Abstract
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that promotes neuronal survival, growth, and differentiation. The role of BDNF in learning and memory suggests that it may also modulate the clinical course of Alzheimer’s disease (AD). This study aimed to determine whether BDNF genetic variants are related to premorbid educational attainment, progression of cognitive and functional decline, and associated neuropsychiatric symptoms in AD patients. A sample of AD subjects (N = 341) was genotyped for the BDNF polymorphisms: Val66Met, C270T, and G-712A. Subjects received tests of cognition and daily function at baseline and at multiple subsequent time points. They were also characterized for the frequency and severity of neuropsychiatric symptoms. There was a significant effect of Val66Met genotype on educational attainment (F = 7.49, df = 2,329, P = 0.00066), with Met/Met homozygotes having significantly lower education than both the Val/Met and Val/Val groups. No association was observed between any BDNF polymorphism and measures of cognitive or functional decline. The T-allele of the C270T polymorphism was associated with a higher prevalence of neuropsychiatric symptoms and specifically with the presence of hallucinations. The effect of the Val66Met polymorphism on premorbid educational attainment is intriguing and should be verified in a larger sample.
Keywords: Activities of daily living, Alzheimer’s disease, brain-derived neurotrophic factor, cognition, education, single nucleotide polymorphism
INTRODUCTION
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that promotes a number of developmental phenomena, including neuronal growth, survival, and differentiation [1–3]. Moreover, BDNF has been discovered to play a critical role in the adult brain, in modulating synaptic transmission and plasticity, especially long-term potentiation (LTP) [4,5] that is important for learning and memory. These properties suggest that BDNF may contribute essentially to neurodegenerative diseases such as Alzheimer’s disease (AD), in which cognitive dysfunction is thought to result from a failure of neuroprotective mechanisms [6]. The brains of AD patients demonstrate decreased levels of BDNF mRNA [7,8] and protein [9–11], and serum levels of BD-NF have been found to correlate with clinical severity of AD [12].
Geneticists have recently identified a number of single nucleotide polymorphisms (SNPs) of BDNF, including C270T, Val66Met (G196A), and G-712A. Previous investigations of the role of these polymorphisms in the pathogenesis of AD have been conflicting. The T-allele of the C270T polymorphism (in the noncoding exon I) has been identified in several studies to be associated with AD [13–16] as well as early-onset AD in patients lacking the apolipoprotein E (ApoE) 4 allele [17]. However, no association has been found in several other studies [18–25]. The G-712A polymorphism is a novel variant that was recently identified by our group in the putative BDNF promoter region [24]. The G-allele and the G/G genotype were found to have a nominally higher frequency in subjects with substance dependence than in controls, but no association was observed with AD or several other neuropsychiatric disorders. The G-712A polymorphism has not yet been studied for functional effects at a molecular level.
Among the identified BDNF polymorphisms, the Val66Met variant in the prepeptide region (in the coding exon V) has received the greatest attention. This is perhaps because it represents a missense mutation (G196A) that produces a non-conservative amino acid substitution (Valine to Methionine) and because evidence has mounted in several models that it constitutes a “functional” mutation. Neurons transfected to express the Met variant show lower depolarization-induced secretion [26,27] and altered intracellular trafficking [28] of BDNF. Healthy subjects who are carriers and/or homozygotes of the Met-allele have been shown to have impaired hippocampal activity and decreased hippocampal volumes [26,29], as well as poorer performance on tests of episodic memory [26,27, 30].
Although most studies examining the association of Val66Met with the risk of AD have found no significant effect at least two reports have suggested higher frequencies of the Val-allele among patients with AD [13, 38]. In addition, some studies have begun to investigate the impact of Val66Met on other characteristics of AD, including age of onset, cognitive test scores, neuropsychiatric symptoms, rate of disease progression, and disease duration [19,31,33,39,40].
Thus, a majority of studies – including a previous report from our group [24] – have failed to link these three BDNF polymorphisms to an increased risk of AD. However, the role of this gene in neuronal function and learning and memory suggests that it may modulate the premorbid or post-onset course of AD. Given the increasing evidence that the process of AD may begin early in life [41,42], we included a measure of early-life cognitive performance in the form of premorbid educational attainment. Higher education has been associated with a reduced risk for AD in several studies [43–50].
The present investigation aimed to determine whether Val66Met, C270T, or G-712A was related to educational attainment, age of disease onset, progression of cognitive and functional decline, and associated neuropsychiatric symptoms in AD. From previously described effects of the Met-allele of the Val66Met polymorphism on BDNF protein secretion and episodic memory performance, we hypothesized that this allele would be associated with lower educational attainment, earlier disease onset, and a more rapid rate of progression. Effects on neuropsychiatric variables and all analyses of C270T and G-712A were considered exploratory.
MATERIALS AND METHODS
Subjects
The study sample was comprised of 341 patients with probable AD [51] who enrolled in a study of the genetics of AD and were initially evaluated in the Yale Alzheimer’s Disease Research Unit between July 1992 and August 2003. Most of these patients then participated in a variety of other research protocols permitting the accumulation of longitudinal cognitive and functional data. Nineteen of these patients have subsequently died and had autopsy confirming definite AD [52]. The demographics and clinical characteristics of patients are displayed in Table 1. The racial composition of the sample was: European-American (n = 333; 97.7%), African-American (n = 6; 1.8%), and Hispanic (n = 2; 0.59%).
Table 1.
