Abstract
Chromium (Cr) is a global marine pollutant, present in marine mammal tissues. Hexavalent chromium [Cr(VI)] is a known human carcinogen. In this study we compare the cytotoxic and clastogenic effects of Cr(VI) in human (Homo sapiens) and sperm whale (Physeter macrocephalus) skin fibroblasts. Our data show that increasing concentrations of both particulate and soluble Cr(VI) induce increasing amounts of cytotoxicity and clastogenicity in human and sperm whale skin cells. Furthermore, the data show that sperm whale cells are resistant to these effects exhibiting less cytotoxicity and genotoxicity than the human cells. Differences in Cr uptake accounted for some but not all of the differences in particulate and soluble Cr(VI) genotoxicity, although it did explain the differences in particulate Cr(VI) cytotoxicity. Altogether the data indicate that Cr(VI) is a genotoxic threat to whales, but also suggest that whales have evolved cellular mechanisms to protect them against the genotoxicity of environmental agents such as Cr(VI).
Keywords: Clastogenicity, hexavalent chromium, human, skin cells, sperm whale
Introduction
Chromium is a marine concern. Chromium contaminated waste waters discharge into rivers that ultimately enter the oceans and chromium contaminated air released from coastal industries deposits in marine ecosystems (ATSDR, 2008; Neff, 2002). Recently, we identified chromium (Cr) as a global marine pollutant, reporting that sperm whales from around the world had measurable chromium levels. Some were remarkably high, reaching levels previously only seen in workers that died of chromate-induced lung cancer (Wise Sr. et al., 2009). In the ocean, Cr(VI) is the predominant state for Cr (Geisler and Schmidt, 1991, Pettine and Millero, 1990), and thus, Cr(VI) is a health threat for marine species such as the sperm whales.
In humans, Cr(VI) is a well known health threat that damages DNA leading to lung cancer and reproductive effects (ATSDR 2008). In marine mammals, the health impacts are uncertain and little studied. We recently demonstrated that both particulate and soluble Cr(VI) are cytotoxic and genotoxic to whale and sea lion cells (Wise Sr. et al., 2008; Li Chen et al., 2009a; Wise et al., 2009; Wise Sr. et al., 2010; Wise Sr. et al., 2011). Considered in conjunction with Cr levels in the animals, the data suggest that the whales may be exposed to potentially genotoxic levels of Cr (Wise Sr. et al., 2008; Wise Sr. et al., 2009; Li Chen et al., 2009a; Wise Sr. et al., 2011).
Cancer incidence in marine mammals is underestimated due to unnoticed deaths in the wild and incomplete necropsies (Newman and Smith, 2006). Tumors have been reported in marine mammals, especially in California sea lions (Zalophus californianus) and St. Lawrence beluga whales (Delphinapterus leucas) (Newman and Smith, 2006). Exposure to environmental chemicals is the primary suspected cause for tumors found in these two species (Martineau et al., 2002, Newman and Smith, 2006, Ylitalo et al., 2005), although the potential role of heavy metals including Cr was not investigated.
One of the two whale species high in Cr, the North Atlantic right whale (Eubalaena glacialis) has been intensively studied on an annual basis for more than thirty years (Kraus and Rolland, 2007). Cancers have generally not been reported in this population, which may reflect either the very small population size, the potential for diseased animals to die at sea, or perhaps a resistance of these animals to the genotoxic effects of Cr. Consistent with the latter possibility, we conducted a comparative study of right whale and human cells and found that, after correcting for differences in uptake, Cr(VI) induced significantly less genotoxicity in the whale cells compared to the human cells (Li Chen et al., 2009b).
The sperm whale is much less understood and studied than the right whale in part because they are more nomadic with a larger geographic range and in part because they live in deeper waters. Tumors occur in sperm whales, but with unknown etiology (Newman and Smith, 2006). The Cr levels observed in sperm whales were much higher than those of the right whale, both as a mean level (8.8 μg /g versus 7.1 μg /g) and as a range (high 122.6 μg /g versus 10 μg /g) (Wise Sr. et al., 2008; Wise Sr. et al., 2009). These high levels raise questions about whether sperm whales have adapted cellular and molecular responses to protect them from the genotoxicity of agents like Cr. The possibility has not been studied before for sperm whale cells. Accordingly, in this study, we compared the cytotoxic and genotoxic effects of Cr(VI) in primary sperm whale and human skin cells.
Materials and Methods
Chemicals and Reagents
Lead chromate (PbCrO4), sodium chromate (Na2CrO4), demecolchicine and potassium chloride (KCl) were purchased from Sigma/Aldrich (St. Louis, MO, USA). Giemsa stain was purchased from Biomedical Specialties Inc. (Santa Monica, CA, USA). Cytoseal 60 slide mounting medium was purchased from VWR (Bridgeport, NJ, USA). Gurr’s buffer, trypsin/EDTA, sodium pyruvate, penicillin/streptomycin, and L-Glutamine were purchased from Invitrogen Corporation (Grand Island, NY). Crystal violet, methanol and acetone were purchased from J.T. Baker (Phillipsburg, NJ, USA). Dulbecco’s minimal essential medium and Ham’s F-12 (DMEM/F-12) 50:50 mixture was purchased from Mediatech Inc. (Herndon, VA, USA). Cosmic calf serum (CCS) was purchased from Hyclone, (Logan, UT, USA). MycoAlert detection kits were purchased from Lonza Rockland, Inc (Rockland, ME, USA). Tissue culture dishes, flasks, and plasticware were purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cells and Cell Culture
We used primary sperm whale skin cells isolated from a free-ranging female sperm whale off the coast of North Carolina previously described in Wise Sr. et al. (2011), and primary human skin cells (BJ cells) previously described in Vaziri and Benchimol (1998). All cells were cultured as adherent monolayers of cells and were subcultured at least once a week. Cells were maintained in Dulbecco’s minimal essential medium and Ham’s F-12 (DMEM/F-12) 50:50 mixture, supplemented with 15% cosmic calf serum, 2 mM L-glutamine, 100 U/mL penicillin/100 μg /mL streptomycin, and 0.1 mM sodium pyruvate. Cells were maintained in a humidified incubator with 5% CO2 set at 33°C for sperm whale cells and 37°C for human cells. Cells were regularly tested for mycoplasma contamination. All experiments were conducted on logarithmically growing cells with a doubling time of 24 h for human cells and 36h for sperm whale cells.
