Abstract
Monocarboxylate transporters (MCTs) are known to mediate the transport of short chain monocarboxylates such as lactate, pyruvate and butyrate. Currently, fourteen members of this transporter family have been identified by sequence homology, of which only the first four members (MCT1- MCT4) have been shown to mediate the proton-linked transport of monocarboxylates. Another transporter family involved in the transport of endogenous monocarboxylates is the sodium coupled MCTs (SMCTs). These act as a symporter and are dependent on a sodium gradient for their functional activity. MCT1 is the predominant transporter among the MCT isoforms and is present in almost all tissues including kidney, intestine, liver, heart, skeletal muscle and brain. The various isoforms differ in terms of their substrate specificity and tissue localization. Due to the expression of these transporters in the kidney, intestine, and brain, they may play an important role in influencing drug disposition. Apart from endogenous short chain monocarboxylates, they also mediate the transport of exogenous drugs such as salicylic acid, valproic acid, and simvastatin acid. The influence of MCTs on drug pharmacokinetics has been extensively studied for γ-hydroxybutyrate (GHB) including distribution of this drug of abuse into the brain and the results will be summarized in this review. The physiological role of these transporters in the brain and their specific cellular localization within the brain will also be discussed. This review will also focus on utilization of MCTs as potential targets for drug delivery into the brain including their role in the treatment of malignant brain tumors.
Keywords: Monocarboxylate transporters, γ-hydroxybutyrate, brain, lactate
Introduction
Monocarboxylic acids play an important role in energy metabolism in various tissues such as skeletal muscle, heart, brain and red blood cells. Among these monocarboxylates, lactate which is the end product of glycolysis is particularly important. This pathway leads to intracellular accumulation of lactate which must be exported out as high levels of lactate result in inhibition of glycolysis. In addition, some of the tissues such as brain, heart and red skeletal muscle utilize lactate as a fuel for respiration, thus requiring its import into the cell [1, 2]. Monocarboxylate transporters facilitate the transport of lactate and other monocarboxylates and therefore play an important role in cellular metabolism. Proton dependent monocarboxylate transporters (MCTs; SLC16A) are a family of transport proteins that contain 14 members which were identified based on sequence homology [3]. Only 4 members of this transporter family (MCT1-4) have been identified as proton dependent MCTs which catalyze the transport of important monocarboxylates such as lactate, pyruvate, and ketone bodies [4]. Another transporter family that has been demonstrated to be involved in monocarboxylate transport is known as sodium coupled monocarboxylate transporters (SMCTs) which contains only two members, SLC5A8 and SLC5A12 [5-7]. MCTs have a ubiquitous distribution in the body when compared to SMCTs which are more limited in their distribution [7, 8]. Apart from endogenous moncarboxylates, MCTs are also involved in the transport of some exogenous drugs such as salicylate, valproic acid and atorvastatin [8]. Monocarboxylate transporters can significantly influence drug pharmacokinetics due to their presence in the kidney, intestine and brain. MCT1, MCT2 and MCT4 are expressed in the brain and play an important role in transport of endogenous monocarboxylates into and out of brain cells [9]. The present review summarizes the function and distribution of monocarboxylate transporters in the brain. The potential role of these transporters in drug delivery to the central nervous system will also be discussed with specific emphasis on γ-hydroxybutyrate (GHB) which has been shown to be a substrate for both MCTs and SMCTs [10-13].
Monocarboxylate Transporters
The presence of proton coupled MCTs was first recognized by lactate and pyruvate transport into human red blood cells with transport being significantly inhibited by α-cyano-4-hydroxycinnamate (CHC) [14-16]. Currently, this family of transporters contains 14 members out of which only 4 members (MCT1-MCT4) have been demonstrated to mediate the proton dependent transport of monocarboxylates such as lactate, pyruvate, and ketone bodies [3, 8]. They provide electroneutral co-transport of monocarboxylates along with protons in a stoichiometric ratio of 1:1. MCT8 is a thyroid hormone transporter and MCT10 is an aromatic amino acid transporter and is also known as T-type amino acid transporter1 (TAT1). The functional characterization of other members of this family has not been done and they are known as orphan transporters. MCTs have 12 transmembrane domains with C- and N-termini within the cytoplasm and an intracellular loop between TMDs 6 and 7 [17]. The conservation of sequence between different isoforms of the mammalian MCTs is the greatest for MCT1-4 whereas sequence is least conserved between other members of the family. The TMDs are highly conserved between the family members with high variations in the C- and N- termini including the intracellular loop [3]. The variations in the sequences of different isoforms may lead to differences in substrate specificity and regulation of MCTs [18]. The regulation of MCTs has been shown to occur both by transcriptional as well as post-transcriptional mechanisms [19, 20]. Although these proteins are not glycosylated, they require association with glycosylated protein, for their functional activity. This ancillary protein is called basigin or CD147 for MCT1 and MCT4 whereas MCT2 differs from its isoforms as it requires embigin instead of basigin for its functional activity [21]. The tissue distribution and substrate specificity of each MCT isoform has been outlined in Table 1. The key features of each functionally characterized MCT isoform will be further discussed in detail in this section.
Table 1. Tissue distribution and substrate specificity of various MCT and SMCT isoforms.
| Protein name | Unigene name | Tissue distribution | Cellular localization in brain | Predominant substrates | Transport mechanism | Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| MCT1 | SLC16A1 | Ubiquitous | Brain endothelial cells, astrocytes, some neurons in rats | Lactate, pyruvate, butyrate, acetoacetate, β-hydroxybutyrate, XP13512, GHB | H+ cotransporter or monocarboxylate exchanger | [8, 9, 87] |
| MCT2 | SLC16A7 | Liver, kidney, brain, testis, heart, spleen, pancreas | Neurons | Pyruvate, lactate | H+ cotransporter | [8, 9, 34] |
| MCT3 | SLC16A8 | Retinal pigment epithelium, choroid plexus | Lactate | H+ cotransporter | [8, 40, 43] | |
| MCT4 | SLC16A3 | Skeletal muscle, brain, kidney, placenta, leukocytes, heart, lung, chondrocytes | Astrocytes | Lactate, pyruvate, acetoacetate, β-hydroxybutyrate | H+ cotransporter | [8, 9, 44, 87] |
| MCT6 | SLC16A5 | Kidney, muscle, brain, heart, placenta, intestine, prostate, lung, pancreas | Bumetanide, nateglinide | Orphan | [8, 46] | |
| MCT8 | SLC16A2 | Liver, brain, heart, kidney, placenta, ovary, prosatate, thymus, pancreas | T3, T4 | Orphan | [8, 48, 49] | |
| MCT10 (TAT1) | SLC16A10 | Skeletal muscle, intestine, kidney, heart, liver, placenta | Aromatic amino acids (L-tryptophan, L-tyrosine, L-phenylalanine, L-DOPA | Facilitated diffusion/exchanger | [8, 50] | |
| SMCT1 | SLC5A8 | Intestine, kidney, brain, retina | Neurons | Lactate, pyruvate, butyrate, nicotinate, acetoacetate, β-hydroxybutyrate,α -ketoisocaproate, salicylates, benzoate, GHB | Na+ cotransporter | [5, 54, 88] |
| SMCT2 | SLC5A12 | Intestine, kidney, brain, retina | Astrocytes and glia | Lactate, pyruvate | Na+ cotransporter | [7, 54, 57] |
MCT1 (SLC16A1)
MCT1 was first identified as a mutation of the wild type protein which enhanced the uptake of mevalonate into Chinese-hamster ovary cells [22]. This protein has been shown to mediate inhibitor sensitive transport of monocarboxylates. MCT1 has now been cloned from mice, rats and humans and shows 95% sequence homology to Chinese-hamster ovary MCT1 [23-26]. The functional activity of MCT1 is dependent on a proton gradient and it acts as a proton dependent cotransporter/exchanger [27]. Transport was determined to follow an ordered, sequential mechanism through kinetic studies of lactate into red blood cells [16, 28]. A proton first binds to the transporter followed by binding of lactate. The proton and lactate are further translocated across the membrane with their sequential release on the other side. The return of the free transporter binding site across the membrane determines the net flux of lactate and thus forms the rate limiting step of transport. Transport can be stimulated by a pH gradient (low to high). The predominant role of MCT1 is to facilitate the unidirectional proton-linked transport of monocarboxylates across the plasma membrane. This may represent either influx or efflux of substrate depending of the intracellular and extracellular substrate concentrations and the existing pH gradient across the plasma membrane. However, MCT1 can also function as an exchanger, with transport occurring bidirectionally with the exchange of one monocarboxylate for another without the net movement of protons [3].