Subject characteristics
| All subjects | Val66Met
|
C270T
|
G-712A
|
|||||
|---|---|---|---|---|---|---|---|---|
| (n = 341) | Val/Val (n = 203) | Val/Met (n = 117) | Met/Met (n = 12) | C/C (n = 301) | T-carrier (n = 31) | G/G (n = 282) | A-carrier (n = 28) | |
| Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | |
| Demographics | ||||||||
| Age | 73.8 ± 8.4 | 73.3 ± 8.4 | 74.4 ± 8.3 | 73.9 ± 7.4 | 73.6 ± 8.4 | 73.9 ± 8.5 | 73.7 ± 8.4 | 74.5 ± 8.1 |
| Sex (% female) | 62.8% | 63.5% | 58.1% | 83.3% | 61.1% | 77.4% | 59.6% | 82.1%a |
| Education (years) | 13.3 ± 3.3 | 13.1 ± 3.3 | 13.8 ± 3.2 | 10.2 ± 2.9b | 13.3 ± 3.4 | 13.1 ± 2.5 | 13.3 ± 3.4 | 12.8 ± 2.2 |
| Disease characteristics | ||||||||
| Onset age | 69.3 ± 8.3 | 68.8 ± 8.3 | 70.0 ± 8.2 | 69.6 ± 8.2 | 69.2 ± 8.3 | 69.1 ± 8.2 | 69.3 ± 8.3 | 69.9 ± 8.2 |
| Duration (years) | 4.5 ± 2.0 | 4.5 ± 2.0 | 4.4 ± 2.1 | 4.3 ± 2.2 | 4.4 ± 2.0 | 4.8 ± 1.9 | 4.4 ± 2.0 | 4.6 ± 1.5 |
| Family history (% positive) | 48.8% | 46.0% | 54.7% | 41.7% | 49.5% | 35.4% | 51.2% | 35.7% |
| ApoE ε4 carrier (%) | 57.4% | 61.4% | 52.6% | 50.0% | 58.3% | 50.0% | 57.5% | 48.1% |
| Concomitant therapies at baseline | ||||||||
| Cholinesterase inhibitors (% use) | 35.2% | 38.4% | 29.1% | 58.3% c | 35.5% | 32.3% | 33.3% | 35.7% |
| Antipsychotics (% use) | 3.5% | 3.9% | 3.4% | 0.0% | 3.3% | 6.5% | 2.8% | 7.1% |
| Antidepressants (% use) | 17.6% | 18.2% | 14.5% | 41.7% | 17.6% | 16.1% | 16.3% | 21.4% |
| Vitamin E (400 IU daily) (% use) | 36.1% | 34.0% | 40.2% | 33.3% | 38.2% | 19.4% | 37.2% | 21.4% |
Family history was positive if primary degenerative dementia was present in a first-degree relative.
A-carriers were disproportionately female (χ2 = 4.57, P = 0.032).
Significant effect of Val66Met genotype on educational attainment (F = 7.49; df = 2,329; P = 0.00066; Observed Power = 0.83 at α = 0.01). Met/Met had significantly lower educational attainment than Val/Met (P = 0.00056) and Val/Val (P = 0.0060) (ANCOVA; post hoc Tukey test).
Met/Met was significantly more likely than Val/Val or Val/Met to be taking cholinesterase inhibitors (χ2 = 6.21, P = 0.045).
Patients received a comprehensive evaluation, including medical history, physical examination, CBC, serum chemistries, thyroid function studies, B12, folate, urinalysis, electrocardiogram, and brain MRI or CT. Subjects were excluded for any neurological or medical disorder (other than AD) judged related to cognitive deterioration or for current psychiatric, alcohol, or substance disorders. Research protocols in which subjects participated following initial evaluation included treatment, neuroimaging, and neuropsychological studies. Some treatments received by subjects – in particular cholinesterase inhibitors, high-dose vitamin E (≥400 IU daily), and psychotropic drugs – may potentially have impacted behavioral variables analyzed in this study. However, these treatments were assumed to be independently distributed with respect to BDNF genotypes, and this assumption was tested statistically. In addition, each subject was assigned an approximate date of disease onset, based on review of medical records and interviews with one or more family members. The date of onset was defined (using a manual available from the corresponding author upon request) as the date at which the “earliest definite symptom” appeared. This could be either a cognitive symptom (e.g., forgetfulness or repetitiousness) or a significant decline in an instrumental activity of daily living (e.g., impaired driving) if cognitively based. All subjects (or their responsible next of kin) provided written informed consent and were studied under protocols approved by the Yale Human Investigation Committee and conducted in accordance with the Helsinki Declaration of 1975.
Behavioral evaluation
Subjects were evaluated using a number of cognitive, functional, and neuropsychiatric tests and rating scales at initial presentation (see Tables 2 and 4). Several of these measures were repeated longitudinally, at varying frequencies, depending on requirements of the different research protocols in which subjects subsequently participated. Many subjects enrolled in multiple studies spanning several years.
Table 2.
Cognitive and functional data at baseline*
| All subjects | Val66Met
|
C270T
|
G-712A
|
|||||
|---|---|---|---|---|---|---|---|---|
| (n = 341) | Val/Val (n = 203) | Val/Met (n = 117) | Met/Met (n = 12) | C/C (n = 301) | T-carrier (n = 31) | G/G (n = 282) | A-carrier (n = 28) | |
| Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | |
| n = 203 | n = 117 | n = 12 | n = 301 | n = 31 | n = 282 | n = 28 | ||
| MMSE (n = 341) | 17.2 ± 5.5 | 17.3 ± 5.4 | 17.0 ± 5.7 | 17.8 ± 5.9 | 17.2 ± 5.5 | 17.2 ± 4.7 | 17.2 ± 5.6 | 17.5 ± 4.5 |
| n = 167 | n = 97 | n = 10 | n = 252 | n = 23 | n = 239 | n = 22 | ||
| ADAS-Cog (n = 282) | 27.1 ± 12.0 | 27.1 ± 12.4 | 27.7 ± 11.5 | 24.5 ± 11.2 | 27.2 ± 12.1 | 27.8 ± 10.4 | 27.4 ± 12.1 | 27.9 ± 10.8 |
| n = 196 | n = 112 | n = 12 | n = 289 | n = 31 | n = 271 | n = 28 | ||
| IADL (n = 329) | 0.65 ± 0.19 | 0.65 ± 0.19 | 0.65 ± 0.19 | 0.65 ± 0.17 | 0.65 ± 0.19 | 0.68 ± 0.18 | 0.65 ± 0.19 | 0.68 ± 0.17 |
| n = 100 | n = 58 | n = 7 | n = 149 | n = 16 | n = 141 | n = 13 | ||
| ADCS-ADL (n = 170) | 57.3 ± 14.4 | 57.8 ± 13.4 | 56.0 ± 16.6 | 62.0 ± 6.7 | 57.3 ± 14.3 | 57.0 ± 12.7 | 57.4 ± 14.6 | 59.5 ± 10.7 |
Abbreviations: MMSE, Mini-Mental State Examination (0–30); ADAS-Cog, Alzheimer’s Disease Assessment Scale – Cognitive Subscale (0–70, lower scores indicate better performance); IADL, Instrumental Activities of Daily Living (0.27–1.00, lower scores indicate better performance; ADCS-ADL, Alzheimer’s Disease Cooperative Study-Activities of Daily Living (0–78).
Data displayed are baseline cognitive and functional measures adjusted for disease duration, patient age, sex, and education.
Table 4.