Preparation of Chromium Compounds
Lead chromate (PbCrO4), a representative particulate Cr(VI) compound (CAS# 7758-97-6, ACS reagent minimum 98% purity), was administered as a suspension of particles as previously described, to ensure that cells were exposed to intact particles (Wise et al., 2002). Briefly, PbCrO4 was weighed, suspended in deionized water and desired treatment concentrations were made from this stock suspension. Sodium chromate (Na2CrO4) , a representative soluble Cr(VI) compound (CAS #7775-11-3, ACS reagent minimum 98% purity), was administered as a solution in water as previously described (Wise et al., 2002). Briefly, Na2CrO4 was weighed and dissolved in deionized water. Desired treatment concentrations were made from a sterile filtered sample of the stock solution.
In accordance with the majority of the published Cr(VI) literature, treatments with particulate Cr(VI) are presented in μg /cm2 and treatments with soluble Cr(VI) in μM. These units reflect that fact that the lead chromate particles only partially dissolve while the sodium chromate fully dissolves. Thus, these chemicals cannot accurately be compared based on administered dose. Lead chromate treatment concentrations were 0.1, 0.5, 1, 5 and 10 μg /cm2, which correspond to 0.068, 0.34, 0.68, 3.4 and 6.8 μg Cr/mL, respectively. Sodium chromate treatment concentrations were 0.1, 0.5, 1, 2.5, 5 and 10 μM, which correspond to 0.005, 0.026, 0.05, 0.13, 0.26 and 0.5 μg Cr/mL, respectively. The best way to compare them both practically and functionally is by the amount of intracellular Cr, which is provided in the results section.
Cytotoxicity
Cytotoxicity was measured using a clonogenic assay that determines the ability of the cell to form colonies in a culture dish following chemical treatment, as described previously (Wise et al., 2002). Briefly, logarithmically growing cells were seeded into each well of 6-well tissue culture plates, cells were allowed to rest for 48 h, and then treated for 24 h with lead chromate or sodium chromate. At the end of the treatment time, cells were collected, counted and re-seeded into 100 mm tissue culture dishes at a density of 1000 cells/dish, four dishes were seeded for each concentration. These dishes were grown for about two weeks until colonies formed, stained and counted. Experiments were repeated at least three times. All treatment groups were compared to the control and expressed as a percentage of the control.
Clastogenicity
Clastogenicity was measured using the chromosome damage assay that determines the ability of Cr to induce chromosomal structural aberrations in metaphase chromosomes, as described previously (Wise et al., 2002). Briefly, logarithmically growing cells were seeded into 100 mm tissue culture dishes, cells were allowed to rest for 48 h, and then treated for 24 h with lead chromate or sodium chromate. Near the end of the treatment time, demecolchicine was added to arrest cells in metaphase. At the end of the treatment, cells were collected, resuspended in a hypotonic solution of 0.075 M KCl, and then fixed with 3:1 methanol:acetic acid. Fixative was changed twice, and then cells were dropped onto wet clean slides, stained and coverslipped. Experiments were repeated at least three times. Chromosomal structural aberrations were scored in 100 metaphases per each treatment dose, according to standard criteria previously described (Wise et al., 2008). Results were expressed as a percentage of metaphases with chromosome damage and as the total chromosome damage observed in the 100 metaphases analyzed.
Determination of Intracellular Chromium Ion Levels
Cell preparation
Intracellular ion levels were measured using the ion uptake assay, as described previously (Holmes et al., 2005), with minor changes. Briefly, logarithmically growing cells were seeded into 100 mm tissue culture dishes, allowed to rest for 48 h and then treated for 24 h. At the end of the treatment, 3 mL of treated culture media was saved for extracellular chromium analysis; cells were collected and the number and volume of cells were determined. Cells were washed twice with PBS, resuspended in 1 mL hypotonic solution followed by 1 mL 2% SDS. Finally the solution was sheered and filtered. Samples were stored at −20°C until analysis.
Ion Level Measurements
Intracellular Cr ion levels were determined using the Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES), equipped with a gem cone low flow nebulizer, according to previously published methods (Holmes et al., 2005). Solutions were introduced to the nebulizer using a peristaltic pump operating at 2 mL/min. Samples of intracellular fluids were diluted 5x in 0.16 M aqueous HNO3 prior to analysis. Chromium was determined using emission wavelength at 267.716 with a minimum detection limit of 2 ppb. Yttrium (Y) was used as an internal standard for chromium determinations. The intracellular concentrations were converted from μg /L to μM by dividing by the volume of the sample, the atomic weight of the chemical, the number of cells in the sample and the average cell volume. To account for the possibility of undissolved particulate Cr(VI) passing through the filter, 0 h treatments were performed and these values were subtracted from the measurements obtained for the 24 h treatments.
Statistical Analyses
Dose-response relationships for both the amount of chromate administered and the estimated intracellular concentrations were assessed using multiple regression methods. Both linear and nonlinear models were fit to the data. Interaction terms were introduced into the models to provide for differences between species in the functional form of the dose-response relationships. Estimates were obtained via maximum likelihood estimation, and 95% confidence intervals (CIs) were calculated using Wald statistics. Linear combinations of parameters were employed for the evaluation of differences between species at particular levels of administered dose or intracellular concentration. P-values were obtained from Wald chi-square statistics and no correction for multiple comparisons was made. All analyses were conducted using SAS (SAS Institute, 2008).