The substrate specificity of MCT1 has been extensively studied in red blood cells by measuring the inhibition of uptake of 14C-lactate [14]. It has been shown that MCT1 is responsible for the transport of a broad range of monocarboxylates including lactate, pyruvate, acetoacetate, β-hydroxybutyrate and GHB [1, 29]. These substrates exist as a monocarboxylate anion under physiological conditions, which is required for a MCT substrate. The Km value for transport decreases with increasing chain lengths of various monocarboxylates. Monocarboxylates that are substituted in the C-2 and C-3 positions with halides, hydroxyl, and carbonyl groups represent good substrates. The C-2 substitution is preferred over C-3, with the carbonyl group being especially favored. Monocarboxylates with longer branched aliphatic or aromatic side chains have also been found to bind to the transporter, but these are not easily released following translocation and may act as potent inhibitors [3]. Lactate transport has been found to be stereospecific with higher affinity for L-lactate when compared to D-lactate [27].
The inhibitors of MCT1 can be classified into three categories: (1) bulky or aromatic monocarboxylates such as 2-oxo-4-methyl-pentanoate, phenyl-pyruvate and α-cyano-4-hydroxycinnamate (CHC) which act as competitive inhibitors and are blockers of transport function of MCTs [30,31]; (2) amphiphilic compounds with divergent structures which include bioflavanoids such as quercetin and phloretin and anion transport inhibitors such as 5-nitro-2-(3-phenylpropylamino)-benzoate and niflumic acid; and (3) 4,40-substituted stilbene 2,20-disulphonates such as 4,40-diisothiocyanostilbene-2,20-disulphonate (DIDS) and 4,40-dibenzamidostilbene-2,20-disulphonate (DBDS) which act as reversible inhibitors of MCT1 in erythrocytes [32, 33]. It is important to note that CHC is not a specific MCT1 inhibitor and may inhibit one or more isoforms of MCTs. One of the important roles of MCT1 is the unidirectional transport of L-lactate (influx or efflux) which depends on the intracellular and extracellular lactate concentrations as well as the proton gradient across the membrane.
MCT2 (SLC16A7)
A second MCT isoform was cloned from a hamster liver cDNA library and was shown to have higher affinity for monocarboxylates than MCT1 [34-36]. This isoform was named MCT2 and was further characterized following the expression of rat isoform in Xenopus oocytes [37]. MCT2 shares 60% identity with MCT1. MCT2 has similar substrate specificity when compared to MCT1. It has also been shown to be inhibited by similar inhibitors such as CHC, DBDS and DIDS but it has been reported to be insensitive to the organomercurial reagent pCMBS [8, 34]. It has been shown that pCMBS inhibits MCT1 by binding to its associated ancillary protein basigin. This may be the reason for insensitivity to pCMBS as MCT2 has been shown to associate with embigin and not basigin [21, 37, 38]. MCT2 has also been cloned from rat, mouse and human tissues [35, 36]. The sequence of MCT2 is conserved to a lesser extent than MCT1 among these species which results in considerable species differences in the tissue distribution of this isoform [8]. MCT2 expression is limited in major human tissues whereas northern and western blot analysis have shown that this isoform is expressed in liver, kidney, brain and sperm tails in rat, mouse and hamster [8].
MCT3 (SLC16A8)
MCT3 has a very limited distribution and is found only in the basolateral membrane of the retinal pigment epithelium and the choroid plexus in humans, rodents and chickens [39]. The Km value of chicken MCT3 for lactate has been found to be around 6 mM in a yeast expression system [40]. It has also been found to be resistant against typical MCT inhibitors such as phloretin, CHC and pCMBS. Further information on substrate kinetics of this MCT isoform is not available and further studies are needed. Based on its localization, it is thought to be responsible for the export of lactate produced as a result of glycolysis from the retina [41, 42].
MCT4 (SLC16A3)
This isoform was initially named MCT3 based on sequence homology to chicken MCT3 but later was renamed as MCT4 [43]. It is mainly found in glycolytic tissues such as white skeletal muscle fibres, astrocytes, white blood cells, and chondrocytes [3, 8]. It has lower affinity for lactate and pyruvate than MCT1 and is believed to be involved in efflux of lactate from these tissues to prevent intracellular accumulation of lactate which would otherwise inhibit glycolysis [44]. This has been studied by expression of this transport protein in Xenopus oocytes [45]. It has a very high Km value for pyruvate (150 mM) which helps in preventing its loss from the cell.
MCT 6 (SLC16A5)
MCT6 was first identified by genomic and EST database screening and is predominantly expressed in the kidney and intestine [43]. It is known to transport pharmaceutical drugs such as bumetanide and nateglinide and does not transport short chain monocarboxylates like the other isoforms [46]. This isoform has also been shown to be present in the intestine implicating its role in drug absorption.
MCT 8 and MCT 10 (SLC16A2 and SLC16A10)
MCT8 was earlier known as XPCT (X-linked PEST containing transporter) because it contains a PEST domain in its N-terminal [47]. This isoform is also known as the thyroid hormone transporter. Substrate kinetic studies through expression in Xenopus oocytes demonstrated that MCT8 transports both the thyroid hormones (T3 and T4) with high affinity with Km values of 2-5 μM [48]. MCT8 is distributed in many tissues including liver, kidney, skeletal muscle, heart, brain, pituitary, and thyroid [49]. MCT10 is also known as TAT1 and was found to transport aromatic amino acids such as phenylalanine and tryptophan. It has also been expressed in Xenopus oocytes which demonstrated Km values of around 5 mM for aromatic amino acid substrates such as tryptophan, tyrosine, and phenylalanine [50]. MCT10 is expressed in a variety of tissues including intestine, kidney, liver, skeletal muscle, heart, and placenta [51]. Both MCT8 and MCT10 are known to mediate proton and sodium independent transport of their substrates. Delayed brain myelination which results in variable degrees of mental retardation, hypotonia, spasticity, ataxia and involuntary movements has been attributed to MCT8 deficiency in the brain [52]. Various tyrosine kinase inhibitors have been shown to noncompetitively inhibit MCT8 leading to reduced thyroid hormone uptake in brain. Hence tyrosine kinase inhibitors can lead to pharmacokinetic drug interactions leading to increased levothyroxine requirement of thyroidectomized patients [53].
Other isoforms of MCTs, MCT5, MCT7, MCT9, and MCT 11-14 have also been identified but their functional characterization has not been performed.
SMCT
The second transport family that is involved in the transport of monocarboxylates is the sodium coupled monocarboxylate transporters (SMCT), part of the solute carrier gene family SLC5. Only two members of this family have been identified as sodium dependent monocarboxylate transporters so far, namely SLC5A8 and SLC5A12 [54]. Characterization of SLC5A8 was done by its expression in Xenopus laevis oocytes and it has been shown to transport short chain monocarboxylates [5]. This transporter is dependent on the sodium gradient and typically transports multiple sodium ions along with monocarboxylates in a stoichiometric ratio of 3:1 making it electrogenic. SLC5A8 is expressed in normal colon tissue, and it functions as a tumor suppressor in human colon with silencing of this gene occurring in colon carcinoma. This transporter is involved in the concentrative uptake of butyrate and pyruvate produced as a product of fermentation by colonic bacteria. These are known to act as inhibitors of histone deacetylases, which supports its suppression in tumor cells [55]. SLC5A8 is also expressed in the brush border membrane of renal tubular cells where it has been suggested to mediate the active reabsorption of lactate and pyruvate to minimize their renal elimination and in the brain [56]. SLC5A8 is a higher affinity transporter when compared to MCT1 with Km values for lactate of 159 μM determined in Xenopus oocytes with heterologous expression of SLC5A8 [5]. The second member of this family, SLC5A12, has been found to be expressed in kidney and intestine with limited distribution in the brain. It is also found to mediate the sodium dependent transport of monocarboxylates but the transport is electroneutral, in contrast to SLC5A8. The affinity of this transporter is lower when compared with SLC5A8, but it exhibits very similar substrate specificity [7, 57].