Analysis of neuropsychiatric symptoms
| NPI Subscore | (n = 248) | Val66Met (n = 241) | C270T (n = 241) | G-712A (n = 222) | |
|---|---|---|---|---|---|
| n | % | OR [95% CI]a | OR [95% CI]b | OR [95% CI]b | |
| Delusions | 76 | 30.6 | 0.64 [0.38–1.09] | 1.39 [0.54–3.60] | 1.44 [0.53–3.91] |
| Hallucinations | 39 | 15.7 | 0.73 [0.38–1.40] | 3.25 [1.22–8.62]c | 2.76 [0.98–7.78] |
| Aggression | 83 | 33.5 | 0.68 [0.41–1.13] | 2.48 [0.97–6.33] | 1.89 [0.70–5.14] |
| Anxiety | 73 | 29.4 | 0.95 [0.58–1.55] | 1.70 [0.68–4.24] | 1.15 [0.41–3.22] |
| Depression | 83 | 33.5 | 0.83 [0.51–1.33] | 1.47 [0.60–3.64] | 1.99 [0.78–5.10] |
| Elation | 18 | 7.3 | * | * | * |
| Apathy | 95 | 38.3 | 0.82 [0.52–1.31] | 1.68 [0.69–4.12] | 1.55 [0.59–4.02] |
| Disinhibition | 53 | 21.4 | 0.86 [0.49–1.50] | 1.73 [0.66–4.58] | 1.43 [0.47–4.33] |
| Irritability | 80 | 32.3 | 0.94 [0.59–1.51] | 1.12 [0.44–2.83] | 1.41 [0.53–3.72] |
| Aberrant motor behavior | 96 | 38.7 | 0.71 [0.44–1.16] | 1.51 [0.61–3.78] | 1.04 [0.39–2.78] |
| Sleep disturbances | 54 | 21.8 | 0.99 [0.57–1.70] | 1.20 [0.43–3.31] | 0.96 [0.30–3.11] |
| Eating disturbances | 50 | 20.2 | 1.42 [0.84–2.42] | 0.57 [0.16–2.02] | 0.48 [0.10–2.19] |
Odds Ratios for Val66Met are for the effect of Met-allele dose (2,1,0).
Odds Ratios for for C270T and A712G are for carrier status of the less common allele (C270T-T, A712G-A).
Genotype significantly increases risk for symptom, p < 0.05.
No carriers of less common allele exhibited Elation.
Cognitive performance of subjects was measured using both the Mini-Mental State Examination (MMSE) [53] and the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog) [54]. Functional capacity was assessed using the Instrumental Activities of Daily Living (IADL) scale [55] and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living inventory (ADCS-ADL) [56]. Both the IADL and ADCS-ADL ratings were obtained by interviewing the patient’s caregiver. The IADL was administered at baseline only (and therefore included in retrospective but not prospective analyses) and scored from 0.27 (no impairment) to 1.00 (maximal impairment) as previously described [57]. Behavioral and psychological symptoms associated with dementia were evaluated using the Neuropsychiatric Inventory (NPI) [58] as previously described [59]. The NPI used scripted questions administered to the patient’s caregiver to assess twelve behavioral domains (Table 4). For each domain in which symptoms were confirmed, the caregiver provided frequency and severity scores, the product of which equaled the domain score (range = 0–12). The sum of all domain scores yielded the total NPI score (range 0–144). Of the cognitive, functional, and neuropsychiatric measures, the MMSE was performed on the entire sample (N = 341), whereas all other measures were available only for subsamples, as detailed in Tables 2 and 4. All subject data were obtained by trained raters who were unaware of genotypes.
Determination of BDNF genotypes
In our recent study, a new SNP variant (G/A) was identified in the 5′ region of the BDNF gene (712 bp from Exon 1, thus the variant was named “G-712A”) using denaturing high performance liquid chromatography (dHPLC) [24]. The newly identified SNP, G-712A, and two previously reported SNPs, C270T [15] and Val66Met (rs6265) [60], were genotyped in patients with AD by PCR and restriction fragment length polymorphism (PCR-RFLP) analysis. Primer pairs and PCR conditions for genotyping the three BDNF SNPs have been previously summarized [24]. ApoE genotypes were determined as previously described [57,59].
Statistical analysis
Analyses of the Val66Met polymorphism involved three-group comparisons. However, the C270T and G-712A variants were studied using two-group comparisons between carriers and non-carriers of the less frequent allele, given the scarcity of homozygotes for the T-allele of the C270T polymorphism (n = 3) and the A-allele of the G-712A polymorphism (n = 2). Subject characteristics were compared across gene dose groups using Student’s t-test or analysis of variance (ANOVA) for continuous variables or chi-square analysis for dichotomous variables. We hypothesized that the Met-allele of the Val66Met polymorphism would be associated with lower educational attainment and an earlier age of disease onset.
The effect of each BDNF polymorphism on rates of AD progression were analyzed using both retrospective and prospective techniques similar to those employed in our previous study of ApoE ε4 [57]. Retrospective analyses examined cross-sectional cognitive (MMSE, ADAS-Cog) and functional (IADL, ADCS-ADL) data obtained at each subject’s initial visit, controlling for the duration of symptoms by analysis of covariance (ANCOVA). While disease duration was the essential covariate in retrospective analyses, age, sex, and educational attainment were also entered in the ANCOVA models. Prospective analyses of disease progression were also conducted for the MMSE, ADAS-Cog, and ADCS-ADL for all subjects who had at least two observations spanning at least six months. For these analyses, an annualized rate of change on each scale was calculated by least-squares regression, using all available measurements for each subject. Rates of change were compared across BDNF genotype groups by AN-COVA, controlling for age, sex, and education. We hypothesized that the Met-allele of the Val66Met polymorphism would confer a more rapid cognitive and functional decline.
Finally, we analyzed the effect of each BDNF polymorphism on neuropsychiatric symptoms in AD, as measured by the NPI. The distribution of total NPI scores in our sample showed a strong positive skew, necessitating nonparametric analyses (Kruskal-Wallis H for three-group analysis of Val66Met, or Mann-Whitney U for two-group analysis of other polymorphisms). The association between BDNF polymorphisms and the presence of symptoms in the twelve NPI domains was further explored using multiple logistic regression models, with domain score (zero or nonzero) as dependent variable and the following independent variables (in addition to BDNF genotype): age, sex, education, and MMSE score.
For the hypothesis-driven analyses of Val66Met effects on educational attainment, age of onset, and seven measures of disease progression, a statistical significance level of α = 0.01 was adopted. With several related dependent variables, full Bonferroni correction (0.05/9 = 0.0056) was considered to confer unacceptably high type II error. Effects on neuropsychiatric variables and all analyses of C270T and G-712A were deemed exploratory (α = 0.05). Since BDNF allele frequencies are known to vary across population groups, and the non-European-American portion of the present sample was too small (n = 8) to control for race statistically, we rechecked all significant results, excluding these subjects. All statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL) and employed two-tailed tests of significance.
