In the United States, more than 1.2 million individuals are infected with HIV, approximately 200,000 remain undiagnosed, and 50,000 new infections occur annually.1 These estimates have not changed dramatically over the last 15 years, and new diagnoses seem to be on the rise in certain populations.1,2 Testing for HIV infection is the first in a series of important interventions aimed at impacting the epidemic. Identifying individuals with HIV infection provides a critical opportunity to link them into care where treatment with antiretroviral medications reduces viral concentrations, thus slowing disease progression and reducing infectivity.3,4 Also, knowing one’s serostatus is thought to attenuate individual behaviors that contribute to transmission of the virus.5
In 2006, the Centers for Disease Control and Prevention (CDC) dramatically shifted its HIV testing paradigm to recommend non–risk-based (i.e., nontargeted) opt-out HIV screening in health care settings where the undiagnosed prevalence was 0.1% or greater.6 This was accompanied by additional suggestions to limit testing barriers and resulted primarily from the following considerations: (1) the number of undiagnosed HIV infections in the United States had not significantly changed during the previous decade; (2) those with undiagnosed infections contributed significantly to forward transmission; and (3) nontargeted opt-out screening would result in larger numbers of individuals tested and identified earlier with HIV infection.7
In 2010, the Office of National AIDS Policy published the National HIV/AIDS Strategy for the United States, where, for the first time, the federal government took an aggressive stance in support of broad screening with goals of reducing the number of individuals with undiagnosed HIV infection to 10% (approximately 100,000 undiagnosed infections) and the number of annual new infections by 25% (approximately 37,500 new annual infections) by 2015.8 Subsequently, in 2013, the US Preventive Services Task Force updated their recommendations to support routine HIV screening based primarily on the consideration that morbidity and transmission may be significantly reduced after diagnosis and initiation of antiretroviral treatment.9
Although the premise of having all individuals know their HIV serostatus and those with HIV infection engaged in care cannot be argued as a critical public health need, our understanding of how best to achieve these results, especially as it relates to actual performance of HIV screening, is still significantly limited. In the nearly 8 years since the CDC’s current recommendation, substantial advocacy, policy, and research efforts have been put forth to help better understand how nontargeted screening should be used in practice. Unfortunately, implementation of these large prevention interventions has proven difficult, with only modest successes limited to relatively few institutions with dedicated resources.10
In this edition of Sexually Transmitted Diseases, Klein et al.11 contribute significantly to this conversation by reporting the impact of routine opt-out HIV screening in sexually transmitted disease (STD) clinics in North Carolina. This before-after study specifically compared nontargeted opt-out HIV screening to more traditional targeted opt-in HIV screening across 102 county-based STD clinics in their state, concluding that nontargeted opt-out screening did not significantly increase the number of patients tested for or newly diagnosed with HIV infection.
This study is unique in that it reports the comparative effectiveness of nontargeted HIV screening on a state wide level, using a multidimensional intervention, and robust analytic methods to account for public health surveillance data. The authors describe using a number of dissemination modalities including webinars, lectures, notices to health departments, contract addendums, and state wide conferences over a 3-month period to supplant targeted screening with nontargeted screening in accordance with the current CDC recommendations. Given that the investigators report essentially no change in the number of HIV tests performed or the number of newly diagnosed patients between the 2 study periods, we wonder whether the intervention was sufficient to change practice or, more likely, whether nontargeted opt-out HIV screening simply is not the panacea once envisioned, especially in this particular clinical setting.
