and subcaudate tractotomy—a clinical and experimental study. Psychological Medicine. 1986;16:439–350. doi: 10.1017/s0033291700009272. [DOI] [PubMed] [Google Scholar]
- Kowalska D, Bachevalier J, Mishkin M. The role of the inferior prefrontal convexity in performance of delayed nonmatching-to-sample. Neuropsychologia. 1991;29:583–600. doi: 10.1016/0028-3932(91)90012-w. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion. In: Mountcastle V, Plum F, Geiger S, editors. Handbook of physiology. Vol. 5. Bethesda, MD: American Physiology Society; 1987. [Google Scholar]
- LeDoux JE. Scientific American. 1994. Jun, Emotion, memory and the brain; pp. 50–54. [DOI] [PubMed] [Google Scholar]
- McDonough L, Mandlers JM, McKee RD, Squire LR. What amnesic patients forget that infants remember: The deferred imitation task as a nonverbal measure of declarative memory. 1994. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy RE, Rogers SJ, Pennington BF. Executive function and social communication deficits in young autistic children. Journal of Child Psychology and Psychiatry. 1993;14:563–578. doi: 10.1111/j.1469-7610.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Infant imitation and memory: Nine-months-olds in immediate and deferred tests. Child Development. 1988a;59:217–225. doi: 10.1111/j.1467-8624.1988.tb03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Infant imitation after a 1-week delay: Long term memory for novel acts and multiple stimuli. Developmental Psychology. 1988b;31:838–850. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Gopnik A. The role of imitation in understanding persons and developing a theory of mind. In: Baron-Cohen S, Tager-Flusberg H, Cohen DJ, editors. Understanding other minds. Oxford: Oxford University Press; 1993. pp. 335–366. [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Is autism an amnesic disorder? Evidence from the California Verbal Learning Test. Neuropsychology. 1993;7:209–216. [Google Scholar]
- Mundy P, Sigman M, Ungerer JA, Sherman T. Defining the social deficits in autism: The contribution of nonverbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Amygdalectomy impairs crossmodal association in monkeys. Science. 1985;228:604–606. doi: 10.1126/science.3983648. [DOI] [PubMed] [Google Scholar]
- Nelson CA, deHaan M. A neurobiological approach to the recognition of facial expressions in infancy. In: Russell JA, editor. The psychology of facial expression. Cambridge: Cambridge University Press; 1996. pp. 176–204. [Google Scholar]
- Ozonoff S, Pennington B, Rogers S. Executive function deficits in high-functioning autistic individuals: Relationship to theory-of-mind. Journal of Child Psychology and Psychiatry. 1991;32:1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Rast M, Meltzoff AN. Memory and representation in young children with Down syndrome; Exploring deferred imitation and object permanence. Development and Psychopathology. 1995;3:137–162. doi: 10.1017/S0954579400006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S, Pennington B. A theoretical approach to the deficits in infantile autism. Developmental and Psychopathology. 1991;3:137–162. [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS) New York: Irvington; 1986. [Google Scholar]
- Sigman M, Kasari C, Kwon J, Yirmiya N. Responses to the negative emotions of others by autistic, mentally retarded and normal children. Child Development. 1992;63:796–807. [PubMed] [Google Scholar]
- Smith I, Bryson S. Imitation and action in autism: A critical review. Psychological Bulletin. 1994;116:259–273. doi: 10.1037/0033-2909.116.2.259. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Survey form manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Squire LR, Zola-Morgan S, Chen KS. Human amnesia and animal models of amnesia: Performance of amnesic patients on tests designed for the monkey. Behavioral Neuroscience. 1988;102:210–221. doi: 10.1037//0735-7044.102.2.210. [DOI] [PubMed] [Google Scholar]
- Teunisse J, DeGelder B. Do autistics have a generalized face processing deficit? International Journal of Neuroscience. 1994;77:1–10. doi: 10.3109/00207459408986014. [DOI] [PubMed] [Google Scholar]
- Waterhouse L, Fein D, Modahl C. Neurofunctional mechanisms in autism. Psychological Review. 1996;103:457–489. doi: 10.1037/0033-295x.103.3.457. [DOI] [PubMed] [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Zimmerman S, et al. Preschool Language Scale—3. San Antonio, TX: Psychological Corp., Harcourt Brace Jovanovich; 1991. [Google Scholar]
- Zola-Morgan S, Squire LR. Neuroanatomy of memory. In: Cowan WM, Shooter EM, Stevens CF, Thompson RF, editors. Annual Review of Neuroscience. Vol. 16. 1993. pp. 547–563. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Lesions of the hippocampal formation but not lesions of the fornix or mammillary nuclei produce long-lasting memory impairment in monkeys. Journal of Neuroscience. 1989;9:897–912. doi: 10.1523/JNEUROSCI.09-03-00898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