RESULTS
Subject characteristics
Table 1 presents the characteristics of the entire sample and each genotype group. Val66Met genotypes were available for n = 332 subjects; C270T genotypes for n = 332 subjects; and G-712A genotypes for n = 310 subjects. The genotypic distribution of each of the three SNPs was consistent with Hardy-Weinberg equilibrium expectations (data not shown). However, as previously detailed for a substantially overlapping sample [24] (291 of 295 AD subjects in that report are also included in the present study), significant linkage disequilibrium (LD) was found between SNPs G-712A and C270T (P < 0.001), with more modest LD between SNP Val66Met and SNP G-712A (P =0.019) or SNP C270T (P = 0.003). ApoE genotypes were also available for n = 338 subjects, and ApoE ε4 carrier status was randomly distributed with respect to BDNF genotypes.
In hypothesis-driven comparisons, there was a significant effect of Val66Met genotype on educational attainment (F = 7.49; df = 2,329; P = 0.00066; Observed Power = 0.83 at α = 0.01; European-Americans: F = 7.30; df = 2,322; P = 0.00079; Observed Power = 0.82 at α = 0.01). Post-hoc Tukey test revealed that Met/Met homozygotes had significantly lower education than both Val/Met heterozygotes (P = 0.00056) and Val/Val homozygotes (P = 0.0060). However, Val/Met heterozygotes and Val/Val homozygotes did not differ from each other (P =0.14). Since males had significantly more education than females (14.0 vs. 12.8 years, P =0.001, t-test), the effect of Val66Met genotype on educational attainment was re-examined with the addition of sex as a covariate and was minimally changed (F = 6.51; df = 2,328; P = 0.0017; ANCOVA). There was no significant effect of either C270T or G-712A genotype on educational attainment or of any polymorphism on age of disease onset.
Otherwise, few significant differences in subject characteristics were observed across genotype groups. Carriers of the A-allele of the G-712A polymorphism were disproportionately female (χ2 = 4.57, df = 1, P = 0.032). In addition, Val66Met Met/Met homozygotes were significantly more likely than Val/Val or Val/Met subjects to be receiving cholinesterase inhibitors at the time of initial assessment (χ2 = 6.21, df = 1, P = 0.045). Apart from this, treatment with cholinesterase inhibitors, antipsychotics, antidepressants, and high-dose vitamin E was distributed independently of genotype.
Retrospective analysis of disease progression
Table 2 summarizes the retrospective analyses of cognitive and functional progression in AD patients. It specifically contains the baseline cognitive and functional data according to BDNF genotypes.
Val66Met
Among n = 332 subjects for whom Val66Met genotypes were available, MMSE performance was analyzed in the overall sample, whereas ADAS-Cog (n = 274), IADL (n = 320), and ADCS-ADL (n =165) performance were analyzed in subsamples. The demographic profile of these sub-samples and all others analyzed below did not differ from that of the overall sample characterized in Table 1. Val66Met genotype groups did not differ significantly in performance on the MMSE (F = 0.68; df = 2,325; P = 0.51), ADAS-Cog (F = 0.78; df = 2,267; P = 0.46), IADL (F = 0.01; df = 2,313; P = 0.99), or ADCS-ADL (F = 0.84; df = 2,158; P = 0.43), controlling for disease duration, age, sex, and education.
C270T
Among n = 332 subjects for whom C270T genotypes were available, MMSE performance was analyzed in the overall sample, whereas ADAS-Cog (n = 275), IADL (n = 320), and ADCS-ADL (n = 165) performance were analyzed in subsamples. T-carriers and C/C homozygotes did not differ significantly in performance on the MMSE (F = 0.01; df = 1,326; P = 0.93), ADAS-Cog (F = 0.04; df = 1,269; P = 0.84), IADL (F = 0.60; df = 1,314; P = 0.44), or ADCS-ADL (F = 0.40; df = 1,159; P = 0.53), controlling for disease duration, age, sex, and education.
G-712A
Among n = 310 subjects for whom G-712A genotypes were available, MMSE performance was analyzed in the overall sample, whereas ADAS-Cog (n = 261), IADL (n = 299), and ADCS-ADL (n = 154) performance were analyzed in subsamples. A-carriers and G/G homozygotes did not differ significantly in performance on the MMSE (F = 0.12; df = 1,304; P = 0.73), ADAS-Cog (F = 0.02; df = 1,255; P = 0.90), IADL (F = 0.78; df = 1,293; P = 0.38), or ADCS-ADL (F = 1.15; df = 1,148; P = 0.29), controlling for disease duration, age, sex, and education.
Prospective analysis of disease progression
Table 3 summarizes the prospective analyses of cognitive and functional progression in AD patients. It specifically contains annualized rates of change for cognitive and functional measures according to BDNF genotypes.
Table 3.
Prospective analysis of cognitive and functional data*
| All subjects | Val66Met
|
C270T
|
G-712A
|
|||||
|---|---|---|---|---|---|---|---|---|
| (n = 341) | Val/Val (n = 203) | Val/Met (n = 117) | Met/Met (n = 12) | C/C (n = 301) | T-carrier (n = 31) | G/G (n = 282) | A-carrier (n = 28) | |
| Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | |
| n = 220 | n = 135 | n = 73 | n = 7 | n = 197 | n = 16 | n = 184 | n = 14 | |
| MMSE annual change | −3.12 ± 3.70 | −3.17 ± 3.93 | −3.03 ± 3.51 | −3.34 ± 1.60 | −3.11 ± 3.73 | −3.93 ± 3.83 | −3.04 ± 3.83 | −4.19 ± 3.98 |
| Number of observations | 6.8 ± 3.5 | 6.7 ± 3.1 | 6.9 ± 4.1 | 6.4 ± 2.7 | 6.9 ± 3.5 | 5.3 ± 2.9 | 7.0 ± 3.6 | 4.7 ± 2.2 |
| Duration of observation (yrs) | 1.79 ± 1.31 | 1.73 ± 1.25 | 1.87 ± 1.45 | 1.51 ± 0.65 | 1.82 ± 1.34 | 1.32 ± 1.02 | 1.84 ± 1.36 | 1.14 ± 0.75 |
| n = 184 | n = 107 | n = 66 | n = 6 | n = 165 | n = 14 | n = 157 | n = 14 | |
| ADAS-Cog annual change* | 5.93 ± 9.03 | 6.13 ± 10.34 | 5.83 ± 7.18 | 5.70 ± 3.77 | 5.63 ± 8.87 | 9.53 ± 11.18 | 5.52 ± 8.97 | 9.81 ± 11.27 |
| Number of observations | 9.4 ± 5.1 | 8.9 ± 5.0 | 10.1 ± 5.3 | 9.7 ± 4.4 | 9.3 ± 5.0 | 10.4 ± 7.2 | 9.5 ± 5.0 | 9.2 ± 5.8 |
| Duration of observation (yrs) | 1.63 ± 1.22 | 1.51 ± 1.23 | 1.78 ± 1.24 | 1.61 ± 0.63 | 1.63 ± 1.22 | 1.55 ± 1.27 | 1.66 ± 1.25 | 1.36 ± 1.07 |
| n = 160 | n = 97 | n = 53 | n = 6 | n = 143 | n = 11 | n = 135 | n = 10 | |
| ADCS-ADL annual change | −10.37 ± 11.17 | −10.10 ± 10.84 | −10.34 ± 11.63 | −14.12 ± 13.03 | −10.68 ± 11.51 | −9.55 ± 8.49 | −10.34 ± 11.59 | −12.41 ± 10.02 |
| Number of observations | 7.1 ± 3.2 | 6.7 ± 2.7 | 7.8 ± 4.0 | 6.7 ± 2.1 | 7.1 ± 3.3 | 5.7 ± 1.7 | 7.2 ± 3.4 | 5.9 ± 1.4 |
| Duration of observation (yrs) | 1.39 ± 1.01 | 1.30 ± 0.89 | 1.57 ± 1.23 | 1.16 ± 0.43 | 1.40 ± 1.04 | 0.90 ± 0.41 | 1.43 ± 1.07 | 0.90 ± 0.34 |
Abbreviations: MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer’s Disease Assessment Scale – Cognitive Subscale; ADCS-ADL, Alzheimer’s Disease Cooperative Study –Activities of Daily Living.