The results reported in their article likely reflect how broad implementation of nontargeted opt-out HIV screening occurs in STD clinics. In a clinical setting where routine HIV screening is and has been commonplace, the incremental benefit of nontargeted screening is likely to be marginal at best, and their results convincingly show that it had no effect. In addition, although opt-out consent methods have been shown to increase proportions of patients who ultimately complete HIV testing when compared with opt-in consent,12,13 additional data indicate that patients are more likely to misunderstand consenting for HIV testing when using opt-out methods.13 Although it is impossible to tease out the individual effects of nontargeted screening and opt-out consent from their study, given the overall negative results, it is clear that neither component significantly impacted testing. Furthermore, a deeper evaluation of their results shows decreased HIV testing during the nontargeted screening period among populations considered most at risk, including males, racial/ethnic minorities (i.e., black and Hispanic), young adults, and those in high-density areas or those with high HIV burden. This finding is particularly troubling and potentially speaks to a saturation effect of testing among those most at risk or to negative effects of nontargeted opt-out screening itself among these populations.
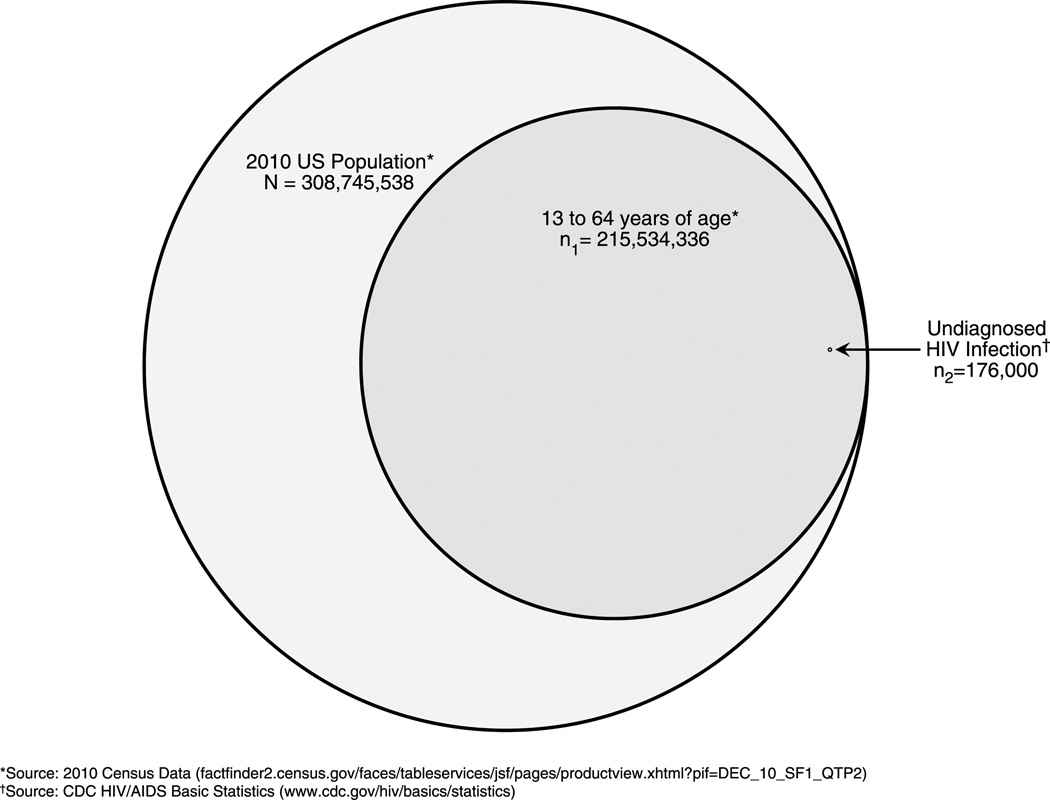
It is not surprising given the aggressive public health efforts put forth by the CDC that several prior surveillance studies have reported increases in HIV testing over the past decade.14–18 However, multiple prior studies, mostly conducted in emergency departments, have also demonstrated relatively limited impact of nontargeted HIV screening in a similarly high-risk clinical setting.10 Since 2006, 17 peer-reviewed studies have reported the feasibility and effectiveness of nontargeted screening in emergency departments, a clinical setting different from STD clinics but one that has also been a central focus of broad HIV screening efforts. In these studies, the median proportion of those eligible for testing who actually completed testing was only 18%, leaving more than 80% of those eligible untested (Table 1). Potentially contributing to this unsettling statistic, at least 2 studies have now reported that most individuals who opt out of HIV testing in emergency department settings do so because they believe they are not at risk.37,38 Although this is less likely in STD clinics where patients seek care specifically for infections, including HIV, the findings by Klein et al. support the notion that nontargeted screening has limited impact and that alternative and more focused HIV screenings methods may be warranted. In fact, as the proportion of undiagnosed HIV infection becomes less (in accordance with goals of the National HIV/AIDS Strategy), the efficiency of non–risk-based HIV screening will become less and we will likely have to shift back toward targeted strategies. Furthermore, of the 17 studies to date, only a few have compared nontargeted screening to alternative screening methods (in most cases, either targeted screening or diagnostic testing), and none have found nontargeted screening to be superior, in terms of identification of newly diagnosed HIV infection.38–40 A nonspecific screening strategy applied to more than 200 million “eligible” individuals to identify a vanishingly small proportion of those infected with HIV seems inefficient (Fig. 1).
