Introduction
Approximately 1 in 1000 pregnancies in the United States are plagued by concurrent neoplastic diseases. These account for the second leading cause of maternal mortality in the United States.1 The majority of these cases are related to solid tumors with only 25% of the cases related to hematologic malignancies.2 Hairy cell leukemia (HCL) is a relatively uncommon lymphoproliferative disorder.3 The outcome of patients with HCL has significantly improved with the introduction of purine nucleoside analogues including cladribine and monoclonal antibodies including rituximab. Remission rates of > 90% have been documented after just 1 course of therapy.4,5 The management of the pregnant patient with hematologic malignancies represents a diagnostic, therapeutic, and social challenge, requiring a multidisciplinary team approach.
There is scant published literature regarding treatment of HCL in pregnancy. The therapeutic efficacy, teratogenicity, and maternal toxicities of purine analogues and rituximab in pregnancy are poorly defined.6,7 Orlowski published the first report of successful pregnancy outcome after treatment of HCL with cladribine, suggesting that fertility may be preserved in some female patients exposed to purine analogues.8 However, to our knowledge this is the first report of a favorable outcome in a pregnant HCL patient treated sequentially with a monoclonal antibody (rituximab) followed by a purine analogue (cladribine).
Case Report
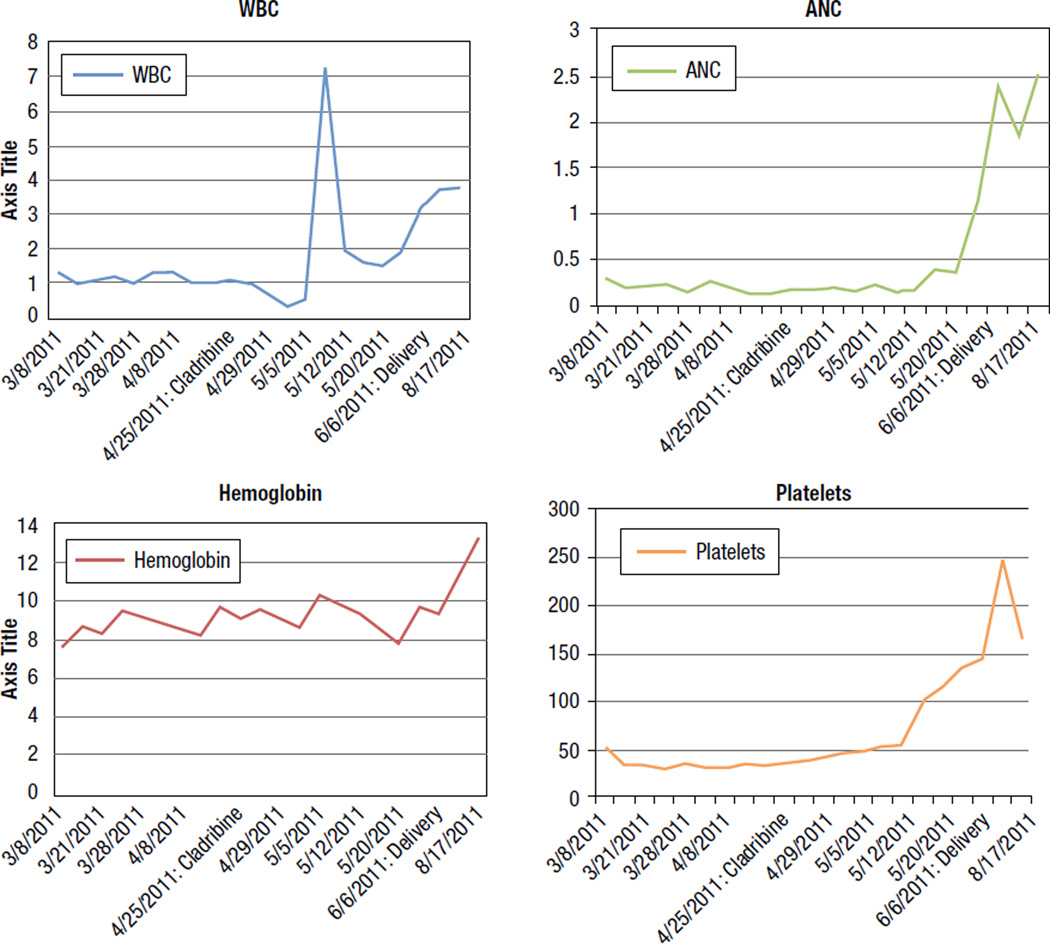
A 28-year-old pregnant female (Gravida 5; Para 3) was in good health until the 23rd week of pregnancy; when routine blood testing revealed pancytopenia with a white cell count (WBC) of 1.3 K/µL, absolute neutrophil count (ANC) 0.25 × 109/L, hemoglobin 8.6 g/dL, and platelet count 65 × 109/L. Further work-up led to a diagnosis of marginal zone lymphoma and she was treated with prednisolone 100 mg by mouth daily for 5 days, to be repeated every 3 weeks. She was subsequently referred to the University of Texas M.D. Anderson Cancer Center (UTMDACC). Review of original bone marrow biopsy at our institution showed hairy cell leukemia. Bone marrow flow cytometry was positive for CD11c, CD20, FMC-7, CD103, CD19, CD22, CD23, CD25, and monotypic lambda light chains. Flow cytometry of peripheral blood was positive for CD11c, CD19, CD20, CD22, CD23, CD25, CD44, CD103, FMC7, and monotypic lambda light chain. On presentation to UTMDACC her WBC count was 1.0 K/µL, ANC 0.21 K/µL, hemoglobin 8.6 g/dL, and platelet count 35 K/µL. She had normal renal and hepatic function. Physical examination revealed an acneiform rash on the face with no lymphadenopathy or hepatosplenomegaly. Ultrasound of the uterus showed a live fetus with appropriate size for gestational age. Laparoscopic splenectomy was considered, but deemed unfeasible