Results
Cr(VI) is more cytotoxic to human skin cells than to sperm whale skin cells
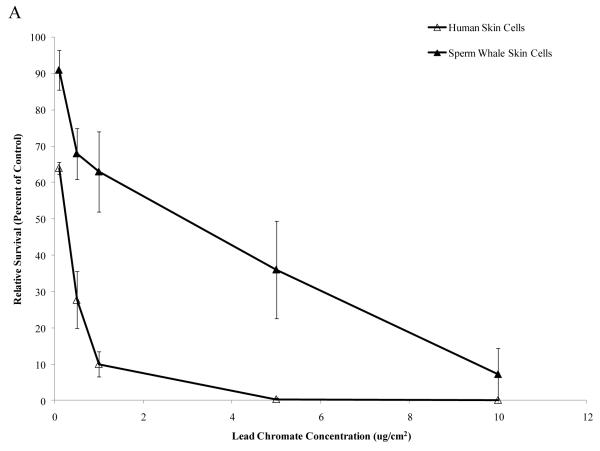
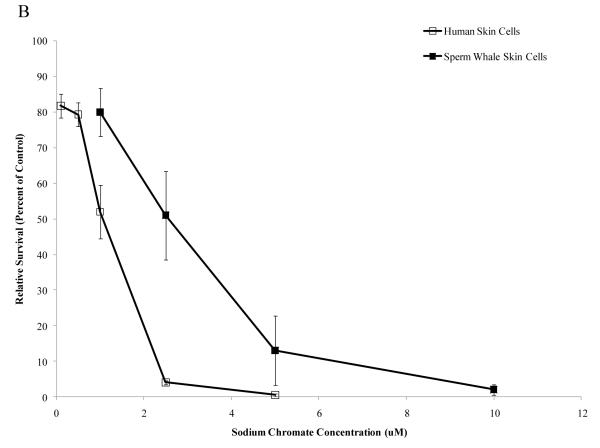
Particulate and soluble Cr(VI) were cytotoxic in a concentration-dependent manner to both whale and human cells. Whale cells were more resistant to both compounds. Concentrations of 0.1, 0.5, 1, 5 and 10 μg /cm2 particulate Cr(VI) induced 64, 28, 10, 0 and 0% relative survival, respectively, in human skin cells. In sperm whale skin cells, these same concentrations induced 91, 68, 63, 36 and 7% relative survival, respectively (Fig. 1A) (difference between sperm whale and human skin cells at 1 μg /cm2 was 40.5% based on the fitted values; CI=27.9% to 53.1%; p<0.001). Concentrations of 1, 2.5 and 5 μM soluble Cr(VI) induced 52, 4 and 0% relative survival, respectively, in human skin cells, while in sperm whale skin cells these concentrations induced 80, 51 and 13% relative survival, respectively (Fig.1B) (difference between sperm whale and human skin cells at 1 μM was 20.5% based on the fitted values; CI=8.2% to 32.8%; p<0.001).
Figure 1. Sperm Whale Skin Cells Are More Resistant to Cr(VI) Cytotoxicity than Human Skin Cells.
This figure shows that exposure to Cr(VI) induces cytotoxicity in human and sperm whale skin cells in a concentration-dependent manner. (A) Particulate Cr(VI) cytotoxicity in human and sperm whale skin cells. Significant differences between the two cell lines were observed (difference between sperm whale and human skin cells at 1 μg /cm2 was 40.5% based on the fitted values; CI=27.9% to 53.1%; p<0.001). (B) Soluble Cr(VI) cytotoxicity in human and sperm whale skin cells. Significant differences between the two cell lines were observed (difference between sperm whale and human skin cells at 1 μM was 20.5% based on the fitted values; CI=8.2% to 32.8%; p<0.001). Data represent the average of 3 independent experiments. Error bars = standard error of the mean.
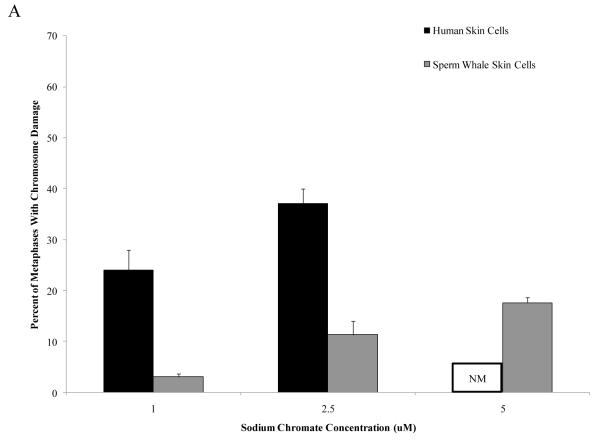
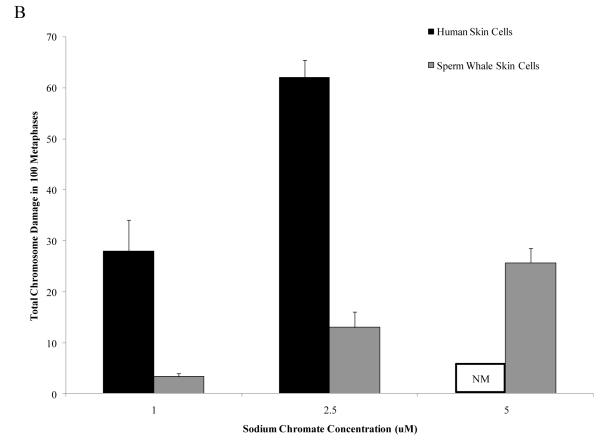
Cr(VI) is more clastogenic to human skin cells than to sperm whale skin cells
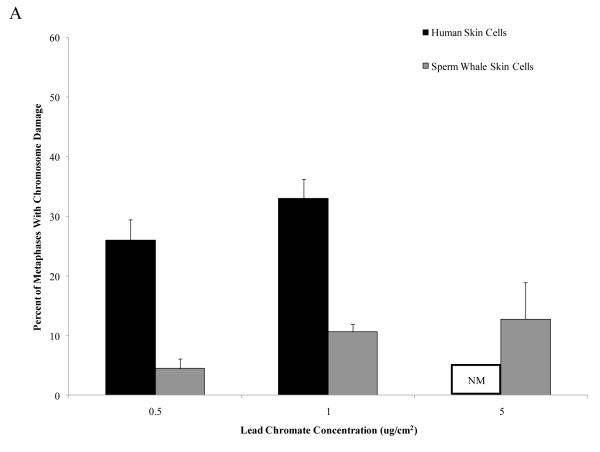
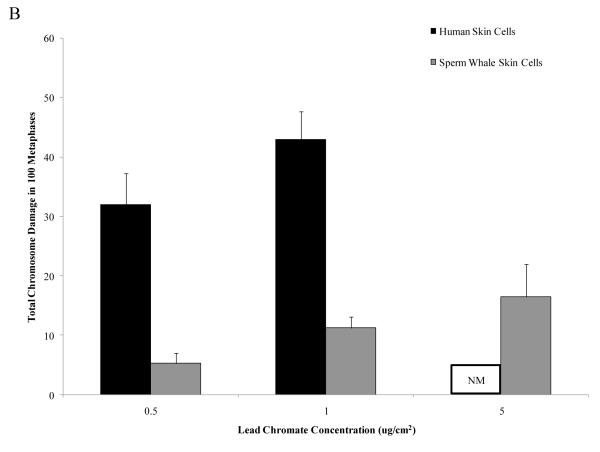
Particulate and soluble Cr(VI) were clastogenic in a concentration-dependent manner and induced a similar spectrum of damage in both whale and human cells. Chromatid and isochromatid gaps and breaks were the predominant types of chromosomal aberrations induced (Table 1). Whale cells were also more resistant to Cr(VI) clastogenicity. For example, in human cells particulate Cr(VI) concentrations of 0.5 and 1 μg /cm2 lead chromate damaged chromosomes in 26 and 33% of metaphases and induced a total of 32 and 43 aberrations in 100 metaphases, respectively. By contrast, in sperm whale cells, these concentrations damaged chromosomes in 11 and 13% of metaphases and induced a total of 11 and 16 