TABLE 1.
Peer-Reviewed Studies (N = 17) Reporting the Effectiveness of Nontargeted HIV Screening in Emergency Departments Since 2006
| Authors | Year | Setting* | Consent Method† |
External Staff |
Eligible Patients‡, n |
Offered Testing |
Patients Tested |
Confirmed Positive |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||||
| Silva et al.19 | 2007 | U, I | OI | Y | 3030 | NR | — | 1428 | 47 | 8 | 0.56 |
| Mehta et al.20 | 2007 | U, A, I | OI | Y | NR | 2924 | — | 1428 | — | 8 | 0.56 |
| Brown et al.21 | 2007 | U, A, I | OO | Y | 13,240 | 4187 | 32 | 2486 | 19 | 9 | 0.36 |
| Walensky et al.22 | 2008 | U, A | OI | Y¶ | 2356 | 1397 | 59 | 854 | 36 | 5 | 0.59 |
| White et al.23 | 2009 | U, A, I | OI | N | 118,324 | 45,159 | 38 | 7923 | 7 | 55 | 0.69 |
| Haukoos et al.24 | 2010 | U, A, I | OO | N | 28,043 | NR | — | 6933 | 25 | 15 | 0.22§ |
| Sattin et al.25‖ | 2011 | U, A, I | OO | Y | 13,035 | 9343 | 72 | 8504 | 65 | 35 | 0.41 |
| Wheatley et al.26 | 2011 | U, A | OO | Y | NR | 8922 | — | 7616 | — | 129 | 1.7 |
| White et al.27** | 2011 | U, A, I | OO | N | 26,757 | 20,280 | 76 | 4679 | 18 | 21 | 0.45§ |
| White et al.27** | 2011 | U, A, I | OI | N | 23,236 | 6479 | 28 | 4053 | 17 | 8 | 0.20§ |
| Hoxhaj et al.28 | 2011 | U, A, I | OO | N | 24,686 | NR | — | 14,093 | 57 | 80 | 0.57§ |
| d’Almeida et al.29 | 2011 | MI | OI | N | 78,411 | 20,962 | 27 | 12,754 | 16 | 18 | 0.14§ |
| Wilbur et al.30 | 2011 | U, A, I | OI | Y | 5794 | 1484 | 26 | 1121 | 19 | 5 | 0.45 |
| Casalino et al.31 | 2012 | MI | OI | N | 183,957 | 11,401 | 6 | 7215 | 4 | 40 | 0.55§ |
| Haukoos et al.32** | 2012 | U, A, I | OO | N | 6842 | 6602 | 97 | 886 | 13 | 2 | 0.23§ |
| Haukoos et al.32** | 2012 | U, A, I | OI | N | 5985 | 5781 | 97 | 389 | 6 | 0 | 0 |
| Hack et al.33 | 2013 | U, A, P | OI | Y | 2645 | 300 | 11 | 224 | 8 | 0 | 0 |
| Haukoos et al.34 | 2013 | U, A, I | OI | N | 29,510 | 19,634 | 67 | 3591 | 12 | 7 | 0.20§ |
| Lyons et al.35 | 2013 | U, A, I | OI | Y | NR | 4692 | — | 1911 | 41 | 6 | 0.31§ |
| Median | 38 | 18 | 0.23§ | ||||||||
| Range | 6–97 | 4–65 | (0–0.57§) | ||||||||
Setting: U, urban; A, academic; I, level 1 trauma center; MI, multiple institutions; P, pediatric-only.