Function of Monocarboxylate Transporters in the Brain
Transport of lactate across the plasma membrane is important under hypoxic conditions when glycolysis becomes the predominant pathway and also for tissues that rely on glycolysis to meet their normal energy demands [3]. Under hypoxic conditions, glycolysis results in the formation of lactate which needs to be exported out of the cell for continued glycolysis to occur [58, 59]. The transporters have lower affinity for pyruvate thus ensuring that it is not lost from the cell and further converted to lactate which results in regeneration of NAD+ and continued glycolysis. In the brain, glucose serves as the major energy source under normal conditions, but during prolonged starvation and diabetic ketoacidosis as observed in diabetes, other monocarboxylates such as lactate and ketone bodies (β-hydroxybutyrate and acetoacetate) become an important energy substrate and their transport into the brain is required [60-62]. The endothelial cells of the blood vessels in the brain have been reported to express MCT1 which probably mediates the transport of lactate and ketone bodies across the blood brain barrier (BBB) [63, 64]. The capacity of the brain to use ketone bodies such as β-hydroxybutyrate was found to increase in starvation and diabetes by 50-60% in rats [62]. This study also showed that BBB permeability to ketone bodies increased by both starvation and diabetes.
Under certain conditions such as hypoxia or ischemia, glycolysis is the only pathway for the production of ATP resulting in increased brain concentrations of lactate [3]. There are different isoforms of MCTs that are expressed in different subcellular regions of the brain with MCT1 and MCT4 being predominantly found in the astrocytes and MCT2 being the major isoform in the neurons [65]. This ensures export of lactate from astrocytes formed as a product of rapid glycolysis which is then taken up by the neurons to be used as a respiratory fuel for further oxidation [9]. Glucose is considered to be the predominant energy fuel for neurons. However, several studies have shown that neurons can efficiently utilize monocarboxylates, especially lactate as oxidative energy substrates in addition to glucose [66]. In contrast, astroglial cells are a major source of lactate and they predominantly metabolize glucose into lactate in the brain followed by lactate efflux [67]. In some cases, it has been shown that astrocytes can use lactate as an energy substrate, but to a very limited extent when compared to neurons [67]. The export of lactate along with a proton also helps in maintaining the intracellular pH by preventing cellular acidification. This has been demonstrated by disrupting the expression of MCT1 or MCT4 in astrocytes in the hippocampus of rats which resulted in loss of memory of learned tasks [68]. This loss in memory could be reversed by injecting L-lactate locally whereas the injection of glucose was not able to reverse this. Similar loss in memory in rats was obtained by disrupting MCT2 in neurons but this could not be reversed by injection of either L-lactate or glucose demonstrating that MCT2 is required for the uptake of these respiratory fuels into the neurons for proper functioning of the brain [68]. This is commonly known as the astrocyte-neuron lactate shuttle hypothesis. Exposure to glutamate has been shown to stimulate glucose utilization and the release of lactate by astrocytes [69]. This provides a coupling mechanism between neuronal activity and glucose utilization. It has also been demonstrated that certain neurotransmitters such as noradrenaline, vasoactive intestinal peptide and adenosine that activate glycogenolysis also increase lactate release [70].
MCTs are also involved in the uptake of ketone bodies in the neurons in conditions with low glucose utilization [8]. Neurons have the ability to oxidize lactate under both physiological and hypoxic conditions similar to heart and red skeletal muscle and they contain the same isoform of lactate dehydrogenase (LDH) as present in heart (LDH-1 subunit) [71]. The LDH-5 subunit (muscle type) is present in glycolytic tissues, favoring the formation of lactate from pyruvate whereas the LDH-l subunit (heart type) preferentially drives the reaction toward the production of pyruvate. It has been shown that LDH-1 subunits are present in neurons. However, LDH-5 subunit is predominantly present in the astrocytes [72]. This selective distribution of lactate dehydrogenase isoenzymes in astrocytes and neurons is consistent with the proposed astrocyte-neuron lactate shuttle.
The utilization of lactate and ketone bodies as energy substrates has been found to be higher in neonates when compared to adults and this is consistent with higher expression of MCT1 in neonates [59, 73, 74]. MCT1 expression in the membrane of capillary endothelium was found to be 25 times higher in 17-day suckling rat pups than adults using electron microscopic immunogold methods. This transporter was found to be equally distributed in both luminal and abluminal membranes [75]. These results were further confirmed by a report of high mRNA and protein expression of MCT1 in the BBB during suckling and reduction in expression with maturation [76]. This also explains the switch in fuel utilization from a combination of glucose, lactate and ketone bodies in the neonatal brain to complete dependence on glucose in adults. It has been shown in rodents that increased susceptibility of the neurons to acute severe hypoxia, which mimics the disorder of sleep apnea, is mediated by decreased expression of MCT2 in the neurons [77]. MCT1 and MCT4 have also been associated with the transport of short chain fatty acids such as acetate and formate which are then metabolized in the astrocytes [78].
Localization of MCTs in the Brain
MCTs are widely expressed in rat, mouse and human brain, both at the cellular and sub-cellular levels. MCT1 has a ubiquitous distribution in the body and is found in the liver, kidney, heart, muscle and brain [3]. Of all the identified isoforms of MCTs, it has been demonstrated that MCT1, MCT2 and MCT4 are expressed in the brain as depicted in (Fig. 1) [9]. The different subcellular regions of the brain express different MCT isoforms. The mRNA of MCT1 has been found in the cortex, hippocampus and cerebellum of adult rat brain [59, 76]. Earlier studies have shown that MCT1 is significantly expressed in cerebral blood vessels with specific localization on the endothelial cells on both luminal and abluminal membranes and ependymocytes lining the four brain ventricles in rats [73]. MCT1 was also found in the glial end feet surrounding capillaries [73, 75] and in brain parenchymal cells [73]. Confocal microscopy studies have also identified the expression of MCT1 in astrocytic processes both in vitro and in vivo [64, 79, 80]. Low expression of MCT1 has also been identified in specific subpopulations of neurons in adult rat brain such as those in the cerebral cortex, hippocampus, and hypothalamus [75]. However, MCT1 expression was not observed in the adult mouse brain neuron [64]. Recently, the absolute protein quantities of MCT1 have been determined in freshly isolated human brain microvessels from patients with epilepsy or glioma using quantitative RT-PCR and LC/MS/MS. The results of this study demonstrated the expression of MCT1 in these samples [81].
Fig. (1).
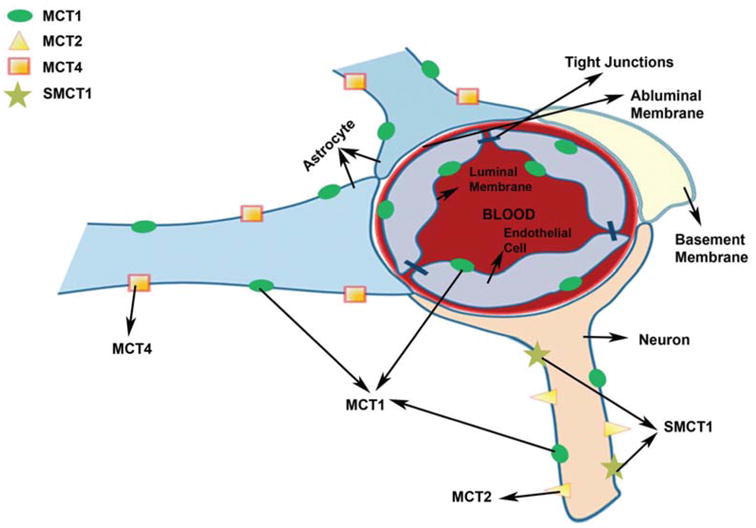
Cellular localization of different MCT isoforms in brain (adapted from Simpson et al. 2007) [125].
Regional distribution of MCT2 in the mouse brain includes cortex, hippocampus and cerebellum [59, 65, 80]. MCT2 is the major neuronal isoform as demonstrated by immunohistochemistry results with major localization in the postsynaptic densities of the neurons [80, 82, 83]. There was no co-localization of MCT2 immunoreactivity with presynaptic elements in the neuron. MCT2 has also been found in immunoreactivity in the postsynaptic membrane of parallel fibre-Purkinje cell synapses in the rat cerebellum and in the postsynaptic δ2-glutamate receptors as demonstrated by electron microscopy [63, 84]. In addition, its presence has also been demonstrated at both mRNA and protein levels in cultured neurons [80]. The expression of MCT2 was also observed in some populations of astrocytes in the white matter and glia but such presence was only detected in rat brain and cultured rat brain astrocytes [79, 85]. The mouse brain or the cultured mouse brain astrocytes failed to show such expression suggesting that there could be species differences in the distribution of MCT2 in the brain [64, 80, 83]. MCT2 has also been found in the Purkinje fibers of the cerebellum as demonstrated by immunohistochemistry [84]. In brain endothelial cells, the presence of MCT2 was only observed in a few studies and thus this still needs to be further examined [82, 86]. Although MCT2 expression has been demonstrated in rodent brain, very little MCT2 expression was observed in human brain as shown by Northern blotting results [43]. It is important to understand that there are some discrepancies in results obtained in different studies. This could be due to the differences in specificity of the antibodies used to identify the MCT isoforms which has been discussed in Bergersen et al. [84]. Species differences in MCT expression could also contribute to some of these differences. These discrepancies remain to be further evaluated in future studies.