Data displayed are longitudinal rates of change per year computed by linear regression. Rates of ADAS-Cog change are positive because lower scores indicate better performance.
Val66Met
Among n = 332 subjects for whom Val66Met genotypes were available, annualized rates of change in MMSE (n = 215), ADAS-Cog (n = 179), and ADCS-ADL (n = 156) performance were measured in subsamples. Val66Met genotype groups did not differ significantly in the rate of change of MMSE (F = 0.00; df = 2,209; P = 1.00), ADAS-Cog (F = 0.04; df = 2,173; P = 0.97), or ADCS-ADL (F = 0.41; df = 2,150; P = 0.67), controlling for age, sex, and education.
C270T
Among n = 332 subjects for whom C270T genotypes were available, annualized rates of change in MMSE (n = 213), ADAS-Cog (n = 179), and ADCS-ADL (n = 154) performance were analyzed in subsamples. T-carriers and C/C homozygotes did not differ significantly in the rate of change in MMSE (F = 0.69; df = 1,208; P = 0.41), ADAS-Cog (F = 2.47; df = 1,174; P = 0.12), or ADCS-ADL (F = 0.01; df = 1,149; P = 0.94), controlling for age, sex, and education.
G-712A
Among n = 310 subjects for whom G-712A genotypes were available, annualized rates of change in MMSE (n = 198), ADAS-Cog (n = 171), and ADCS-ADL (n = 145) were analyzed in subsamples. A-carriers and G/G homozygotes did not differ significantly in the rate of change in MMSE (F = 1.33; df = 1,193; P = 0.25), ADAS-Cog (F = 3.24; df = 1,166; P = 0.07), or ADCS-ADL (F = 0.72; df = 1,140; P = 0.40), controlling for age, sex, and education.
Analysis of neuropsychiatric symptoms
NPI data were available for n = 248 subjects. Of these, Val66Met and C270T genotypes were available for n = 241 subjects, and G-712A genotypes for n = 222. There was no significant effect of Val66Met on total NPI score (χ2=1.61, df = 2, P = 0.45, Kruskal-Wallis H test). Conversely, carriers of the T-allele of the C270T polymorphism had higher NPI scores than C/C homozygotes (24.5 ± 21.2 vs. 14.9 ± 15.1; Z = −2.11, P = 0.035, Mann-Whitney U test; European-Americans: Z = −1.87, P = 0.062). G-712A genotype groups did not differ in total NPI score (Z = −1.66, P = 0.098).
Our exploratory analysis of individual NPI domains is summarized in Table 4. This analysis suggests that the higher prevalence of neuropsychiatric symptoms for the C270 T-allele may accrue specifically from the presence of hallucinations (T-carriers: 36.4%; C/C homozygotes: 13.7%) (OR = 3.25, 95% CI = [1.22–8.62], P = 0.018; European-Americans: OR = 3.08, 95% CI = [1.12–8.48], P = 0.029) but not from disturbances in other domains. Neither Val66Met nor G-712A genotype was associated with the presence of behavioral symptoms in any NPI domain.
DISCUSSION
We evaluated the role of BDNF variants in modulating several features of the clinical course of AD. We specifically investigated effects of three BDNF polymorphisms – Val66Met, C270T, and G-712A – on educational attainment, age of onset, progression of cognitive and functional decline, and associated neuropsychiatric symptoms in AD patients. Val66Met demonstrated a significant effect on education (with Met/Met homozygotes having fewer years of education than both the Val/Met and Val/Val groups) but not age of onset or rates of cognitive or functional progression. Other polymorphisms were not associated with any of these variables. The T-allele of the C270T polymorphism was associated with a higher prevalence of neuropsychiatric symptoms and specifically with the presence of hallucinations.
Education
Perhaps the most intriguing finding of this study was the highly significant effect of the Val66Met polymorphism on premorbid educational attainment, with Met/Met homozygotes having fewer years of education than other genotype groups (by 2.9 and 3.6 years). Previous studies have seldom provided information pertaining to educational attainment as a function of BD-NF genotypes. The AD samples studied by Chuu et al. [39] and Borroni et al. [40] both evidenced no statistically significant differences in education according to Val66Met genotype, although in each case the Met/Met homozygotes (n = 5, and n = 19 respectively) had numerically fewer years of education than the other groups by approximately one year. In a large (N = 641) non-AD sample (that included patients with schizophrenia, their unaffected siblings, and healthy controls), Egan and colleagues observed no significant effect of Val66Met on educational attainment [26]. However, genetic effects on education may be quite different in AD populations insofar as they are skewed toward lower “cognitive reserve” as discussed below.
An association between Met/Met homozygosity and lower educational attainment in patients with AD would suggest that the previously-described effect of the Met-allele on episodic memory [26,27,30] might have long-term impact on academic performance. Notably, in the report of Egan et al. the effect of Val66Met on episodic memory performance accrued from poorer performance by Met/Met homozygotes compared to other genotype groups [26].
Interestingly, higher educational attainment has been shown in several cohorts of varying ethnicities to be associated with a reduced risk for AD [43–50]. This phenomenon has been linked to the more general hypothesis of “cognitive reserve,” which holds that a reduced risk of incident AD may be conferred by increased educational and occupational attainment [44], and ultimately by additional neural networks that become active when other brain regions become too damaged by AD neuropathology to function [61]. Although, this hypothesis is often viewed in terms of higher education, occupation, and leisure activity as a lifestyle that protects against later development of AD, it might also have genetic determinants. A gene or a number of genes (including Val66Met) might predispose both to greater cognitive reserve throughout the lifespan as well as to a reduced risk of AD. Arguing against Val66Met as such a gene is the preponderance of AD studies that demonstrate no association [14,19–22,24,31–36]. However, these studies, particularly those conducted in populations of European origin, have contained comparatively few Met/Met homozygotes, and further research may be necessary to rule out Met/Met homozygosity as a risk factor for AD.