Consent method: OO, opt-out; OI, opt-in.
Eligibility varied by study.
Combined use of external staff and emergency department staff in a randomized manner.
Specifically indicates new HIV diagnoses.
Reports more complete results that overlap with a prior publication.36
Reported in the same study.
NR indicates not reported.
FIGURE 1.
Venn diagram representing the total number of undiagnosed HIV infections in the United States relative to the total population and the total number of those between 13 and 64 years of age as the recommended age range by the CDC for performing nontargeted screening. Areas represent precise proportions.
With this in mind, our group recently developed, validated, and preliminarily tested the comparative effectiveness of enhanced targeted HIV screening using an empirically derived clinical prediction instrument (Denver HIV Risk Score) to nontargeted HIV screening.40,42,43 The preliminary results demonstrate a significantly stronger association between enhanced targeted HIV screening and new HIV diagnoses than to nontargeted HIV screening in an urban emergency department.40 A multicenter clinical trial is currently underway to evaluate the broader comparative effectiveness of structured targeted screening versus nontargeted screening.44 The Denver HIV Risk Score includes only demographics (age, sex, race/ethnicity), 2 risk behaviors (sex with men and injection drug use), and history of HIV testing and has been shown to accurately stratify patients into 5 distinct risk groups from several different clinical settings, including emergency departments and STD clinics.
Although the study by Klein et al. does not provide details related to why HIV testing decreased among high-risk groups, it also raises the question as to whether a more structured risk-based approach would facilitate conversations about HIV testing, thus improving rates of testing and identification of newly diagnosed cases. Such standardized risk-based screening may also improve how clinicians identify patients for repeat screening, as recommended by the CDC for “high-risk” patients.6
Although HIV screening is feasible in clinical venues and will likely be the only way to achieve the goals set forth by the Office of National AIDS Policy, routine screening can take many forms and will likely need to vary depending on the venue. However, nontargeted HIV screening has had little impact even in settings where prevalence is highest. We must continue to work to understand which screening strategies are most effective at identifying patients with HIV infection. High-impact prevention must prioritize effectiveness and costs, feasibility of implementation, and coverage of target populations, and we believe that the study by Klein et al. provides more evidence in support of using more structured approaches to screen for HIV infection in health care settings.
Acknowledgments
This work was supported, in part, by an Investigator-Initiated Grant (R01AI105067) from the National Institute of Allergy and Infectious Diseases.
Financial disclosures: Dr Haukoos, Ms Hopkins, and Ms Bucossi are supported, in part, by the National Institute of Allergy and Infectious Diseases (R01AI106057).
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Author contributions: Dr Haukoos drafted the manuscript, and all authors contributed to critical revisions and important intellectual content.
REFERENCES
- 1.HIV in the United States. [Accessed April 7, 2014];2011 Available at: http://www.cdc.gov/hiv/resources/factsheets/PDF/us.pdf.