given the advanced stage of pregnancy and the presence of significant thrombocytopenia. During the 26th week of gestation, she was treated with rituximab at a dose of 375 mg/m2 intravenously; given weekly for 4 weeks with no improvement in her blood counts. She was monitored closely with blood and platelet transfusions as needed. Treatment with cladribine was considered. To minimize the risk of developing severe cytopenias at the time of labor, cladribine was initiated early—during the 32nd week of pregnancy. She received 5 consecutive days of cladribine at a dose of 5.6 mg/m2 per day. She tolerated the therapy well and subsequently had improvement in her blood counts. She was monitored closely and eventually underwent spontaneous labor during the 40th week of gestation resulting in the delivery of a healthy baby. Her blood counts at delivery had significantly improved to WBC of 3.7 K/µL, ANC of 1.8 K/µL, hemoglobin (HGB) of 11.5 g/dL, and platelet (PLT) of 248 K/µL (Figure 1: Trend of WBC, ANC, platelet, and hemoglobin from presentation at UTMDACC through delivery).
Figure 1.
Trend of White Cell Count (WBC), Absolute Neutrophil Count (ANC), Hemoglobin, and Platelets, From Presentation at University of Texas M.D. Anderson Cancer Center Through Delivery
Discussion
Hairy cell leukemia is a chronic lymphoproliferative disorder characterized by splenomegaly and pancytopenia. Pathologic diagnosis is based on characteristic ‘hairy’ appearance of white blood cells on peripheral blood film, presence of increased bone marrow fibrosis, and localized or diffuse infiltration of bone marrow with cells that have characteristic cytoplasmic halo. Confirmation is by means of immunohistochemistry with anti-CD20/DBA-44 and tartrate-resistant acid phosphatase (TRAP) stain. Immunophenotyping, showing strong expression of CD11c, CD25, HC2+, CD123, cyclin D1, annexin A4, and lack of expression of CD5, CD10, CD23,9 differentiates HCL from other lymphoproliferative disorders. Therapy is not recommended unless the patient develops significant cytopenias (HGB < 10 g/dL, ANC < 1.0 K/µL, PLT < 100 K/µL), symptomatic splenomegaly, recurrent infections, extralymphatic involvement, autoimmune complications, or progressive disease.4,5 Management includes supportive measures in addition to specific treatment. Treatment options include purine analogues (cladribine), interferon-α (IFN-α), splenectomy, or monoclonal antibodies. Cladribine is widely used as first line therapy, with complete remission rates of 76%–80%, partial remission rates of 7%–24%,10–12 and overall survival of 79%–87%; with a 12-year follow-up.13 Purine analogues are classified as pregnancy class D drugs because teratogenic effects and fetal mortality were observed in animal studies.6 However, we were unable to find data regarding use of cladribine in gestating women.