Age of onset and rate of progression
Contrary to expectations, we observed no effect of Val66Met on age of disease onset or subsequent rates of cognitive or functional progression. Our finding that BDNF polymorphisms were unrelated to age of onset accords with several other studies that reported no effect of Val66Met [19,31,39] or C270T [19] on this variable.
Our observations that BDNF polymorphisms were unrelated to rates of cognitive and functional progression – either retrospectively or prospectively – is also consistent with the extant research. Nacmias and colleagues examined the effect of Val66Met on the cognitive profile of mild to moderate AD using a cross-sectional design and reported no effect [33]. However, they did not control for duration of symptoms to attempt a retrospective analysis of disease progression. Similarly, Desai and collaborators [19] found no association between the Val66Met and C270T polymorphisms and MMSE scores but again did not covary for duration of symptoms. However, these investigators included a unique quantitative measure of AD progression – overall disease duration (from onset to death) – and again observed no differences across Val66Met and C270T genotype subgroups.
Our finding that Val66Met was unrelated to prospective rates of cognitive decline in AD is consistent with one previous study [39]. Chuu et al. determined rate of change by sequential MMSE scores in 149 AD patients followed for an average of 3.9 years and demonstrated no effect on the rate of cognitive change. Our study is the first to measure cognitive change prospectively for C270T or G712-A and the first to measure change in activities of daily living for any BDNF polymorphism in AD.
Collectively, the available research for Val66Met on AD progression suggests that, although the Met-allele is associated with impaired episodic memory performance in non-AD populations [26,27,30] and – if the present finding is replicated – Met/Met homozygosity with lower educational attainment in AD, this does not alter the age of AD onset or the rate of post-onset progression. Postmortem AD brain specimens demonstrate decreased levels of BDNF mRNA [7,8] and protein [9–11]; however, these alterations may be unrelated to Val66Met genotype. Although the Met-allele has been linked to reduced BDNF secretion in vitro [26–28], one recent study observed no effect of this allele on BDNF concentrations in AD brain (in a sample that included only 3 Met/Met homozygotes) [11]. Genetic influences on the post-onset progression of AD remain elusive, as no polymorphism has yet been shown to alter rates of deterioration. Even ApoE ε4, despite conferring an increased risk and a lower age of onset of AD [62,63], has broadly not been shown to alter cognitive and functional progression (reviewed in [57]).
Neuropsychiatric symptoms
Our finding that carriers of the T-allele of the C270T polymorphism had modestly greater behavioral disturbance than C/C homozygotes as measured by total NPI score (Z = −2.11, p = 0.035, Mann-Whitney U test) must be interpreted with caution. We did not correct for multiple comparisons to reduce the risk of Type II error in this exploratory analysis, and the nominal significance of this finding was lost when European-Americans were considered separately (Z = −1.87, P = 0.062). Examination of NPI subscores by logistic regression suggested that the T-allele was specifically associated with increased risk for the presence of hallucinations. That the T-allele of C270T has been documented in higher frequency among patients with schizophrenia than healthy controls in several studies [60,64–66] raises the possibility that this allele might predispose broadly to psychotic symptoms. However, other studies have failed to corroborate this association in schizophrenia [24,67,68].
We did not replicate the finding of Borroni et al. of a significant effect of the Val66Met polymorphism on depressive symptoms in AD [40]. Those authors reported significantly higher risk for depression in both Met carriers and Met/Met homozygotes compared to Val/Val homozygotes, using both the NPI and the Geriatric Depression Scale. We observed no effect of Val66Met on the presence of depressive symptoms using the NPI.
Limitations
A number of potential limitations of this investigation deserve comment. First, our subject sample was drawn from an academic medical center and may differ demographically from a population sample [69]. In particular, our subject observations were performed mainly during the course of trials of investigational drugs whose effects on AD progression may obscure those of BDNF genotypes. However, most of the experimental treatments in question were ultimately found not to influence the progression of AD symptoms, and any small effects were likely distributed randomly with respect to BDNF genotypes. Second, our retrospective analysis of disease progression was susceptible to recall bias, as disease onset was estimated by recollection of relatives. However, prospective and retrospective ratings by relatives have shown reasonably close agreement [70], and inaccuracies in recollection are unlikely to be systematically biased with regard to BDNF genotypes. Finally, our most noteworthy finding – of an effect of Val66Met on educational attainment – was based on only twelve Met/Met homozygotes. It is perhaps all the more remarkable that such a highly significant difference emerged, despite the limited power of the sample to detect an effect that was present only in Met/Met homozygosity (~4% of subjects of European origin). Nonetheless, this finding will certainly need to be replicated with additional AD samples.
In conclusion, we observed that Val66Met – in particular Met/Met homozygosity – was associated with lower premorbid educational attainment in AD patients. However, it did not confer an earlier age of onset or a more rapid cognitive and functional decline. The T-allele of the C270T polymorphism was associated with a higher prevalence of neuropsychiatric symptoms and specifically with the presence of hallucinations. Otherwise, these three polymorphisms did not impact the clinical course of AD in our sample.
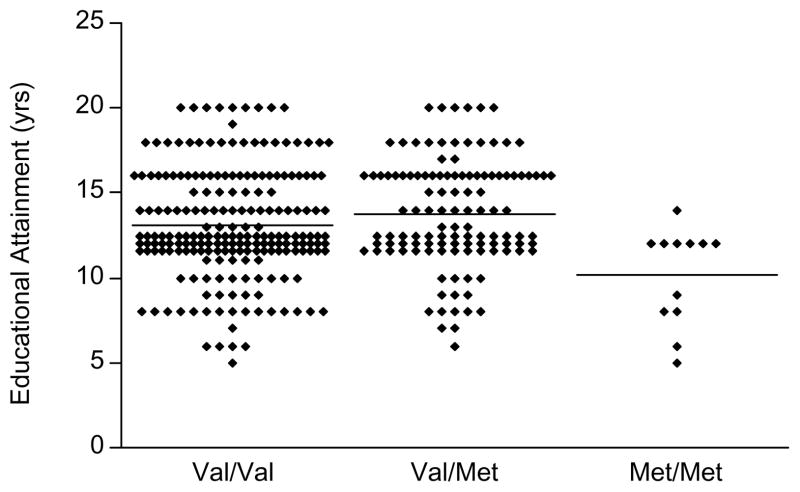
Fig. 1.