aberrations in 100 metaphases, respectively (Fig. 2) (difference between sperm whale and human skin cells for percent of metaphases with damage at 1 μg /cm2 was −22.7% based on the fitted values; CI = −17.0% to −28.3%; p<0.001; difference for total damage in 100 metaphases at 1 μg /cm2 was −31.4 based on the fitted values; CI= −25.2 to −37.7; p<0.001). In other words, particulate Cr(VI) damaged chromosomes in 3 to 5-fold fewer metaphases and induced 4 to 6-fold fewer total aberrations in whale cells compared to human cells. This decreased clastogenicity is also reflected in the observations of the amount of cells with multiple aberrations. For human cells, increasing concentrations of Cr(VI) showed an increase in metaphases with multiple chromosomal aberrations, while cells with multiple chromosome aberrations were less common in whale cells. For example, in human cells, 1 μg /cm2 lead chromate produced an average of 8 metaphases with multiple aberrations, compared to an average of 1 for sperm whale cells.
Table 1.
Spectrum of Chromosome Damage in Sperm Whale and Human Skin Cells After Particulate and Soluble Cr(VI).
|
Chromosome Aberration Type
|
Total
aberrations observed |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Chromatid Break |
Chromatid Gap |
Isochromatid Break |
Isochromatid Gap |
Acentric Fragment |
Double Minute |
Dicentric | Chromatid Exchange |
|
| Sperm Whale Skin Cells | |||||||||
| Lead Chromate (μg /cm2) | |||||||||
| 0 | 1±0.5 | 1±0.3 | 0±0.2 | 0±0.1 | 0±0.0 | 0±0.0 | 0±0.1 | 0±0.0 | 2±0.6 |
| 0.5 | 3±0.7 | 3±1.0 | 0±0.3 | 0±0.3 | 0±0.3 | 0±0.0 | 0±0.0 | 0±0.3 | 7±1.3 |
| 1 | 7±1.0 | 2±0.8 | 2±0.7 | 0±0.0 | 0±0.2 | 1±0.4 | 0±0.0 | 0±0.0 | 13±1.6 |
| 5 | 9±5.1 | 7±1.3 | 2±0.9 | 2±1.7 | 0±0.3 | 0±0.0 | 0±0.0 | 0±0.0 | 20±4.7 |
| Sodium Chromate (μM) | |||||||||
| 0 | 1±0.5 | 2±0.5 | 0±0.0 | 0±0.0 | 0±0.0 | 0±0.0 | 0±0.3 | 0±0.0 | 4±0.8 |
| 1 | 3±0.3 | 3±0.3 | 0±0.0 | 0±0.3 | 0±0.3 | 0±0.0 | 0±0.3 | 0±0.0 | 8±0.7 |
| 2.5 | 6±1.5 | 10±12 | 0±0.3 | 0±0.3 | 1±0.7 | 0±0.0 | 0±0.3 | 0±0.0 | 17±2.7 |
| 5 | 11±0.8 | 17±2.6 | 1±1.0 | 0±0.4 | 0±0.3 | 0±0.3 | 0±0.0 | 0±0.0 | 30±3.1 |
| Human Skin Cells | |||||||||
| Lead Chromate (μg /cm2) | |||||||||
| 0 | 1±0.3 | 1±0.9 | 0±0.0 | 0±0.0 | 0±0.0 | 1±0.7 | 0±0.0 | 0±0.0 | 3±1.3 |
| 0.5 | 18±2.3 | 12±1.2 | 3±1.5 | 1±0.3 | 1±0.6 | 0±0.0 | 0±0.0 | 1±0.0 | 35±4.5 |
| 1 | 30±3.6 | 12±2.2 | 2±0.8 | 1±1.0 | 0±0.0 | 1±0.3 | 0±0.0 | 0±0.3 | 45±3.7 |
| 5 | NM | NM | NM | NM | NM | NM | NM | NM | NM |
| Sodium Chromate (μM) | |||||||||
| 0 | 0±0.3 | 1±0.3 | 1±0.7 | 0±0.3 | 0±0.0 | 0±0.0 | 0±0.0 | 0±0.0 | 2±1.0 |
| 1 | 16±4.3 | 11±1.0 | 1±1.0 | 1±0.4 | 1±0.4 | 0±0.0 | 0±0.0 | 0±0.4 | 31±6.7 |
| 2.5 | 33±3.7 | 13±2.5 | 5±0.6 | 2±1.8 | 1±0.6 | 1±0.7 | 0±0.5 | 1±1.0 | 64±3.5 |
| 5 | NM | NM | NM | NM | NM | NM | NM | NM | NM |
Values represent the number of occurrences for each type of chromosome aberration.