- 2.Prejean J, Song R, Hernandez A, et al. Estimated HIV Incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: A meta-analytic review. JAMA. 2004;292:224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 5.Marks G, Crepaz N, Senterfitt JW, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 6.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 7.Bartlett JG, Branson BM, Fenton K, et al. Opt-out testing for human immunodeficiency virus in the United States: Progress and challenges. JAMA. 2008;300:945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 8.National HIV/AIDS Strategy for the United States. [Accessed April 7 2014];2010 Available at: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 9.Moyer VA. U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;159:51–60. doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- 10.Haukoos JS. The impact of nontargeted HIV screening in emergency departments and the ongoing need for targeted strategies. Arch Intern Med. 2012;172:20–22. doi: 10.1001/archinternmed.2011.538. [DOI] [PubMed] [Google Scholar]
- 11.Klein P, Messer L, Myers E, et al. Impact of a routine, opt-out HIV teseting program on HIV testing and case detection in North Carolina sexually transmitted disease clinics. Sex Transm Dis. 2014;41:395–402. doi: 10.1097/OLQ.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White DA, Scribner AN, Martin ME, et al. A comparison of patient satisfaction with emergency department opt-in and opt-out rapid HIV screening. AIDS Res Treat. 2012;2012:904916. doi: 10.1155/2012/904916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haukoos JS, Hopkins E, Bender B, et al. Use of kiosks and patient understanding of opt-out and opt-in consent for routine rapid human immunodeficiency virus screening in the emergency department. Acad Emerg Med. 2012;19:287–293. doi: 10.1111/j.1553-2712.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Results of the expanded HIV testing initiative—25 jurisdictions, United States, 2007–2010. MMWR Morb Mortal Wkly Rep. 2011;60:805–810. [PubMed] [Google Scholar]
- 15.High-Impact HIV Prevention: CDC’s Approach to Reducing HIV Infection in the United States. [Accessed April 7, 2014];2011 Available at: http://www.cdc.gov/hiv/nhas/dhap/pdf/nhas_booklet.pdf.
- 16.Centers for Disease Control and Prevention. HIV testing among men who have sex withmen—21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60:694–699. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Expanded HIV testing and trends in diagnoses of HIV infection—District of Columbia, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59:737–741. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Rapid HIV testing in emergency departments—Three U.S, sites, January 2005–March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597–601. [PubMed] [Google Scholar]
- 19.Silva A, Glick NR, Lyss SB, et al. Implementing an HIV and sexually transmitted disease screening program in an emergency department. Ann Emerg Med. 2007;49:564–572. doi: 10.1016/j.annemergmed.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Mehta SD, Hall J, Lyss SB, et al. Adult and pediatric emergency department sexually transmitted disease and HIV screening: programmatic overview and outcomes. Acad Emerg Med. 2007;14:250–258. doi: 10.1197/j.aem.2006.10.106. [DOI] [PubMed] [Google Scholar]
- 21.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention Guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46:395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 22.Walensky RP, Arbelaez C, Reichmann WM, et al. Revising Expectations from Rapid HIV Tests in the Emergency Department. Ann Intern Med. 2008;149:153–160. doi: 10.7326/0003-4819-149-3-200808050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White DA, Scribner AN, Schulden JD, et al. Results of a Rapid HIV Screening and Diagnostic Testing Program in an Urban Emergency Department. Ann Emerg Med. 2009;54:56–64. doi: 10.1016/j.annemergmed.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Haukoos JS, Hopkins E, Conroy AA, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304:284–292. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 25.Sattin RW, Wilde JA, Freeman AE, et al. Rapid HIV Testing in a Southeastern Emergency Department Serving a Semiurban-Semirural Adolescent and Adult Population. Ann Emerg Med. 2011;58(Suppl 1):S60–S64. doi: 10.1016/j.annemergmed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Wheatley MA, Copeland B, Shah B, et al. Efficacy of an Emergency Department-Based HIV Screening Program in the Deep South. J Urban Health. 