MCT4 expression has been demonstrated in the astrocytes of adult rat and mouse brain in the cerebral cortex, striatum, hippocampus, paraventricular nucleus in the hypothalamus and capsula internalis [87]. MCT4 has been found to be exclusively expressed in the astrocytes [63, 84]. This is consistent with the high rate of glycolysis in astrocytes, thus requiring continuous efflux of lactate.
Studies have shown that a developmental switch exists in the expression of different MCT isoforms in various regions of the rat brain [76]. The mRNA and protein expression of MCT1 in the BBB has been found to be maximum during suckling followed by a decline with maturation in rats [75]. However, MCT2 found predominantly in the neurons shows constant expression during maturation, thus demonstrating that they play an important role in energy metabolism in the adult brain. In contrast, Pellerin et al have observed a decline in expression of both MCT1 and 2 during maturation by Northern blot analysis [87].
SMCT1 has recently been shown to be expressed exclusively in the neurons of mouse brain through immunofluorescence studies and it was reported to co-localize with MCT2 [88]. Studies in mixed cultures of rat brain neurons and astrocytes have also demonstrated its localization in the neurons. This suggests that SMCT1 can also play a role in the entry of lactate and other monocarboxylates into the neurons thus maintaining their energy status.
MCTs in Drug Disposition
Apart from their role in the transport of endogenous short chain monocarboxylates, MCTs also play a role in the transport of drugs such as valproic acid, salicylate, bumetanide, nateglinide, simvastatin and atorvastatin [8, 46]. The presence of these transporters in major organs such as kidney, liver, brain and intestine suggests that they may have a potential impact on the pharmacokinetics of substrate drug molecules. This may be due to the influence of these transporters on intestinal absorption, blood-brain and tissue transport, and the renal reabsorption of these drugs. In addition, due to the widespread distribution of MCT1 in various tissues, it may be targeted for drug delivery into specific tissues. Presence of MCTs at the BBB implies that they can serve as potential targets in order to achieve optimum delivery of their substrates into the brain. Earlier studies in rats have shown that acidic drugs such as valproic acid, benzoic acid, nicotinic acid or beta-lactam antibiotics including benzylpenicillin, propicillin and cefazolin could be transported into the brain utilizing a carrier mediated transport system in the BBB in a pH dependent manner with transport being significantly reduced in the presence of their respective unlabeled compounds [89]. The uptake of acetic acid was studied in primary cultured bovine brain capillary endothelial cells and was found to be significantly inhibited by a number of monocarboxylates including nicotinic acid further suggesting a role of MCTs in the transport of these monocarboxylates into the brain [90]. The uptake of nicotinate was also studied in primary cultures of astrocytes from rat cerebral cortex [91]. The nicotinate uptake was found to be saturable and pH dependent with uptake being significantly inhibited by CHC, suggesting that nicotinate uptake by rat astrocytes is mediated by protondependent monocarboxylate transport system. Recent studies in SMCT1 expressing Xenopus laevis oocytes, suggest the involvement of this transporter in nicotinic acid uptake [92], in addition to proton dependent MCTs. SMCT1-mediated uptake of nicotinate was found to be saturable and sodium dependent and significantly inhibited by lactate and pyruvate. As SMCT1 is expressed in neurons [88], it may play a role in neuronal uptake of this vitamin in the brain. A deficiency of nicotinic acid can cause serious neurological complications such as dementia, psychosis and ataxia which can be resolved through nicotinic acid supplementation. Dietary nicotinic acid has also been shown to have a protective effect on the development of Alzheimer disease and cognitive decline in a large prospective clinical study [93]. This suggests that the role of MCTs in mediating the entry of nicotinic acid into the brain may have clinical relevance in the treatment of neurological disorders.
HMG-CoA inhibitors such as simvastatin and lovastatin exhibit sleep disturbances as their side effect which suggests that they may cross the BBB. Also, such CNS side effects have been correlated with BBB permeability of these drugs using an in vivo brain perfusion technique [94]. In vitro studies utilizing primary cultures of bovine capillary endothelial cells showed that HMG-CoA inhibitors such as simvastatin in their acidic form are transported across the BBB through MCTs [95]. The lipophilic statins such as simvastatin acid, atorvastatin and lovastatin also have the potential to inhibit MCT4 in cell lines expressing this MCT isoform [96]. Recent studies suggest that statins can act as antioxidants mediated via free radical scavenger-like mechanism [97]. This function has been shown to be independent of their effects on cholesterol biosynthesis. Statins have been proposed as novel agents for the treatment of Alzheimer disease due to their antioxidant properties. A recent study demonstrated that treatment with atorvastatin significantly reduced lipoperoxidation, protein oxidation and nitration and also resulted in increased levels of glutathione in parietal cortex of aged beagles that represent a natural higher mammalian model of the disease [98]. This drug also resulted in upregulation of the inducible isoform of haemoxygenase (HO-1) which is an enzyme with significant neuroprotective activity. Thus, statins might be useful in the treatment of Alzheimer disease mediated by reduction of oxidative damage. Since the transport of statins in their acidic form across the BBB has been suggested to be mediated by MCTs [95], the MCT-mediated delivery of statins into the brain for the treatment of neurodegenerative disorders such as Alzheimer disease remains an important area of investigation.
SMCT1 has been shown to be involved in the transport of pharmaceutical drugs such as benzoate, salicylate, 5-aminosalicylate and γ- hydroxybutyrate (GHB). The Km values for these drugs range from 1-7 mM [54]. Non-steroidal anti-inflammatory drugs such as ibuprofen, ketoprofen, and fenoprofen do not serve as transportable substrates for this transporter but block the transport function of SMCT1 by competing with its substrates. The findings that ibuprofen can serve as a blocker of monocarboxylate transport by SMCT1 suggests potential drug-drug interactions with a potential influence on oral bioavailability and renal reabsorption of monocarboxylate drugs, owing to the expression of this transporter in these tissues, and remains to be investigated.
Human MCT6 has recently been isolated and has been found to transport bumetanide in a pH and membrane potential-sensitive manner but the transport is not dependent on proton gradient. The uptake of bumetanide in Xenopus oocytes expressing MCT6 was inhibited by drugs such as furosemide, probenecid, glibenclamide, and nateglinide [46]. This isoform is not involved in the transport of short chain monocarboxylic acids such as lactate and thus has different substrate specificity compared to other MCT isoforms that are involved primarily in the transport of short chain monocarboxylates.
MCTs may also be involved in the efflux of certain drugs across the BBB as illustrated by studies carried out with probenecid. Microdialysis studies suggest that the restricted entry of probenecid into the brain is due to MCT mediated efflux from the brain [99]. It has also been hypothesized that MCTs play a role in the efflux of 6-mercaptopurine, a drug used to treat acute myeloid leukemia [100]. This could be one of the reasons for CNS relapses observed in these patients, but such a role needs to be confirmed through further studies. Thus transport by MCTs may play an important role in transport of drugs across the BBB thereby playing an important role in drug disposition.