Interferon-α has a documented response rate of 75%–90%14,15 but often produces only partial remission. The slower onset of action, need for long-term treatment ranging from 12 to 18 months, and side effects such as depression, fatigue, and flu-like syndrome have made IFN-α a second line therapeutic option in HCL. Interferon-α does not inhibit DNA synthesis and theoretically may be safe to use during pregnancy. A case report of 2 pregnant patients with hairy cell leukemia treated with IFN-α showed good tolerance, uncomplicated pregnancy and delivery, and normal child development after delivery.16
Splenectomy was the first effective therapy for hairy cell leukemia.17,18 Although it does not produce pathologic remission, peripheral blood counts return to normal in 40%–70% of patients,18 and overall 5-year survival rates are 60%–70%.18,19 On review of medical literature, we identified 4 cases of therapeutic splenectomy in pregnant patients with HCL.20–23 Two of these patients needed cladribine after delivery.20,21 One patient underwent laparoscopic splenectomy at 25 weeks of gestation, with transient response followed by relapse of pancytopenia at week 34. She was induced to deliver vaginally. She went on to receive cladribine 6 weeks after delivery.21
Monoclonal antibodies directed against CD20, CD22, and CD25 have shown good tolerability and efficacy; even in HCL patients resistant to purine analogues.24 Rituximab as a single agent produces an overall response rate of 64% in HCL.25
Our patient was initiated on rituximab in the 26th week of pregnancy and received weekly rituximab for 4 doses. Rituximab is classified as a pregnancy class C drug by the FDA. Data regarding safety of rituximab in gestating women remains scant. In animal studies cynomolgus monkeys exposed to therapeutic doses of rituximab experienced no teratogenicity.26 However, B cells were depleted in the offspring at birth and returned to normal within 6 months after birth. Chakravarty et al reported outcomes of 231 pregnancies with preconceptional or antepartum exposure to rituximab.7 Most resulted in uncomplicated live births. The mothers exposed to rituximab had a slightly increased preterm delivery rate than the general obstetric population (19% vs. 12%). However, there was no difference in the incidence or type of congenital anomalies identified. In this context it is important to recognize that rituximab is predominantly used in the treatment of chronic autoimmune conditions or malignant disease and is frequently administered in combination with cytotoxic chemotherapy or immunotherapy with known teratogenic potential. Furthermore, transportation of IgG-based monoclonal antibodies such as rituximab across the placenta depends on the phase of gestation. Transportation increases progressively through the first and second trimester and reaches its peak at the 26th week of gestation. Thus, effects of rituximab on pregnancy may vary based on the timing of the exposure. These factors may act as confounding variables making it difficult to assess the true impact of antepartum rituximab exposure on eventual pregnancy outcome. Therefore, at this time most manufacturer guidelines and clinical trials recommend that patients be counseled to avoid pregnancy for the duration of treatment and for at least 12 months after last rituximab exposure.
Rituximab often acts synergistically with purine analogues including cladribine in the treatment of HCL with improved outcomes. This synergistic benefit has been best defined in the treatment of relapsed HCL and for eradication of minimal residual disease.27–29 In the 26th week of pregnancy our patient started treatment with rituximab. She received a total of 4 weekly doses with no improvement in peripheral cytopenias. At week 32 she continued to have significant pancytopenia and we decided that further treatment was warranted. We chose cladribine after failure of rituximab for the following reasons: (1) the advanced stage of pregnancy theoretically reduced teratogenic risks to the fetus, (2) the potential synergism between already administered rituximab and purine analogues cladribine, (3) the need to achieve rapid improvement in blood counts which is difficult to accomplish with interferon, and (4) the potentially increased risk of bleeding or surgical complications with splenectomy secondary to enlarged uterus. Given the sequential nature of administration it is difficult to determine whether the response achieved was attributable to cladribine alone or the combination of rituximab followed by purine analogue cladribine.