Figure 2. Sperm Whale Skin Cells Are More Resistant to Particulate Cr(VI) Clastogenicity than Human Skin.
This figure shows that exposure to particulate Cr(VI) induces clastogenicity in human and sperm whale skin cells in a concentration-dependent manner. Clastogenicity is presented as a percent of metaphases with chromosome damage and total chromosome damage in 100 metaphases for each cell line. (A) Particulate Cr(VI)-induced percent of metaphases with chromosome damage. Significant differences between the two cell lines were observed (difference between sperm whale and human skin cells for percent of metaphases with damage at 1 μg /cm2 was −22.7% based on the fitted values; CI = −17.0% to −28.3%; p<0.001). Untreated control values were subtracted from each concentration. The percent metaphases with damage for untreated controls was 2% for both sperm whale and human cells. (B) Particulate Cr(VI)-induced total chromosome damage in 100 metaphase. Significant differences between the two cell lines were observed (difference for total damage in 100 metaphases at 1 μg /cm2 was −31.4 based on the fitted values; CI= −25.2 to −37.7; p<0.001.). Untreated control values were subtracted from each concentration. The total damage in 100 metaphases for untreated controls was 2 for sperm whale cells and 3 for human cells. Data represent the average of at least 3 independent experiments. Error bars = standard error of the mean. NM – no metaphases observed.
The outcome was similar for soluble Cr(VI). Concentrations of 1 and 2.5 μM sodium chromate damaged chromosomes in 24 and 37 percent of metaphases and induced a total of 28 and 62 aberrations in 100 metaphases, respectively, in human skin cells. In sperm whale skin cells, these concentrations damaged chromosomes in 11 and 18% metaphases and induced a total of 13 and 26 aberrations in 100 metaphases, respectively (Fig. 3) (difference between sperm whale and human skin cells for percent of metaphases with damage at 1 μM was −20.2% based on the fitted values; CI= −16.7% to −23.7%; p<0.001; difference for total damage in 100 metaphases at 1 μM was −24.3 based on the fitted values; CI= −19.4 to −29.2; p<0.001). In other words, soluble Cr(VI) damaged chromosomes in 3 to 8-fold fewer metaphases and induced 5 to 9-fold fewer total aberrations in whale cells compared to human cells.
Figure 3. Sperm Whale Skin Cells Are More Resistant to Soluble Cr(VI) Clastogenicity than Human Skin.
This figure shows that exposure to soluble Cr(VI) induces clastogenicity in human and sperm whale skin cells in a concentration-dependent manner. Clastogenicity was measured as a percent of metaphases with chromosome damage and total chromosome damage in 100 metaphases for each cell line. (A) Soluble Cr(VI)-induced percent of metaphases with chromosome damage. Significant differences between the two cell lines were observed (difference between sperm whale and human skin cells for percent of metaphases with damage at 1 μM was −20.2% based on the fitted values; CI= −16.7% to −23.7%; p<0.001). Untreated control values were subtracted from each concentration. The percent metaphases with damage for untreated controls was 2% for sperm whale cells and 4% for human cells. (B) Soluble Cr(VI)-induced total chromosome damage in 100 metaphases. Significant differences between the two cell lines were observed (difference for total damage in 100 metaphases at 1 μM was −24.3 based on the fitted values; CI= −19.4 to −29.2; p<0.001). Untreated control values were subtracted from each concentration. The total damage in 100 metaphases for untreated controls was 2 for sperm whale cells and 4 for human cells. Data represent the average of at least 3 independent experiments. Error bars = standard error of the mean. NM – no metaphases observed.
This decreased clastogenicity is again also reflected in the observations of the amount of cells with multiple aberrations. For human cells, increasing concentrations of Cr(VI), showed an increase in metaphases with multiple chromosomal aberrations, while cells with multiple chromosome aberrations were again less common in whale cells. For example, in human cells, 2.5 μM sodium chromate produced an average of 14 metaphases with multiple aberrations, compared to an average of 1 for sperm whale cells.
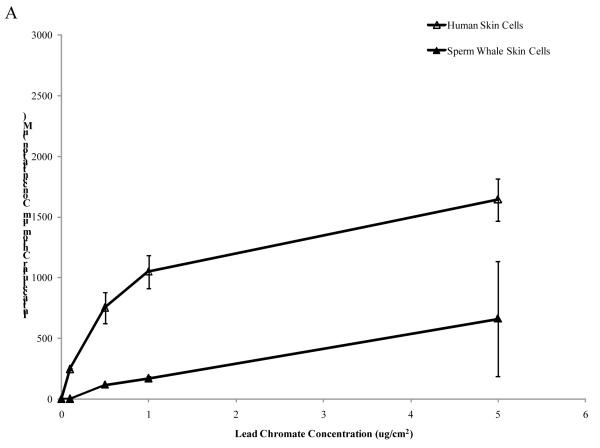
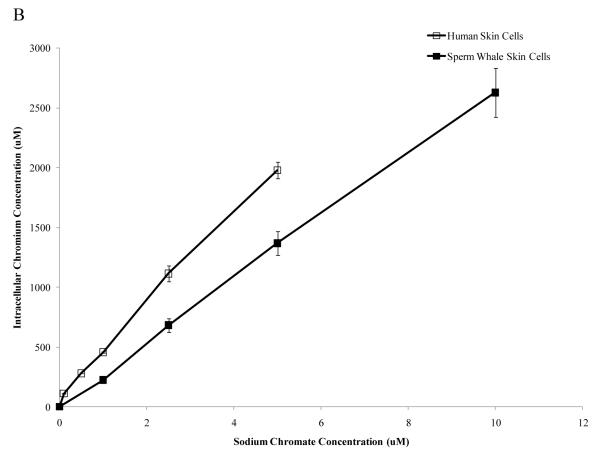
Human skin cells take up more Cr than sperm whale skin cells
One possible explanation for the reduced amount of cytotoxicity and genotoxicity in the whale cells is that they may take up less Cr(VI). To eliminate possible differences in Cr(VI) uptake, we measured the intracellular Cr ion concentrations in human and whale cells (Fig. 4) after 24 h exposures. Both cell lines took up increasing amounts of Cr after both particulate and soluble Cr treatments. After exposure to 0.1, 0.5, 1 and 5 μg /cm2 particulate Cr(VI) human cells had intracellular levels of 245, 753, 1049 and 1644 μM Cr, respectively. Sperm whale skin cells treated with 0.5, 1, 5 and 10 μg /cm2 particulate Cr(VI) had intracellular Cr levels of 116, 169, 661 and 963 μM, respectively (Fig. 4A) (linear component of the relationship between administered dose and intracellular concentration was 275 μM less per μg /cm2 for sperm whale cells than for human cells; CI = 153 to 397; p<0.001). Concentrations of 0.1, 0.5, 1, 2.5 and 5 μM soluble Cr(VI), produced intracellular Cr levels of 111, 280, 456, 1116 and 1979 μM, respectively, in human skin cells. In sperm whale skin cells, concentrations of 1, 2.5, 5 and 10 μM produced 224, 682, 1369 and 2629 uM of intracellular Cr levels, respectively (Fig. 4B) (linear component of the relationship between administered dose and intracellular concentration was 235 μM less per μM for sperm whale cells than for human cells; CI = 134 to 337; p<0.001).