MCTs have been utilized for optimizing drug delivery through the oral route. This is illustrated by the development of XP13512, a novel prodrug of gabapentin which is designed to be absorbed throughout the intestine by the high capacity nutrient transporter MCT1 [101]. Gabapentin is an antiepileptic drug which is otherwise absorbed through low capacity solute transporters located in the upper small intestine. The bioavailability of gabapentin has been found to be dose dependent possibly due to the saturation of the transporters involved in its intestinal absorption at clinical doses, owing to their low capacity. This also leads to unpredictable exposure of the drug in patients and inefficient therapy. This drug also exhibits large inter-individual variability which could be due to differences in transporter expression in individuals [101]. The limitations in the oral absorption of this drug have been overcome by developing its prodrug, gabapentin enacarbil which is now approved under the trade name of Horizant. This prodrug was designed to be transported through two transporters in the intestine, sodium-dependent multivitamin transporter (SMVT) and MCT1 which are high capacity transporters and are expressed along the entire length of the intestine in rats and humans. At physiological pH values, gabapentin is present as a zwitterion and several studies have demonstrated that it is a substrate of the low capacity solute transporters that are expressed in intestine and BBB. Transport of gabapentin into the brain possibly involves L-type amino acid transporter, LAT-1 [101]. The prodrug, XP13512 was synthesized by the reversible masking of the amine group of gabapentin with acyloxyalkylcarbamate promoiety (Fig. 2) which yielded a monoanionic compound at physiological pH making it a potential substrate for monocarboxylate transporters. In vitro studies in Caco-2 cells and chinese hamster ovary cells overexpressing SMVT have demonstrated that this prodrug is a substrate for both MCT1 and SMVT [101]. In monkeys, the oral bioavailability of gabapentin following the administration of its prodrug was found to be 84.2% compared with 25.4% after a similar oral dose of gabapentin [102]. The exposure of gabapentin was 17 fold higher in rats and 34 fold higher in monkeys following intracolonic administration of the prodrug when compared to intracolonic gabapentin. In healthy human volunteers, the immediate release formulation of this prodrug resulted in a dose proportional exposure whereas the absorption of oral gabapentin decreased with increasing doses as shown in (Fig. 3). The extended release formulation of the prodrug was found to provide extended gabapentin exposure and higher oral bioavailability when compared to an equimolar dose of gabapentin (74.5% vs 36.6%) [103]. This suggests that MCTs may be targeted in order to optimize drug delivery into various tissues based on their widespread tissue distribution both in humans and rodents and high capacity for transport. Thus MCTs may play an important role in drug delivery to various tissues including transport across the BBB.
Fig. (2).
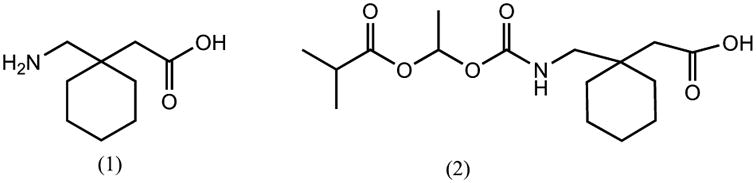
Structure of gabapentin (1) and its prodrug, XP13512 (2).
Fig. (3).
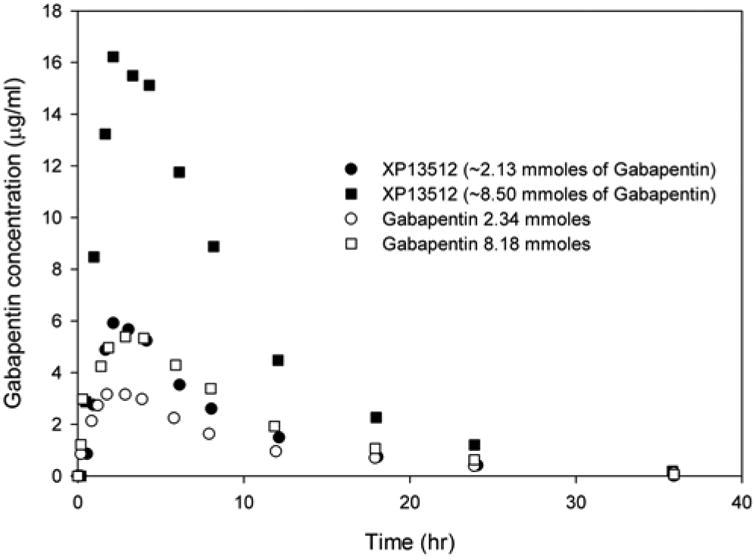
Mean concentrations of gabapentin in blood after oral administration of its prodrug, XP13512 immediate release capsules or oral gabapentin (data modified from Cundy et al 2008) [93].
There is very limited knowledge on the impact of MCTs on the pharmacokinetics of drugs that are substrates for such transporters. In addition, very few studies have examined the role of MCTs in the BBB transport of drugs and their potential use in drug delivery to the brain. One such drug where the influence of MCTs on drug pharmacokinetics has been extensively studied is γ-hydroxybutyrate (GHB). In the next section, we will discuss the impact of MCTs on the pharmacokinetics of GHB including its transport into the brain.
GHB is a naturally occurring short chain fatty acid present in the mammalian brain and is formed from γ-aminobutyric acid (GABA). It is also found in other tissues such as heart, liver and kidney [104]. It is approved in the United States for the treatment of narcolepsy associated with cataplexy, and in Europe for the treatment of alcohol withdrawal [105]. However, it is widely abused due to its sedative and euphoric effects [106]. It has also been used as a means of drug-facilitated sexual assaults. The pharmacological actions of GHB have been shown to be mediated by its binding to GABAB receptors. It is also known to bind to GHB receptors, and this binding is thought to mediate its physiological role in the body [106]. Overdose of GHB can lead to serious adverse effects such as nausea, sedation, dizziness, seizure, respiratory depression, hypothermia, coma and death [106]. There are numerous reports in the clinic of GHB-related fatality among drug abusers. Currently, there is no antidote for the treatment of GHB overdose and treatment is limited to supportive care.
GHB exhibits nonlinear pharmacokinetics in rats [107] and humans [108, 109] which is due to its capacity limited metabolism [107-110], saturable absorption [111] and carrier-mediated renal reabsorption [112]. The renal clearance of GHB increases with increasing dose. The saturable intestinal absorption and renal reabsorption is due to MCT-mediated transport of GHB [11, 113].
The transport mechanism of GHB across the BBB was investigated using in situ rat brain perfusion technique. The kinetics of GHB BBB transport was found to be a saturable carrier-mediated process with a Km value of around 11 mM [114]. This suggests that GHB transport into the brain involves a low affinity high capacity transporter protein. The transport of GHB was inhibited by short chain monocarboxylic acids such as lactate, pyruvate and β-hydroxybutyrate, known substrates of MCT1. The transport was also inhibited by CHC, a specific inhibitor of MCTs, suggesting that transport of GHB across the BBB is mediated by MCTs. GHB also inhibited the transport of benzoic acid, which is a well-known MCT substrate, further confirming the involvement of MCTs in the transport of these compounds. Administration of salicylic acid, a known substrate of MCTs, along with GHB was able to reduce GHB-induced sleep time in rats [115].
GHB distribution into the brain was recently investigated in our laboratory using in vivo microdialysis in rats. In vitro studies were also performed using rat (RBE4) and human brain endothelial cells (hCMEC/D3) to understand the BBB uptake of GHB. Both these cell lines are known to express MCTs. The uptake of GHB into these cells was found to be saturable, and pH and concentration dependent. GHB uptake exhibited typical Michaelis-Menten kinetics with a Km value around 23 mM in RBE4 cells (Fig. 4A) and 18 mM in hCMEC/D3 cells at pH 7.4 (Fig. 4B). The uptake of GHB into these cell lines was found to be significantly inhibited by CHC [116]. These data suggest the involvement of MCTs in GHB uptake into the brain. The unbound brain concentration of GHB was measured using microdialysis in frontal cortex of rat brain following intravenous dosing of GHB. The extracellular fluid (ECF) concentrations demonstrated some nonlinearity as the dose normalized concentrations for the lower GHB dose (400 mg/kg) did not overlap with those of the higher doses (600 and 800 mg/kg). However, the overall partition coefficient of GHB into the brain was not significantly different at the doses studied which suggested that the distribution of GHB into brain was not capacity limited at the doses studied. Although, based on the Km values that were obtained, the distribution of GHB into the brain could be saturated at higher concentrations such as those observed in overdose situations [116].
Fig. (4).
