Abstract
A small-molecule drug mimics the beneficial effects of adiponectin in cells and in animal models of diabetes and obesity.
Since its initial description in 1995 (1), few molecules in metabolism research have received as much continued interest as adiponectin. The focus of more than 12,000 publications, this adipokine has been widely studied in preclinical and clinical settings under a variety of physiological and pathophysiological conditions (2). Adiponectin is produced by adipocytes and released into circulation. It is considered protective, based on the potent insulin-sensitizing, anti-lipotoxic, anti-apoptotic, and anti-inflammatory actions it exerts on different cell types. These compelling effects marked adiponectin as a possible drug target for diabetes and other obesity-associated diseases. The long wait for a small-molecule agonist for adiponectin receptors may soon be over. Okada-Iwabu et al. (3) have identified a compound that is an adiponectin receptor agonist in rodent and cell culture models. It represents an important step toward filling an unmet clinical need for additional therapeutic options against diabetes, obesity, and other associated disorders.
Unlike the vast majority of other adipocyte-derived factors, adiponectin enjoys a reputation of being a “friendly” adipokine whose circulating concentration increases under metabolically favorable conditions and decreases under conditions of obesity-induced metabolic stress (as compared to other adipokines, adiponectin secretion is unusual; the more adipose tissue one has, the less adiponectin is found in circulation). Its actions on hepatocytes, endothelial cells, pancreatic β cells, and cardiac myocytes have been reported in rodent studies and are substantiated by clinical correlations. Indeed, mice that constitutively overexpress adiponectin are protected against metabolic challenges, including those imposed by a high-fat diet. A modest amount of adiponectin also conquered the genetic challenge of the ob/ob mouse, rescuing its diabetic phenotype (4). Adiponectin improved survival in mouse models of cell-type–specific apoptosis as well (5). Not only have genetic gain-of-function mutations in animals demonstrated the potent actions of this protein, but adiponectin produced in vitro from recombinant DNA can induce responses in animals that are comparable to those elicited by genetic overexpression of the adipokine (6,7).
Many of the cellular effects of adiponectin became better understood when the receptors for adiponectin, AdipoR1 and AdipoR2, were identified in 2003 (8). This spurred efforts to produce recombinant bioactive adiponectin (9). Although these preparations were active and insulin sensitizing, several issues made its large-scale production challenging. Adiponectin is difficult to produce in its full-length form in bacteria. It also requires several posttranslational modifications in its collagenous amino terminus, which can only be achieved if produced in mammalian cells. It is also a homo-oligomer that assembles into higher-order structures that consist of trimers, hexamers, and high molecular weight species of 12 to 36 oligomers that circulate in plasma as large complexes of ∼800 kD (10). And although adiponectin circulates at microgram per milliliter concentrations in plasma, it turns over quickly, with a half-life of 45 to 60 min in the mouse (11). The complex quaternary structure and rapid turnover are major disavantages to producing and administering adiponectin in amounts that can be sustained over time and in a cost-effective manner. Thus, the field has been awaiting the advent of low molecular weight agonists for adiponectin receptors that would overcome production bottlenecks.
Okada-Iwabu et al. screened a compound library and identified several molecules that activate adiponectin receptors but focused their in-depth analysis on one, “AdipoRon.” AdipoRon binds, at a low micromolar concentration, to both AdipoR1 and AdipoR2. Like adiponectin, it activates 5′-adenosine monophosphate–activated protein kinase (AMPK) in cultured mammalian cells, an enzyme that is involved in many metabolic processes including the release of insulin, inhibition of lipid synthesis, and stimulation of glucose uptake. It also activates the transcriptional coactivator peroxisome proliferator–activated receptor gamma coactivator 1–alpha (PGC1α), which boosts mitochondrial proliferation and energy metabolism. Like adiponectin, AdipoRon improved glucose metabolism, lipid metabolism, and insulin sensitivity in cultured cells and in mice by mechanisms requiring the presence of adiponectin receptors. When db/db mice (an animal model for type II diabetes and obesity) fed a high-fat diet were treated with AdipoRon (by oral administration), the metabolic improvements also extended their life span. Furthermore, the authors demonstrated that giving chow-fed wild-type mice AdipoRon enhanced their exercise endurance capacity. The study makes a convincing case that targeting adiponectin receptors with low molecular weight agonists is a viable strategy, and that developing higher-affinity agonists with improved pharmacokinetics should be pursued.