Scatter plot of premorbid educational attainment (yrs) by Val66Met genotype in AD patients (N = 332). Each symbol denotes an individual subject. Val/Val and Val/Met subjects with 12 yrs education are separated into three rows for legibility. Horizontal bars indicate group means. There was a significant effect of Val66Met genotype on educational attainment (F = 7.49; df = 2,329; P = 0.00066, ANOVA). Post-hoc Tukey test showed that the Met/Met homozygote group had significantly lower education than both the Val/Met heterozygotes (P = 0.00056) and the Val/Val homozygotes (P = 0.0060). However, the Val/Met heterozygote and Val/Val homozygote groups did not differ from each other (P = 0.14).
Acknowledgments
The authors thank Ann Marie Lacobelle, Benjamin Black, and Tracy Rightmer for technical assistance. This research was supported in part by NIH Clinical Neuroscience Mental Health Research Training Grant, as well as K24-DA15105 (JG), and P30-MH30929.
Footnotes
Author disclosures available online (http://www.j-alz.com/disclosures/view.php?id=29).
References
- 1.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 2.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalb R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends Neurosci. 2005;28:5–11. doi: 10.1016/j.tins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 5.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fumagalli F, Racagni G, Riva MA. The expanding role of BDNF: a therapeutic target for Alzheimer’s disease? Pharmacogenomics J. 2006;6:8–15. doi: 10.1038/sj.tpj.6500337. [DOI] [PubMed] [Google Scholar]
- 7.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF messenger RNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 8.Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 9.Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- 10.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Fukumoto H, Orne J, Klucken J, Raju S, Vanderburg CR, Irizarry MC, Hyman BT, Ingelsson M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita S, Arai H, Matsui T, Yuzuriha T, Urakami K, Masaki T, Higuchi S. Brain-derived neurotrophic factor gene polymorphisms and Alzheimer’s disease. J Neural Transm. 2005;112:703–711. doi: 10.1007/s00702-004-0210-3. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura M, Kuno S, Kaji R, Kawakami H. Brain-derived neurotrophic factor gene polymorphisms in Japanese patients with sporadic Alzheimer’s disease, Parkinson’s disease, and multiple system atrophy. Mov Disord. 2005;20:1031–1059. doi: 10.1002/mds.20491. [DOI] [PubMed] [Google Scholar]
- 15.Kunugi H, Ueki A, Otsuka M, Isse K, Hirasawa H, Kato N, Nabika T, Kobayashi S, Nanko S. A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer’s disease. Mol Psychiatry. 2001;6:83–86. doi: 10.1038/sj.mp.4000792. [DOI] [PubMed] [Google Scholar]
- 16.Olin D, MacMurray J, Comings DE. Risk of late-onset Alzheimer’s disease associated with BDNF C270T polymorphism. Neurosci Lett. 2005;381:275–278. doi: 10.1016/j.neulet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Riemenschneider M, Schwarz S, Wagenpfeil S, Diehl J, Muller U, Forstl H, Kurz A. A polymorphism of the brain-derived neurotrophic factor (BDNF) is associated with Alzheimer’s disease in patients lacking the Apolipoprotein E epsilon 4 allele. Mol Psychiatry. 2002;7:782–785. doi: 10.1038/sj.mp.4001073. [DOI] [PubMed] [Google Scholar]
- 18.Bagnoli S, Nacimas B, Tedde A, Guarnieri BM, Cellini E, Petruzzi C, Bartoli A, Ortenzi L, Sorbi S. Brain-derived neurotrophic factor genetic variants are not susceptibility factors to Alzheimer’s disease in Italy. Ann Neurol. 2004;55:447–448. doi: 10.1002/ana.10842. [DOI] [PubMed] [Google Scholar]
- 19.Desai P, Nebes R, DeKosky ST, Kamboh MI. Investigation of the effect of brain-derived neurotrophic factor (BDNF) polymorphisms on the risk of late-onset Alzheimer’s disease (AD) and quantitative measures of AD progression. Neurosci Lett. 2005;379:229–234. doi: 10.1016/j.neulet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Bodner SM, Berrettini W, van Deerlin V, Bennett DA, Wilson RS, Trojanowski JQ, Arnold SE. Genetic variation in the brain derived neurotrophic factor gene in Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2005;134:1–5. doi: 10.1002/ajmg.b.30154. [DOI] [PubMed] [Google Scholar]
- 21.Saarela MS, Lehtimaki T, Rinne JO, Huhtala H, Rontu R. No association between the brain-derived neurotrophic factor 196 G>A or 270 C>T polymorphisms and Alzheimer’s or Parkinson’s disease. Folia Neuropathol. 2006;44:12–16. [PubMed] [Google Scholar]
- 22.Akatsu H, Yamagata HD, Kawamata J, Kamino K, Takeda M, Yamamoto T, Miki T, Tooyama I, Shimohama S, Kosaka K. Variations in the BDNF gene with Lewy Bodies in Japan. Dement Geriatr Cogn Disord. 2006;22:216–222. doi: 10.1159/000094933. [DOI] [PubMed] [Google Scholar]
- 23.Tsai SJ, Hong CJ, Liu HC, Liu TY, Liou YJ. The brain-derived neurotrophic factor gene as a possible susceptibility candidate for Alzheimer’s disease in a Chinese population. Dement Geriatr Cogn Disord. 2006;21:139–143. doi: 10.1159/000090673. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, Rosenheck R, Price LH, Southwick S, Yang B-Z, Gelernter J. Brain Derived Neurotrophic Factor (BD-NF) genetic variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang R, Huang J, Cathcart H, Smith S, Poduslo SE. Genetic variants in brain-derived neurotrophic factor associated with Alzheimer’s disease. J Med Genet. 2007;44:e66. doi: 10.1136/jmg.2006.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 27.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val(66)met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA. Association between BDNF val66met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134:73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- 31.Tsai SJ, Hong CJ, Liu HC, Liu TY, Hsu LE, Lin CH. Association analysis of brain-derived neurotrophic factor Val66Met polymorphisms with Alzheimer’s disease and age of onset. Neuropsychobiology. 2004;49:10–12. doi: 10.1159/000075332. [DOI] [PubMed] [Google Scholar]
- 32.Combarros O, Infante J, Llorca J, Berciano J. Polymorphism at codon 66 of the brain-derived neurotrophic factor gene is not associated with sporadic Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;18:55–58. doi: 10.1159/000077736. [DOI] [PubMed] [Google Scholar]