Figure 4. Sperm Whale Skin Cells Take Up Less Cr than Human Skin Cells.
This figure shows that after exposure to Cr(VI) human and sperm whale skin cells take up Cr in a concentration-dependent manner. (A) Cr uptake after particulate Cr(VI) treatment. Significant differences between the two cell lines were observed (linear component of the relationship between administered dose and intracellular concentration was 275 μM less per μg /cm2 for sperm whale cells than for human cells; CI = 153 to 397; p<0.001). (B) Cr uptake after soluble CrVI) treatment. Significant differences between the two cell lines were observed (linear component of the relationship between administered dose and intracellular concentration was 235 μM less per μM for sperm whale cells than for human cells; CI = 134 to 337; p<0.001). Data represent the average of 3 independent experiments. Error bars = standard error of the mean.
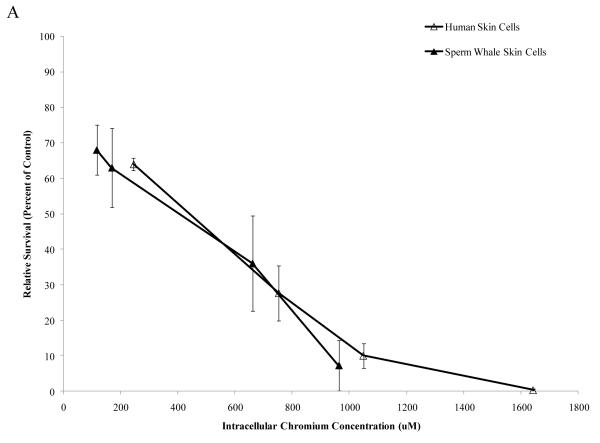
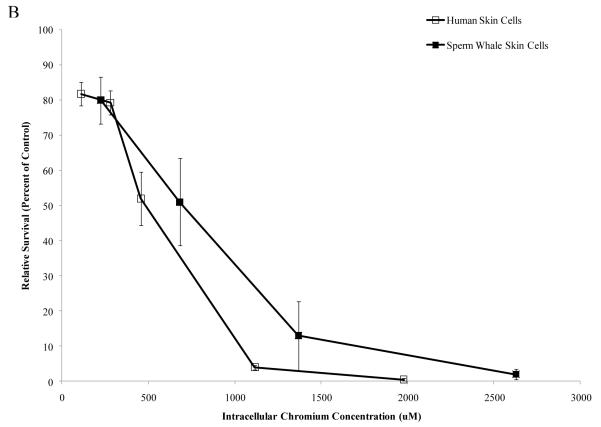
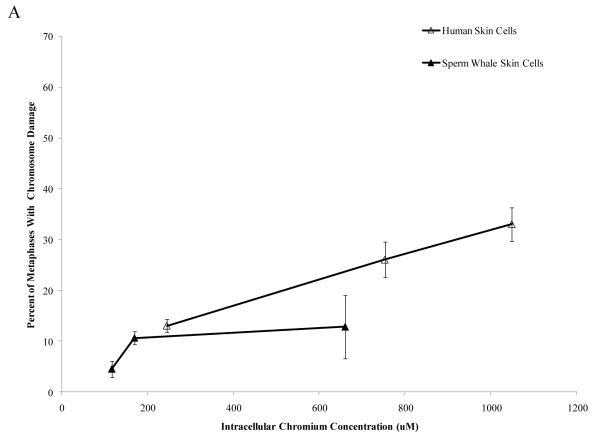
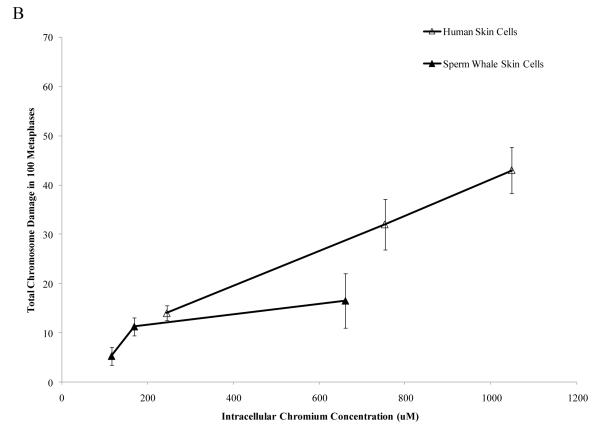
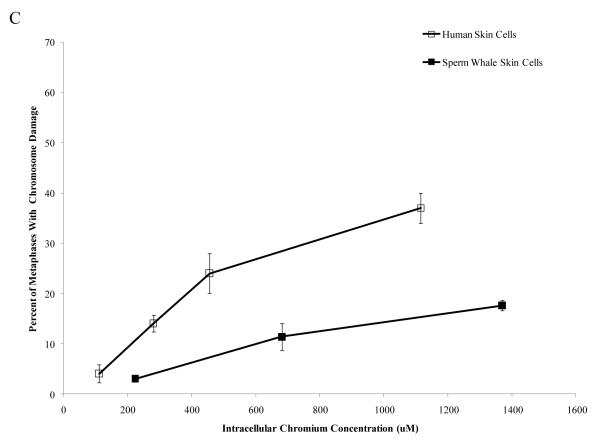
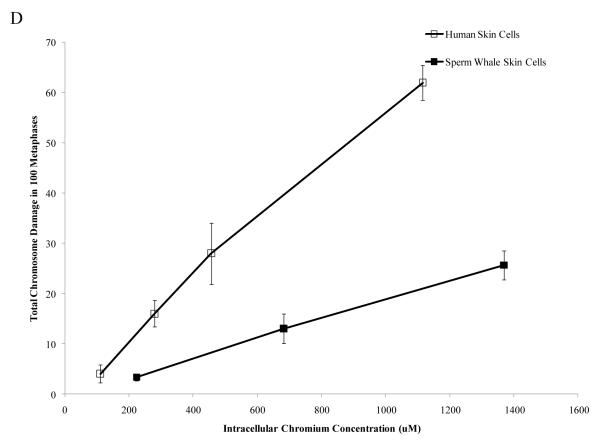
Interestingly, when data were corrected for difference in intracellular Cr levels, particulate Cr(VI) was equipotent in inducing cytotoxicity for both human and sperm whale skin cells indicating that the observed differences were due to differential uptake (difference between sperm whale and human cells in survival at 400 μM was 3.1% based on fitted values; CI = −12.1% to 18.2%; p = 0.69). By contrast, soluble Cr(VI) was still more toxic to human cells than to sperm whale skin cells indicating that these differences were not due to differences in uptake (Fig. 5) (difference between sperm whale and human cells in survival at 800 μM was 21.7% based on fitted values; CI = 10.4% to 33.1%; p<0.001). The difference between these effects for the particulate versus the soluble forms was statistically reliable; p = 0.01). Similarly, correcting the clastogenicity data for intracellular Cr levels, sperm whale skin cells still show a lower amount of chromosome damage for both Cr(VI) forms (Fig. 6), indicating that the observed differences were not due to a differential uptake (difference in percent of metaphase with damage for the particulate form at 800 μM was −31.3%; CI = −9.2% to −53.4%; p<0.006. Difference in total damage for the particulate form at 800 μM was −32.3; CI = −6.9 to −57.8; p = 0.01. Difference in percent of metaphase with damage for the soluble form at 800 μM was −21.3%; CI = −17.0% to −25.6%; p<0.001. Difference in total damage for the soluble form at 800 μM was −31.6; CI = −25.7 to −37.4; p<0.001).
Figure 5. Differences in Cr Uptake Explain the Resistance of Whale Cells to Particulate Cr(VI) Cytotoxicity but Not Soluble Cr(VI) Cytotoxicity.
This figure shows that Cr(VI) induced cytotoxicity in human and sperm whale skin cells as a function of intracellular Cr concentration. In accordance with the majority of the published Cr(VI) literature, treatments with particulate Cr(VI) are presented in μg /cm2 and treatments with soluble Cr(VI) in μM. These units reflect that fact that the lead chromate particles only partially dissolve while the sodium chromate fully dissolves. Thus, these chemicals cannot accurately be compared based on administered dose. Lead chromate treatment concentrations of 0.1, 0.5, 1, 5 and 10 μg /cm2 correspond to 0.068, 