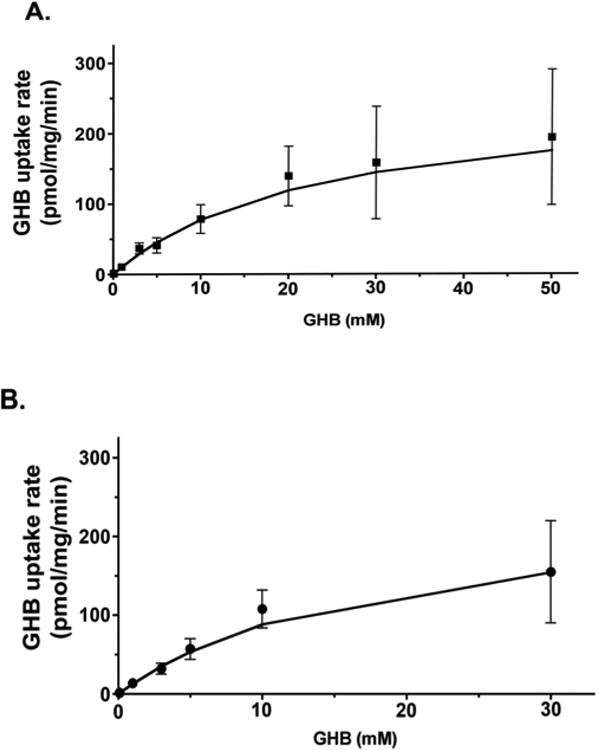
Concentration-dependent uptake of GHB in RBE4 (A) and hCMEC/D3 (B) cells. Uptake was performed at pH 7.4 by incubating cells with up to 50 mM GHB for 15 s at room temperature. Data displayed as mean ± S.D. of three experiments, each experiment performed in triplicate. (Figure taken from Roiko et al 2012 with permission) [116].
Unpublished data from our laboratory has shown that L-lactate administration as a bolus followed by a continuous intravenous infusion to rats treated with GHB resulted in a decrease in plasma as well as frontal cortex ECF concentrations when compared to GHB alone. The reduction in plasma and ECF GHB concentrations were greater with a higher dose of lactate. This higher lactate dose also significantly reduced GHB brain to plasma partition coefficient whereas no such change was observed with lower lactate doses. These data suggest that L-lactate at higher doses can alter the BBB transport of GHB at higher concentrations which can act as a potential treatment strategy for GHB overdose. The Km value for GHB uptake has been shown to increase at pH 7.4 when compared to pH 6.5 in red blood cells [117]. As the physiologically relevant pH at the BBB is 7.4, higher concentrations of lactate may be required to inhibit MCT-mediated transport of GHB across the BBB, compared with the intestine or kidneys. Consistent with the reduction in plasma and brain ECF concentrations of GHB, L-lactate also significantly reduced GHB induced sleep time measured as difference in return and loss of righting reflex. L-lactate was also able to inhibit GHB uptake into RBE4 cells in vitro at pH 7.4 at concentrations of 5 and 10 mM. The renal clearance of GHB was also increased by L-lactate administration due to inhibition of MCT-mediated active reabsorption in the proximal tubule of kidney as demonstrated previously. These results together suggest that the transport of GHB across the BBB is mediated by MCTs. Since MCT1 is the predominant transporter expressed in the BBB, it is most likely responsible for the observed effects. The knowledge of the transport mechanism of GHB and specific MCT isoforms involved in its entry into the brain can lead to the development of potential treatment strategies for its overdose.
MCTs in Brain Tumors
Malignant tumors are known to be highly dependent on glycolysis to meet their energy demands. As a result of glycolysis, lactate accumulates in such tumors leading to intracellular acidification. Lactate therefore needs to be continuously effluxed out of the tumor cells for continued glycolysis to occur facilitating the rapid differentiation of tumor cells. MCTs have been demonstrated to be the most important in mediating lactate efflux in highly metabolizing and glycolytic tumors thereby facilitating their rapid differentiation and proliferation [118]. Expression patterns in primary human brain tumors (Glioblastoma multiforme) and glioma-derived cell lines (U87- MG) showed the presence of MCT1 and MCT2 as the major MCT isoforms [119]. Small interfering ribonucleic acid (siRNA) specific for MCT1 and MCT2 resulted in decreased expression of these isoforms in U87-MG cells. Silencing of both MCT1 and MCT2 together led to a reduction in lactate efflux from these cells by 85% and a decrease in intracellular pH. Consistent with the proposed hypothesis, these authors observed significant cell death when both the MCT isoforms were silenced, demonstrated by a 92% reduction in cell viability. This hypothesis was tested in vivo in immunodeficient rats with stereotaxic intracranial implantation of the glioma cells to develop the tumor [120]. Intratumoral administration of a specific MCT inhibitor, CHC, resulted in tumor necrosis and 50% of the animals survived beyond the experimental targeted end point of 30 days after drug application with no tumor recurrence. These results suggest that targeting lactate efflux mediated by MCTs can serve as a promising treatment strategy for highly invasive brain tumors and may be of clinical relevance. Recent studies have shown that under hypoxic conditions present in tumors, the expression levels of MCT1 and MCT4 are upregulated as compared to cancer cells exposed to normoxia [121]. In fact, prolonged ischemia which also results in hypoxic conditions has also been shown to increase the expression of MCT8 mRNA in rat brain [122]. As MCTs are expressed throughout the brain, it is important to evaluate that normal energy metabolism in the brain is not disturbed due to global inhibition of MCTs. Again, isoform specific MCT inhibitors are needed in order to ensure normal energy metabolism owing to the importance of MCTs in cellular metabolism in various tissues.
Recently a class of specific and potent MCT1 inhibitors with nanomolar affinity has been developed by AstraZeneca and has shown to inhibit the proliferation of activated T-lymphocyte [123]. It is known that activated T-lymphocytes are highly dependent on aerobic glycolysis for their energy demands. The results of this study demonstrated a direct association of blockade of lactate efflux by MCT1 and inhibition of T-lymphocyte proliferation. This demonstrates that MCT1 can serve as a promising target for immunosuppressive therapy. Ovens et al characterized the properties of one of these inhibitors, AR-C155858 [124]. This inhibitor demonstrated Ki value of 2.3 nM which was measured by studying inhibition of L-lactate transport by MCT1 in rat erythrocytes. The application of such potent and isoform specific inhibitors in targeting MCTs at the BBB needs to be further investigated in order to develop pharmacologically useful therapies utilizing MCTs as potential targets for drug delivery into the brain.
Conclusion
The role of MCTs in cellular energy metabolism in various tissues including the brain is fairly well established. The knowledge about the localization and function of each isoform within the brain is important in understanding their role in mediating the transport of exogenous drug molecules that act as their substrates. Development of isoform specific inhibitors will allow us to determine the specific role of MCT isoforms in metabolic functions and as pharmacological targets for drug delivery into the brain. Recent studies show the utilization of such transporters to develop anticancer and immunosuppressant therapies. These transporters can also be probed in order to optimize delivery of drug molecules otherwise incapable of crossing the BBB. Based on the results obtained with GHB, the inhibition of these transporters represents a potential treatment strategy for overdose situations mediated by reduced distribution of GHB into the brain and increased renal elimination. Further studies on the effect of MCTs on the brain distribution of various drug molecules will lead to a better understanding of the effect of these transporters on BBB transport and development of potential drug delivery strategies for enhanced entry into the brain.
Acknowledgments
Support was provided by National Institutes of Health grant DA023223. NV received a graduate fellowship from Pfizer Global Research Inc.
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflicts of interest.