2011;88:1015–1019. doi: 10.1007/s11524-011-9588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White DA, Scribner AN, Vahidnia F, et al. HIV Screening in an Urban Emergency Department: Comparison of Screening Using an Opt-In Versus an Opt-Out Approach. Ann Emerg Med. 2011;58(Suppl 1):S89–S95. doi: 10.1016/j.annemergmed.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Hoxhaj S, Davila JA, Modi P, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med. 2011;58:S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 29.d’Almeida KW, Kierzek G, de Truchis P, et al. Modest Public Health Impact of Nontargeted Human Immunodeficiency Virus Screening in 29 Emergency Departments. Arch Intern Med. 2012;172:12–20. doi: 10.1001/archinternmed.2011.535. [DOI] [PubMed] [Google Scholar]
- 30.Wilbur L, Huffman G, Lofton S, et al. The Use of a Computer Reminder System in an Emergency Department Universal HIV Screening Program. Ann Emerg Med. 2011;58(Suppl 1):S71–S73 e1. doi: 10.1016/j.annemergmed.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Casalino E, Bernot B, Bouchaud O, et al. Twelve months of routine HIV screening in 6 emergency departments in the Paris area: results from the ANRS URDEP study. PLoS One. 2012;7:e46437. doi: 10.1371/journal.pone.0046437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haukoos JS, Hopkins E, Bender B, et al. Use of kiosks and patient understanding of opt-out and opt-in consent for routine rapid human immunodeficiency virus screening in the emergency department. Acad Emerg Med. 2012;19:287–293. doi: 10.1111/j.1553-2712.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 33.Hack CM, Scarfi CA, Sivitz AB, et al. Implementing routine HIV screening in an urban pediatric emergency department. Pediatr Emerg Care. 2013;29:319–323. doi: 10.1097/PEC.0b013e3182850910. [DOI] [PubMed] [Google Scholar]
- 34.Haukoos JS, Hopkins E, Bender B, Thrun MW, et al. Comparison of enhanced targeted rapid HIV screening using the Denver HIV risk score to nontargeted rapid HIV screening in the emergency department. Ann Emerg Med. 2013;61:353–361. doi: 10.1016/j.annemergmed.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons MS, Lindsell CJ, Ruffner AH, et al. Randomized Comparison of Universal and Targeted HIV Screening in the Emergency Department. J Acquir Immune Defic Syndr. 2013;64:315–323. doi: 10.1097/QAI.0b013e3182a21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman AE, Sattin RW, Miller KM, Dias JK, Wilde JA. Acceptance of rapid HIV screening in a southeastern emergency department. Acad Emerg Med. 2009;16:1156–1164. doi: 10.1111/j.1553-2712.2009.00508.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown J, Kuo I, Bellows J, et al. Patient perceptions and acceptance of routine emergency department HIV testing. Public Health Rep. 2008;123(suppl 3):21–26. doi: 10.1177/00333549081230S304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haukoos JS, Hopkins E, Conroy AA, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304:284–292. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 39.White DA, Scribner AN, Vahidnia F, et al. HIV screening in an urban emergency department: Comparison of screening using an opt-in versus an opt-out approach. Ann Emerg Med. 2011;58(suppl 1):S89–S95. doi: 10.1016/j.annemergmed.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Haukoos JS, Hopkins E, Bender B, et al. Comparison of enhanced targeted rapid HIV screening using the Denver HIV risk score to nontargeted rapid HIV screening in the emergency department. Ann Emerg Med. 2013;61:353–361. doi: 10.1016/j.annemergmed.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons MS, Lindsell CJ, Ruffner AH, et al. Randomized comparison of universal and targeted hiv screening in the emergency department. J Acquir Immune Defic Syndr. 2013;64:315–323. doi: 10.1097/QAI.0b013e3182a21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haukoos JS, Lyons MS, Lindsell CJ, et al. Derivation and validation of the Denver Human Immunodeficiency Virus (HIV) risk score for targeted HIV screening. Am J Epidemiol. 2012;175:838–846. doi: 10.1093/aje/kwr389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haukoos J, Lyons M, Rothman R, et al. Validation of the refined Denver HIV Risk Score using a national HIV testing cohort. Conference on Retroviruses and Opportunistic Infections; Boston, MA. March 2014.2014. [Google Scholar]
- 44.The HIV TESTED Trial Investigators. The HIV Testing using Enhanced Screening Techniques in Emergency Departments (TESTED) Trial, 2014. [Accessed April 7, 2014]; Available at www.DenverEDHIV.org. ClinicalTrials.gov Identifier: NCT01781949.