Adiponectin receptor activation is a promising potential therapy for diabetes, nonalcoholic fatty liver disease, cardiovascular disease, anti-inflammatory action in macrophages, and cytoprotective effects on pancreatic β cells (see the figure). Moreover, AdipoRon is orally administered and readily absorbed and delivered to relevant target tissues, making the approach even more attractive (some compounds are highly partitioned into certain tissues, are poorly absorbed, or are too rapidly altered to be of therapeutic benefit). The next steps should include evaluating whether AdipoRon stimulates a ceramidase activity in cells (thereby decreasing the amount of the sphingolipid ceramide), the most proximal cellular response to receptor activation (5,12). There are also several concerns that must be addressed. Notably, mice genetically engineered to overexpress adiponectin show reduced bone density. This is also true of molecules that stimulate adiponectin secretion in rodents (such as thiazolidinediones and fibroblast growth factor 21) (13,14). Furthermore, heart damage (left ventricular hypertrophy) has been observed in rodents upon chronic increase of adiponectin production. Lastly, adiponectin may promote adipogenesis and angiogenesis associated with weight gain and growth of established tumors, respectively (7). Chronically elevated adiponectin concentrations can trigger infertility (15). These are potential side effects that will need to be scrutinized in great detail.
Despite these concerns, AdipoRon does not promote weight gain in mice, and the reported observation of a prolonged life span in db/db mice helps obviate some of these concerns, even though many of these effects require a more chronic exposure to be manifest. Nevertheless, the weight-neutral improvements in insulin sensitivity evoked by this adiponectin mimetic represent an important proof-of-principle and pave the way for developing much needed therapies.
Adiponectin mimetic.
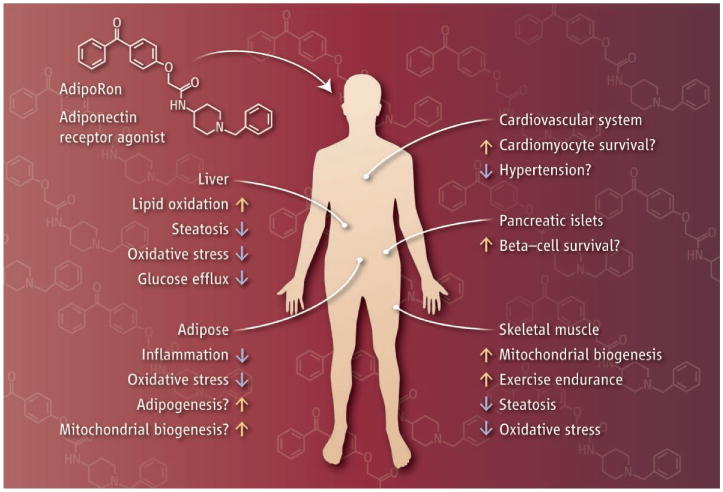
Muscle, heart, pancreas, liver, and adipocytes are key target tissues for adiponectin receptor activation. The small molecule AdipoRon has the potential to induce insulin sensitivity, as well as anti-inflammatory and anti-apoptotic effects, in a wide range of tissues due to the widespread expression of adiponectin receptors.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. J Biol Chem. 1995;270:26746. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Ye R, et al. Mol Metab. 2013;2:133. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada-Iwabu M, et al. Nature. 2013;503:493. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, et al. J Clin Invest. 2007;117:2621. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland WL, et al. Nat Med. 2011;17:55. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. Nat Med. 2001;7:947. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, et al. Nat Med. 2001;7:941. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, et al. Nature. 2003;423:762. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 9.Mancia F, et al. Structure. 2004;12:1355. doi: 10.1016/j.str.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Pajvani UB, et al. J Biol Chem. 2003;278:9073. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 11.Halberg N, et al. Diabetes. 2009;58:1961. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland WL, et al. Cell Metab. 2013;17:790. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ealey KN, Kaludjerovic J, Archer MC, Ward WE. Exp Biol Med. 2008;233:1546. doi: 10.3181/0806-RM-192. [DOI] [PubMed] [Google Scholar]
- 14.Wei W, et al. Proc Natl Acad Sci U S A. 2012;109:3143. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combs TP, et al. Endocrinology. 2004;145:367. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]