References
- 1.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993;264:C761–82. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 2.Juel C. Lactate-proton cotransport in skeletal muscle. Physiol Rev. 1997;77:321–58. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- 3.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343 Pt 2:281–99. [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem. 1996;271:861–8. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- 5.Coady MJ, Chang MH, Charron FM, et al. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol. 2004;557:719–31. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopal E, Fei YJ, Sugawara M, et al. Expression of slc5a8 in kidney and its role in Na(+)-coupled transport of lactate. J Biol Chem. 2004;279:44522–32. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas SR, Gopal E, Zhuang L, et al. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2) Biochem J. 2005;392:655–64. doi: 10.1042/BJ20050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–28. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 9.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Darling IM, Morris ME. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J Pharmacol Exp Ther. 2006;318:751–61. doi: 10.1124/jpet.106.105965. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Lu Y, Morris ME. Monocarboxylate transporter (MCT) mediates the transport of gamma-hydroxybutyrate in human kidney HK-2 cells. Pharm Res. 2007;24:1067–78. doi: 10.1007/s11095-006-9228-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Morris ME. The role of monocarboxylate transporter 2 and 4 in the transport of gamma-hydroxybutyric acid in mammalian cells. Drug Metab Dispos. 2007;35:1393–9. doi: 10.1124/dmd.107.014852. [DOI] [PubMed] [Google Scholar]
- 13.Cui D, Morris ME. The drug of abuse gamma-hydroxybutyrate is a substrate for sodium-coupled monocarboxylate transporter (SMCT) 1 (SLC5A8): characterization of SMCT-mediated uptake and inhibition. Drug Metab Dispos. 2009;37:1404–10. doi: 10.1124/dmd.109.027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deuticke B. Monocarboxylate transport in erythrocytes. J Membr Biol. 1982;70:89–103. doi: 10.1007/BF01870219. [DOI] [PubMed] [Google Scholar]
- 15.Halestrap AP. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976;156:193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Bruijne AW, Vreeburg H, Van Steveninck J. Kinetic analysis of L-lactate transport in human erythrocytes via the monocarboxylate-specific carrier system. Biochim Biophys Acta. 1983;732:562–8. doi: 10.1016/0005-2736(83)90232-8. [DOI] [PubMed] [Google Scholar]
- 17.Poole RC, Sansom CE, Halestrap AP. Studies of the membrane topology of the rat erythrocyte H+/lactate cotransporter (MCT1) Biochem J. 1996;320(Pt 3):817–24. doi: 10.1042/bj3200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juel C, Halestrap AP. Lactate transport in skeletal muscle - role and regulation of the monocarboxylate transporter. J Physiol. 1999;517(Pt 3):633–42. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. J Physiol. 2006;577:433–43. doi: 10.1113/jphysiol.2006.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chenal J, Pellerin L. Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. J Neurochem. 2007;102:389–97. doi: 10.1111/j.1471-4159.2007.04495.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70) J Biol Chem. 2005;280:27213–21. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 22.Kim CM, Goldstein JL, Brown MS. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of the point mutation responsible for its gain of function. J Biol Chem. 1992;267:23113–21. [PubMed] [Google Scholar]
- 23.Jackson VN, Price NT, Halestrap AP. cDNA cloning of MCT1, a monocarboxylate transporter from rat skeletal muscle. Biochim Biophys Acta. 1995;1238:193–6. doi: 10.1016/0005-2736(95)00160-5. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter L, Poole RC, Halestrap AP. Cloning and sequencing of the monocarboxylate transporter from mouse Ehrlich Lettre tumour cell confirms its identity as MCT1 and demonstrates that glycosylation is not required for MCT1 function. Biochim Biophys Acta. 1996;1279:157–63. doi: 10.1016/0005-2736(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 25.Takanaga H, Tamai I, Inaba S, et al. cDNA cloning and functional characterization of rat intestinal monocarboxylate transporter. Biochem Biophys Res Commun. 1995;217:370–7. doi: 10.1006/bbrc.1995.2786. [DOI] [PubMed] [Google Scholar]
- 26.Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–73. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 27.Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333(Pt 1):167–74. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bruijne AW, Vreeburg H, van Steveninck J. Alternative-substrate inhibition of L-lactate transport via the monocarboxylate-specific carrier system in human erythrocytes. Biochim Biophys Acta. 1985;812:841–4. doi: 10.1016/0005-2736(85)90280-9. [DOI] [PubMed] [Google Scholar]
- 29.Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008;10:311–21. doi: 10.1208/s12248-008-9035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974;138:313–6. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halestrap AP, Brand MD, Denton RM. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and alpha-ketoisocaproate. Biochim Biophys Acta. 1974;367:102–8. doi: 10.1016/0005-2736(74)90140-0. [DOI] [PubMed] [Google Scholar]
- 32.Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halestrap AP, Denton RM. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-Cyano-4-hydroxycinnamate and related compounds. Biochem J. 1975;148:97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–9. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 35.Jackson VN, Price NT, Carpenter L, Halestrap AP. Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochem J. 1997;324(Pt 2):447–53. doi: 10.1042/bj3240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin RY, Vera JC, Chaganti RS, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem. 1998;273:28959–65. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- 37.Broer S, Broer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341(Pt 3):529–35. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 39.Yoon H, Fanelli A, Grollman EF, Philp NJ. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem Biophys Res Commun. 1997;234:90–4. doi: 10.1006/bbrc.1997.6588. [DOI] [PubMed] [Google Scholar]
- 40.Grollman EF, Philp NJ, McPhie P, Ward RD, Sauer B. Determination of transport kinetics of chick MCT3 monocarboxylate transporter from retinal pigment epithelium by expression in genetically modified yeast. Biochemistry. 2000;39:9351–7. doi: 10.1021/bi000464+. [DOI] [PubMed] [Google Scholar]
- 41.Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am J Physiol. 1998;274:R1824–8. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- 42.Bergersen L, Johannsson E, Veruki ML, et al. Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience. 1999;90:319–31. doi: 10.1016/s0306-4522(98)00427-8. [DOI] [PubMed] [Google Scholar]
- 43.Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329(Pt 2):321–8. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350 Pt 1:219–27. [PMC free article] [PubMed] [Google Scholar]
- 45.Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol. 2000;529 Pt 2:285–93. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami Y, Kohyama N, Kobayashi Y, et al. Functional characterization of human monocarboxylate transporter 6 (SLC16A5) Drug Metab Dispos. 2005;33:1845–51. doi: 10.1124/dmd.105.005264. [DOI] [PubMed] [Google Scholar]
- 47.Lafreniere RG, Carrel L, Willard HF. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Hum Mol Genet. 1994;3:1133–9. doi: 10.1093/hmg/3.7.1133. [DOI] [PubMed] [Google Scholar]
- 48.Friesema EC, Ganguly S, Abdalla A, et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–35. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 49.Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem. 2001;276:17221–8. doi: 10.1074/jbc.M009462200. [DOI] [PubMed] [Google Scholar]
- 51.Meredith D, Christian HC. The SLC16 monocaboxylate transporter family. Xenobiotica. 2008;38:1072–106. doi: 10.1080/00498250802010868. [DOI] [PubMed] [Google Scholar]
- 52.Tonduti D, Vanderver A, Berardinelli A et al. MCT8 Deficiency: Extrapyramidal Symptoms and Delayed Myelination as Prominent Features. J Child Neurol. 2012 doi: 10.1177/0883073812450944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun D, Kim TD, le Coutre P, Kohrle J, Hershman JM, Schweizer U. Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J Clin Endocrinol Metab. 2012;97:E100–5. doi: 10.1210/jc.2011-1837. [DOI] [PubMed] [Google Scholar]
- 54.Ganapathy V, Thangaraju M, Gopal E, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–9. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Myeroff L, Smiraglia D, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A. 2003;100:8412–7. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barac-Nieto M, Murer H, Kinne R. Lactate-sodium cotransport in rat renal brush border membranes. Am J Physiol. 1980;239:F496–506. doi: 10.1152/ajprenal.1980.239.5.F496. [DOI] [PubMed] [Google Scholar]
- 57.Gopal E, Umapathy NS, Martin PM, et al. Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim Biophys Acta. 2007;1768:2690–7. doi: 10.1016/j.bbamem.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Prog Neurobiol. 2004;73:397–445. doi: 10.1016/j.pneurobio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci U S A. 1998;95:3990–5. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–87. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez E, Medina JM. Lactate utilization by the neonatal rat brain in vitro Competition with glucose and 3-hydroxybutyrate. Biochem J. 1986;234:489–92. doi: 10.1042/bj2340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hawkins RA, Mans AM, Davis DW. Regional ketone body utilization by rat brain in starvation and diabetes. Am J Physiol. 1986;250:E169–78. doi: 10.1152/ajpendo.1986.250.2.E169. [DOI] [PubMed] [Google Scholar]