Conclusion
HCL during pregnancy is an exceedingly rare, but potentially manageable condition. Treatment should be initiated only when truly warranted. Therapeutic options include purine analogues (cladribine), splenectomy, IFN-α, or rituximab. The appropriate treatment choice depends on a multitude of factors, including stage of pregnancy, rate of disease progression, and response to previous therapies. We initially administered rituximab in the 26th week of pregnancy with no improvement in blood cytopenias after 4 doses given on a weekly schedule. Persistent significant cytopenias and advanced gestational age prompted us to use cladribine in the third trimester. The cladribine was well tolerated and resulted in improvement in blood counts with no harm to the mother or fetus culminating in favorable pregnancy outcome. More data are needed to support the use of this agent in pregnant patients with HCL in earlier stages of pregnancy.
Clinical Practice Points.
The Occurrence of Neoplastic Diseases In Pregnant Women Can Present a Significant Diagnostic and Therapeutic Challenge for the Treating physician.
We Describe the Case of a Pregnant Woman Who Presented With Hairy Cell Leukemia (HCL) In the Second Trimester of Pregnancy. In the 26th Week of Pregnancy She Started Treatment With Weekly Rituximab. She Remained Pancytopenic and Was Then Treated With Cladribine During the 32nd Week With Favorable outcome.
We Found Scant Published Data Regarding Use of Rituximab Or Cladribine for the Treatment of Hcl In Pregnant women.
We Further Discuss Potential Treatment Indications and Therapeutic Options for Hcl In pregnancy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
All authors have no conflicts of interest.
References
- 1.Pavlidis NA. Cancer and pregnancy. Ann Oncol. 2000;11(Suppl 3):247–253. doi: 10.1093/annonc/11.suppl_3.247. [DOI] [PubMed] [Google Scholar]
- 2.Rizack T, Mega A, Legare R, et al. Management of hematological malignancies during pregnancy. Am J Hematol. 2009;84:830–841. doi: 10.1002/ajh.21547. [DOI] [PubMed] [Google Scholar]
- 3.Dores GM, Matsuno RK, Weisenburger DD, et al. Hairy cell leukaemia: a heterogeneous disease? Br J Haematol. 2008;142:45–51. doi: 10.1111/j.1365-2141.2008.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grever MR. How I treat hairy cell leukemia. Blood. 2010;115:21–28. doi: 10.1182/blood-2009-06-195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones G, Parry-Jones N, Wilkins B, et al. Revised guidelines for the diagnosis and management of hairy cell leukaemia and hairy cell leukaemia variant*. Br J Haematol. 2012;156:186–195. doi: 10.1111/j.1365-2141.2011.08931.x. [DOI] [PubMed] [Google Scholar]
- 6.Lau C, Narotsky MG, Lui D, et al. Exposure-disease continuum for 2-chloro-2’-deoxyadenosine (2-CdA), a prototype teratogen: induction of lumbar hernia in the rat and species comparison for the teratogenic responses. Teratology. 2002;66:6–18. doi: 10.1002/tera.10039. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499–1506. doi: 10.1182/blood-2010-07-295444. [DOI] [PubMed] [Google Scholar]
- 8.Orlowski RZ. Successful pregnancy after cladribine therapy for hairy cell leukemia. Leuk Lymphoma. 2004;45:187–188. doi: 10.1080/1042819031000149458. [DOI] [PubMed] [Google Scholar]
- 9.Robbins BA, Ellison DJ, Spinosa JC, et al. Diagnostic application of two-color flow cytometry in 161 cases of hairy cell leukemia. Blood. 1993;82:1277–1287. [PubMed] [Google Scholar]
- 10.Hoffman MA, Janson D, Rose E, et al. Treatment of hairy-cell leukemia with cladribine: response, toxicity, and long-term follow-up. J Clin Oncol. 1997;15:1138–1142. doi: 10.1200/JCO.1997.15.3.1138. [DOI] [PubMed] [Google Scholar]
- 11.Chadha P, Rademaker AW, Mendiratta P, et al. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine (2-CdA): long-term follow-up of the Northwestern University experience. Blood. 2005;106:241–246. doi: 10.1182/blood-2005-01-0173. [DOI] [PubMed] [Google Scholar]