0.34, 0.68, 3.4 and 6.8 μg Cr/mL, respectively. Sodium chromate treatment concentrations of 0.1, 0.5, 1, 2.5, 5 and 10 μM correspond to 0.005, 0.026, 0.05, 0.13, 0.26 and 0.5 μg Cr/mL, respectively. (A) Particulate Cr(VI)-induced cytotoxicity in human and sperm whale skin cells based on intracellular Cr levels. Significant differences between the two cell lines were not observed (difference between sperm whale and human cells in survival at 400 μM was 3.1% based on fitted values; CI = −12.1% to 18.2%; p = 0.69). (B) Soluble Cr(VI)-induced cytotoxicity in human and sperm whale skin cells based on intracellular Cr levels. Significant differences between the two cell lines were observed (difference between sperm whale and human cells in survival at 800 μM was 21.7% based on fitted values; CI = 10.4% to 33.1%; p<0.001. The difference between these effects for the particulate versus the soluble was statistically reliable p = 0.01). Data represent the average of 3 independent experiments. Error bars = standard error of the mean.
Figure 6. Differences in Cr Uptake Cannot Explain the Resistance of Whale Cells to Cr(VI) Clastogenicity.
This figure shows that exposure to Cr(VI) induces clastogenicity in human and sperm whale skin cells as a function of intracellular Cr concentration. Clastogenicity is presented as a percent of metaphases with chromosome damage and total chromosome damage in 100 metaphases for each cell line. (A) Particulate Cr(VI)-induced percent metaphases with chromosome damage as a function of intracellular Cr concentration. Significant differences between the two cell lines were observed (difference in percent of metaphase with damage for the particulate form at 800 μM was −31.3%; CI = −9.2% to −53.4%; p=0.006). Untreated control values were subtracted from each concentration. The percent metaphases with damage for untreated controls was 2% for both sperm whale and human cells. (B) Particulate Cr(VI)-induced total chromosome damage in 100 metaphases as a function of intracellular Cr concentration. Significant differences between the two cell lines were observed (difference in total damage for the particulate form at 800 μM was −32.3; CI = −6.9 to −57.8; p = 0.01). Untreated control values were subtracted from each concentration. The total damage in 100 metaphases for untreated controls was 2 for sperm whale cells and 3 for human cells. (C) Soluble Cr(VI)-induced percent metaphases with chromosome damage as a function of intracellular Cr concentration. Significant differences between the two cell lines were observed (difference in percent of metaphase with damage for the soluble form at 800 μM was −21.3%; CI = −17.0% to −25.6%; p<0.001). Untreated control values were subtracted from each concentration. The percent metaphases with damage for untreated controls was 2% for sperm whale cells and 4% for human cells. (D) Soluble Cr(VI)-induced total chromosome damage in 100 metaphases as a function on intracellular Cr concentration. Significant differences between the two cell lines were observed (difference in total damage for the soluble form at 800 μM was −31.6; CI = −25.7 to −37.4; p<0.001). Untreated control values were subtracted from each concentration. The total damage in 100 metaphases for untreated controls was 2 for sperm whale cells and 4 for human cells. Data represent the average of at least 3 independent experiments. Error bars = standard error of the mean.