- 33.Nacmias B, Piccini C, Bagnoli S, Tedde A, Cellini E, Bracco L, Sorbi S. Brain-derived neurotrophic factor, apolipoprotein E genetic variants and cognitive performance in Alzheimer’s disease. Neurosci Lett. 2004;367:379–383. doi: 10.1016/j.neulet.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 34.Bagnoli S, Nacmias B, Tedde A, Guarnieri BM, Cellini E, Petruzzi C, Bartoli A, Ortenzi L, Sorbi S. Brain-derived neurotrophic factor genetic variants are not susceptibility factors to Alzheimer’s disease in Italy. Ann Neurol. 2004;55:447–448. doi: 10.1002/ana.10842. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Rowland C, Tacey K, Catanese J, Sninsky J, Hardy J, Powell J, Lovestone S, Morris JC, Thal L, Goate A, Owen M, Williams JGA. The BDNF val66met polymorphism is not associated with late-onset Alzheimer’s disease in three case-control samples. Mol Psychiatry. 2005;10:809–810. doi: 10.1038/sj.mp.4001702. [DOI] [PubMed] [Google Scholar]
- 36.Bian J-T, Zhang J-W, Zhang Z-X, Zhao H-L. Association analysis of brain-derived neurotrophic factor (BDNF) gene 196 A/G polymorphism with Alzheimer’s disease (AD) in mainland Chinese. Neurosci Lett. 2005;387:11–16. doi: 10.1016/j.neulet.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37.He XM, Zhang ZX, Zhang JW, Zhou YT, Tang MN, Wu CB, Hong Z. Lack of association between the BDNF gene Val66Met polymorphism and Alzheimer disease in a Chinese Han population. Neuropsychobiology. 2007;55:151–155. doi: 10.1159/000106473. [DOI] [PubMed] [Google Scholar]
- 38.Ventriglia M, Chiavetto LB, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M. Association between the BD-NF 196 A/G polymorphism and sporadic Alzheimer’s disease. Mol Psychiatry. 2002;7:136–137. doi: 10.1038/sj.mp.4000952. [DOI] [PubMed] [Google Scholar]
- 39.Chuu JY-J, Taylor JL, Tinklenberg J, Noda A, Yesavage J. The brain-derived neurotrophic factor val66met polymorphism and rate of decline in Alzheimer’s disease. J Alzheimers Dis. 2006;9:43–49. doi: 10.3233/jad-2006-9104. [DOI] [PubMed] [Google Scholar]
- 40.Borroni B, Archetti S, Costanzi C, Grassi M, Ferrari M, Radeghieri A, Caimi L, Caltagirone C, Di Luca M, Padovani A. Role of BDNF Val66Met functional polymorphism in Alzheimer’s disease-related depression. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.11.023. in press. [DOI] [PubMed] [Google Scholar]
- 41.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 42.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, Qu GY, Grant I, Yu E, Levy P, Klauber MR, Liu WT. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 44.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 45.Letenneur L, Commenges D, Dartigues JF, Barberger-Gateau P. Incidence of dementia and Alzheimer’s disease in elderly community residents of south-western France. Int J Epidemiol. 1994;23:1256–1261. doi: 10.1093/ije/23.6.1256. [DOI] [PubMed] [Google Scholar]
- 46.Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Pilgrim DM, Taylor JO. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 48.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURO-DEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 49.Qiu C, Bäckman L, Winblad B, Agüero-Torres H, Fratiglioni L. The influence of education on clinically diagnosed dementia incidence and mortality data from the Kungsholmen Project. Arch Neurol. 2001;58:2034–2039. doi: 10.1001/archneur.58.12.2034. [DOI] [PubMed] [Google Scholar]
- 50.Lindsay J, Laurin D, Verrault R, Hebert R, Helliwell B, Mc-Dowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 51.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 52.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 53.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 54.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 55.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 56.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:33–39. [PubMed] [Google Scholar]
- 57.Kleiman TG, Zdanys KF, MacAvoy MG, Rightmer TE, Black BT, Grey M, Morgan K, Gelernter J, van Dyck CH. Apolipoprotein E e4 allele does not affect cognitive or functional decline in Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2006;22:73–82. doi: 10.1159/000093316. [DOI] [PubMed] [Google Scholar]
- 58.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 59.Zdanys K, Kleiman T, MacAvoy M, Black B, Rightmer T, Grey M, Garman K, Tampi R, Gelernter J, van Dyck C. Apolipoprotein E epsilon4 allele increases risk for psychotic symptoms in Alzheimer’s disease. Neuropsychopharmacology. 2007;32:172–179. doi: 10.1038/sj.npp.1301148. [DOI] [PubMed] [Google Scholar]
- 60.Nanko S, Kunugi H, Hirasawa H, Kato N, Nabika T, Kobayashi S. Brain-derived neurotrophic factor gene and schizophrenia: polymorphism screening and association analysis. Schizophr Res. 2003;62:281–283. doi: 10.1016/s0920-9964(02)00349-3. [DOI] [PubMed] [Google Scholar]
- 61.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 62.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 63.Farrer LA, Cupples LA, Haines JL, Hyman BT, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch NJ, van Duijn CM. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease – A meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 64.Szekeres G, Juhasz A, Rimanoczy A, Keri S, Janka Z. The C270T polymorphism of the brain-derived neurotrophic factor gene is associated with schizophrenia. Schizophr Res. 2003;65:15–18. doi: 10.1016/s0920-9964(02)00505-4. [DOI] [PubMed] [Google Scholar]
- 65.Durany N, Thome J. Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry. 2004;19:326–337. doi: 10.1016/j.eurpsy.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 66.Jonsson EG, Edman-Ahlbom B, Sillen A, Gunnar A, Kulle B, Frigessi A, Vares M, Ekholm B, Wode-Helgodt B, Schumacher J, Cichon S, Agartz I, Sedvall GC, Hall H, Terenius L. Brain-derived neurotrophic factor gene variants and schizophrenia: an association study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:924–933. doi: 10.1016/j.pnpbp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Szczepankiewicz A, Skibinska M, Czerski PM, Kapelski P, Leszczynska-Rodziewicz A, Slopien A, Dmitrzak-Weglarz M, Rybakowski JK, Hauser J. No association of the brain-derived neurotrophic factor (BDNF) gene C-270T polymorphism with schizophrenia. Schizophr Res. 2005;76:187–193. doi: 10.1016/j.schres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe Y, Muratake T, Kaneko N, Nunokawa A, Someya T. No association between the brain-derived neurotrophic factor gene and schizophrenia in a Japanese population. Schizophr Res. 2006;84:29–35. doi: 10.1016/j.schres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Kokmen E, Ozsarfati Y, Beard CM, O’Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer’s disease. J Clin Epidemiol. 1996;49:79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 70.Watson JS, Matsuyama SS, Dirham PM, Liston EH, La Rue A, Jarvik LF. Relatives’ descriptions of changes in symptoms of dementia of the Alzheimer type: a comparison of retrospective and concurrent ratings. Alzheimer Dis Assoc Disord. 1987;1:98–102. doi: 10.1097/00002093-198701020-00005. [DOI] [PubMed] [Google Scholar]