- 12.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145:733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 13.Jehn U, Bartl R, Dietzfelbinger H, et al. An update: 12-year follow-up of patients with hairy cell leukemia following treatment with 2-chlorodeoxyadenosine. Leukemia. 2004;18:1476–1481. doi: 10.1038/sj.leu.2403418. [DOI] [PubMed] [Google Scholar]
- 14.Golomb HM, Ratain MJ, Fefer A, et al. Randomized study of the duration of treatment with interferon alfa-2b in patients with hairy cell leukemia. J Natl Cancer Inst. 1988;80:369–373. doi: 10.1093/jnci/80.5.369. [DOI] [PubMed] [Google Scholar]
- 15.Ratain MJ, Golomb HM, Vardiman JW, et al. Relapse after interferon alfa-2b therapy for hairy-cell leukemia: analysis of prognostic variables. J Clin Oncol. 1988;6:1714–1721. doi: 10.1200/JCO.1988.6.11.1714. [DOI] [PubMed] [Google Scholar]
- 16.Baer MR, Ozer H, Foon KA. Interferon-alpha therapy during pregnancy in chronic myelogenous leukaemia and hairy cell leukaemia. Br J Haematol. 1992;81:167–169. doi: 10.1111/j.1365-2141.1992.tb08202.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Norman AS, Nagorney DM, Martin JK, et al. Splenectomy for hairy cell leukemia. A clinical review of 63 patients. Cancer. 1986;57:644–648. doi: 10.1002/1097-0142(19860201)57:3<644::aid-cncr2820570341>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Golomb HM, Vardiman JW. Response to splenectomy in 65 patients with hairy cell leukemia: an evaluation of spleen weight and bone marrow involvement. Blood. 1983;61:349–352. [PubMed] [Google Scholar]
- 19.Magee MJ, McKenzie S, Filippa DA, et al. Hairy cell leukemia. Durability of response to splenectomy in 26 patients and treatment of relapse with androgens in six patients. Cancer. 1985;56:2557–2562. doi: 10.1002/1097-0142(19851201)56:11<2557::aid-cncr2820561103>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Alothman A, Sparling TG. Managing hairy-cell leukemia in pregnancy. Ann Intern Med. 1994;120:1048–1049. doi: 10.7326/0003-4819-120-12-199406150-00019. [DOI] [PubMed] [Google Scholar]
- 21.Adeniji BA, Fallas M, Incerpi M, et al. Laparoscopic splenectomy for hairy cell leukemia in pregnancy. Case Report Med. 2010;2010:136823. doi: 10.1155/2010/136823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustamante A, Rodríguez MA, Ocqueteau M, et al. Hairy cell leukemia during pregnancy. Report of one case [in Spanish] Rev Med Chil. 2010;138:1422–1426. [PubMed] [Google Scholar]
- 23.Stiles GM, Stanco LM, Saven A, et al. Splenectomy for hairy cell leukemia in pregnancy. J Perinatol. 1998;18:200–201. [PubMed] [Google Scholar]
- 24.Robak T. Monoclonal antibodies in the treatment of chronic lymphoid leukemias. Leuk Lymphoma. 2004;45:205–219. doi: 10.1080/1042819031000139666. [DOI] [PubMed] [Google Scholar]
- 25.Hagberg H, Lundholm L. Rituximab, a chimaeric anti-CD20 monoclonal antibody, in the treatment of hairy cell leukaemia. Br J Haematol. 2001;115:609–611. doi: 10.1046/j.1365-2141.2001.03143.x. [DOI] [PubMed] [Google Scholar]
- 26.Schröder C, Azimzadeh AM, Wu G, et al. Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transpl Immunol. 2003;12:19–28. doi: 10.1016/S0966-3274(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 27.Else M, Osuji N, Forconi F, et al. The role of rituximab in combination with pentostatin or cladribine for the treatment of recurrent/refractory hairy cell leukemia. Cancer. 2007;110:2240–2247. doi: 10.1002/cncr.23032. [DOI] [PubMed] [Google Scholar]
- 28.Ravandi F, Jorgensen JL, O’Brien SM, et al. Eradication of minimal residual disease in hairy cell leukemia. Blood. 2006;107:4658–4662. doi: 10.1182/blood-2005-11-4590. [DOI] [PubMed] [Google Scholar]
- 29.Cervetti G, Galimberti S, Andreazzoli F, et al. Rituximab as treatment for minimal residual disease in hairy cell leukaemia: extended follow-up. Br J Haematol. 2008;143:296–298. doi: 10.1111/j.1365-2141.2008.07333.x. [DOI] [PubMed] [Google Scholar]