- 63.Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. 2002;27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- 64.Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100:617–27. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- 65.Broer S, Rahman B, Pellegri G, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272:30096–102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- 66.Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 67.Itoh Y, Esaki T, Shimoji K, et al. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100:4879–84. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magistretti PJ, Sorg O, Yu N, Martin JL, Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev Neurosci. 1993;15:306–12. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- 71.McKenna MC, Tildon JT, Stevenson JH, Hopkins IB, Huang X, Couto R. Lactate transport by cortical synaptosomes from adult rat brain: characterization of kinetics and inhibitor specificity. Dev Neurosci. 1998;20:300–9. doi: 10.1159/000017325. [DOI] [PubMed] [Google Scholar]
- 72.Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16:1079–89. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E207–13. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- 74.Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J Neurochem. 1979;33:439–45. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- 75.Leino RL, Gerhart DZ, Drewes LR. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Dev Brain Res. 1999;113:47–54. doi: 10.1016/s0165-3806(98)00188-6. [DOI] [PubMed] [Google Scholar]
- 76.Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–34. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Guo SZ, Bonen A, et al. Monocarboxylate transporter 2 and stroke severity in a rodent model of sleep apnea. J Neurosci. 2011;31:10241–8. doi: 10.1523/JNEUROSCI.1462-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moschen I, Broer A, Galic S, Lang F, Broer S. Significance of Short Chain Fatty Acid Transport by Members of the Monocarboxylate Transporter Family (MCT) Neurochem Res. 2012 doi: 10.1007/s11064-012-0857-3. [DOI] [PubMed] [Google Scholar]
- 79.Hanu R, McKenna M, O'Neill A, Resneck WG, Bloch RJ. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am J Physiol Cell Physiol. 2000;278:C921–30. doi: 10.1152/ajpcell.2000.278.5.C921. [DOI] [PubMed] [Google Scholar]
- 80.Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–55. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- 81.Shawahna R, Uchida Y, Decleves X, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8:1332–41. doi: 10.1021/mp200129p. [DOI] [PubMed] [Google Scholar]
- 82.Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. Am J Physiol. 1998;275:E516–24. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- 83.Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–95. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 84.Bergersen L, Waerhaug O, Helm J, et al. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res. 2001;136:523–34. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- 85.Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia. 1998;22:272–81. [PubMed] [Google Scholar]
- 86.Mac M, Nalecz KA. Expression of monocarboxylic acid transporters (MCT) in brain cells. Implication for branched chain alpha-ketoacids transport in neurons. Neurochem Int. 2003;43:305–9. doi: 10.1016/s0197-0186(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 87.Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- 88.Martin PM, Gopal E, Ananth S, et al. Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J Neurochem. 2006;98:279–88. doi: 10.1111/j.1471-4159.2006.03878.x. [DOI] [PubMed] [Google Scholar]
- 89.Kang YS, Terasaki T, Tsuji A. Acidic drug transport in vivo through the blood-brain barrier. A role of the transport carrier for monocarboxylic acids. J Pharmacobiodyn. 1990;13:158–63. doi: 10.1248/bpb1978.13.158. [DOI] [PubMed] [Google Scholar]
- 90.Terasaki T, Takakuwa S, Moritani S, Tsuji A. Transport of monocarboxylic acids at the blood-brain barrier: studies with monolayers of primary cultured bovine brain capillary endothelial cells. J Pharmacol Exp Ther. 1991;258:932–7. [PubMed] [Google Scholar]
- 91.Shimada A, Nakagawa Y, Morishige H, Yamamoto A, Fujita T. Functional characteristics of H+ -dependent nicotinate transport in primary cultures of astrocytes from rat cerebral cortex. Neurosci Lett. 2006;392:207–12. doi: 10.1016/j.neulet.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 92.Gopal E, Fei YJ, Miyauchi S, Zhuang L, Prasad PD, Ganapathy V. Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochem J. 2005;388:309–16. doi: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morris MC, Evans DA, Bienias JL, et al. Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline. J Neurol Neurosurg Psychiatry. 2004;75:1093–9. doi: 10.1136/jnnp.2003.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saheki A, Terasaki T, Tamai I, Tsuji A. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res. 1994;11:305–11. doi: 10.1023/a:1018975928974. [DOI] [PubMed] [Google Scholar]
- 95.Tsuji A, Saheki A, Tamai I, Terasaki T. Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:1085–90. [PubMed] [Google Scholar]
- 96.Kobayashi M, Otsuka Y, Itagaki S, Hirano T, Iseki K. Inhibitory effects of statins on human monocarboxylate transporter 4. Int J Pharm. 2006;317:19–25. doi: 10.1016/j.ijpharm.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 97.Kassan M, Montero MJ, Sevilla MA. In vitro antioxidant activity of pravastatin provides vascular protection. Eur J Pharmacol. 2010;630:107–11. doi: 10.1016/j.ejphar.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 98.Barone E, Cenini G, Di Domenico F, et al. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: a novel mechanism of action. Pharmacol Res. 2011;63:172–80. doi: 10.1016/j.phrs.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deguchi Y, Nozawa K, Yamada S, Yokoyama Y, Kimura R. Quantitative evaluation of brain distribution and blood-brain barrier efflux transport of probenecid in rats by microdialysis: possible involvement of the monocarboxylic acid transport system. J Pharmacol Exp Ther. 1997;280:551–60. [PubMed] [Google Scholar]
- 100.Deguchi Y, Yokoyama Y, Sakamoto T, et al. Brain distribution of 6-mercaptopurine is regulated by the efflux transport system in the blood-brain barrier. Life Sci. 2000;66:649–62. doi: 10.1016/s0024-3205(99)00637-2. [DOI] [PubMed] [Google Scholar]
- 101.Cundy KC, Branch R, Chernov-Rogan T, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid]], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311:315–23. doi: 10.1124/jpet.104.067934. [DOI] [PubMed] [Google Scholar]
- 102.Cundy KC, Annamalai T, Bu L, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311:324–33. doi: 10.1124/jpet.104.067959. [DOI] [PubMed] [Google Scholar]
- 103.Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48:1378–88. doi: 10.1177/0091270008322909. [DOI] [PubMed] [Google Scholar]
- 104.Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–61. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 105.Mamelak M, Scharf MB, Woods M. Treatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findings. Sleep. 1986;9:285–9. doi: 10.1093/sleep/9.1.285. [DOI] [PubMed] [Google Scholar]
- 106.Okun MS, Boothby LA, Bartfield RB, Doering PL. GHB: an important pharmacologic and clinical update. J Pharm Pharm Sci. 2001;4:167–75. [PubMed] [Google Scholar]
- 107.Lettieri JT, Fung HL. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther. 1979;208:7–11. [PubMed] [Google Scholar]
- 108.Palatini P, Tedeschi L, Frison G, et al. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45:353–6. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- 109.Ferrara SD, Zotti S, Tedeschi L, et al. Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. Br J Clin Pharmacol. 1992;34:231–5. doi: 10.1111/j.1365-2125.1992.tb04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lettieri J, Fung HL. Improved pharmacological activity via pro-drug modification: comparative pharmacokinetics of sodium gamma-hydroxybutyrate and gamma-butyrolactone. Res Commun Chem Pathol Pharmacol. 1978;22:107–18. [PubMed] [Google Scholar]
- 111.Arena C, Fung HL. Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci. 1980;69:356–8. doi: 10.1002/jps.2600690331. [DOI] [PubMed] [Google Scholar]
- 112.Morris ME, Hu K, Wang Q. Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther. 2005;313:1194–202. doi: 10.1124/jpet.105.083253. [DOI] [PubMed] [Google Scholar]
- 113.Lam WK, Felmlee MA, Morris ME. Monocarboxylate transporter-mediated transport of gamma-hydroxybutyric acid in human intestinal Caco-2 cells. Drug Metab Dispos. 2010;38:441–7. doi: 10.1124/dmd.109.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhattacharya I, Boje KM. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther. 2004;311:92–8. doi: 10.1124/jpet.104.069682. [DOI] [PubMed] [Google Scholar]
- 115.Bhattacharya I, Boje KM. Potential gamma-hydroxybutyric acid (GHB) drug interactions through blood-brain barrier transport inhibition: a pharmacokinetic simulation-based evaluation. J Pharmacokinet Pharmacodyn. 2006;33:657–81. doi: 10.1007/s10928-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 116.Roiko SA, Felmlee MA, Morris ME. Brain uptake of the drug of abuse gamma-hydroxybutyric acid in rats. Drug Metab Dispos. 2012;40:212–8. doi: 10.1124/dmd.111.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morse BL, Felmlee MA, Morris ME. gamma-Hydroxybutyrate blood/plasma partitioning: effect of physiologic pH on transport by monocarboxylate transporters. Drug Metab Dispos. 2012;40:64–9. doi: 10.1124/dmd.111.041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–39. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 119.Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55:1410–9. doi: 10.1227/01.neu.0000143034.62913.59. discussion 1419. [DOI] [PubMed] [Google Scholar]
- 120.Colen CB, Shen Y, Ghoddoussi F, et al. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia. 2011;13:620–32. doi: 10.1593/neo.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng C, Edin NF, Lauritzen KH, et al. Alterations of monocarboxylate transporter densities during hypoxia in brain and breast tumour cells. Cell Oncol (Dordr) 2012;35:217–27. doi: 10.1007/s13402-012-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cui D, Guan Y, Jang H, Wang J, Xi L, Wang Q. Expression of monocarboxylate transporter 8 mRNA in the brain tissue of rats with cerebral ischemia. J Southern Med Univ. 2012;32:913–5. [PubMed] [Google Scholar]
- 123.Murray CM, Hutchinson R, Bantick JR, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol. 2005;1:371–6. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 124.Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10. Biochem J. 2010;425:523–30. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–91. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]