Discussion
Sperm whales are exposed to remarkably high levels of Cr suggesting that they may have evolved protective cellular and molecular mechanisms to prevent Cr toxicity. We investigated this possibility by directly comparing the cytotoxic and clastogenic effects of particulate and soluble Cr(VI) in primary human and sperm whale skin fibroblasts. Our data show that Cr(VI) is cytotoxic and clastogenic to skin cells from both organisms; however, sperm whale cells are significantly more resistant to Cr(VI)-induced cytotoxicity and genotoxicity than human cells. Some of the difference is due to the fact that whale cells take up less Cr(VI) than human cells. However, differential uptake could not explain the differences in genotoxicity for either compound or the differences in cytotoxicity for soluble Cr(VI).
Only two previous studies have considered the cellular toxic effects of heavy metals in marine mammals and compared it to human model systems. One study found that primary North Atlantic right whale lung and skin cells were more resistant than human cells to the cytotoxic and genotoxic effects of particulate and soluble Cr(VI) and the other found that primary Steller sea lion lung cells were similarly more resistant (Li Chen et al., 2009b, Wise Sr. et al, 2010). This study is consistent with those studies and extends the observations of protective cellular mechanisms in marine mammals from a baleen whale (right whale) and a pinniped (sea lion) to include sperm whales, a toothed whale. Thus, the data suggest that marine mammals, in general, may have novel cellular mechanisms to protect them from genotoxic environmental agents.
Sperm whale cells were resistant to the genotoxic effects of both particulate and soluble Cr(VI). Differences in intracellular Cr levels accounted for some of the difference, but could not explain all of the difference we observed. The underlying explanation for this increased resistance is uncertain. The mechanism for Cr(VI)-induced toxicity was recently reviewed (Wise et al., 2008). Cr(VI) ions cross the cell membrane through an anion transport system and are reduced to Cr(III) generating a series of reactive intermediates, including reactive oxygen species. Then Cr(III) and/or other intermediates cause DNA adducts, which lead to a stalled replication fork. The cell attempts to repair this damage, and during that repair, DNA double strand breaks are formed. These breaks then go on to form chromosomal aberrations. One possible explanation for the lower amount of chromosome damage observed in the sperm whale skin cells could be that the whales have evolved a more efficient DNA repair system that protects them against Cr(VI)-induced DNA damage. This possibility is consistent with the hypothesis that larger animals have evolved protective mechanisms against cancer (Leroi et al., 2003).
Alternatively, it may be that marine mammals have altered detoxifying pathways for Cr(VI). Antioxidants are important in reducing Cr(VI) toxicity by neutralizing reactive Cr intermediates and reactive oxygen species generated by Cr(VI) intracellular metabolism. Marine mammals synthesize ascorbic acid (Nakajima et al., 1969) and higher levels of ascorbic acid may protect against reactive oxygen species (Bodannes and Chan, 1979, Kaczmarek et al., 2007) or shift the spectrum of reactive intermediates produced during Cr(VI) reduction (Sugiyama, 1992). Similarly, glutathione metabolism plays a role in decreasing the harmful effects of Cr(VI) by reducing Cr(VI) reactive intermediates to less reactive Cr(III) (Lushchak et al., 2008). Thus, marine mammals may also have higher levels of antioxidants and/or associated enzymes. Further investigation is needed to elucidate these possible detoxifying cellular mechanisms.
We also observed that whale cells were resistant to the cytotoxic effects of particulate and soluble Cr(VI). For soluble Cr(VI), the differences in uptake could not fully explain the increased resistance. Most likely, the decreased cytotoxicity is due to the fact that there is less genotoxicity in the whale cells. However, for particulate Cr(VI) the outcome was different. The difference in intracellular Cr uptake did account for all of the increased resistance. Previous studies in human lung cells indicated that for particulate Cr(VI) compounds, the cytotoxic effect was due to the chromate anion (Holmes et al., 2005). In that study, lead chromate and sodium chromate induced the same amount of cell death based on intracellular chromium levels. Thus, one would predict that the particulate form would behave just as the soluble form did in whale cells. However, this outcome was not the case. In fact, in considering our data, in the whale cells, particulate Cr(VI) was more cytotoxic than soluble Cr(VI) based on intracellular Cr levels. One possible explanation is that in whale cells, the lead counter ion in the particulate compound is more active. The lead cation might be enhancing the cytotoxic response to the Cr(VI) anion. Lead ions have been shown to induce apoptosis in mammalian cells (Pulido and Parrish, 2003; Singh et al., 1999). It may be that whale cells are more sensitive to lead, causing it to appear that the difference in Cr(VI) uptake can account for the cytotoxic differences between whale and human cells, when in actuality both lead and chromium are causing toxicity in the whale cells and only chromium is causing toxicity in the human cells. Another possible explanation is that whale cells are more sensitive to the uptake of undissolved particles than human cells, though we have not located any data to support this hypothesis.
We also found differences in Cr(VI) uptake between the whale and human cells with less uptake in the whale cells. The mechanism for this difference is uncertain. Cr(VI) enters cells via facilitated diffusion involving anion transport proteins (Wise et al., 2008). It may be that whale cells have fewer of these transport proteins in their membranes. Alternatively, it may be that the whales have evolved transport proteins with a lesser affinity for Cr(VI). Further research is needed to distinguish between these possibilities.
In summary, our study shows that Cr compounds induce cytotoxicity and clastogenicity in both human and sperm whale skin cells and that sperm whale cells have different, and perhaps more efficient mechanisms against the genotoxic effects of Cr. Possible mechanisms include better DNA repair or a different intracellular metabolism of Cr(VI). Our data also suggest that whale cells may be sensitive to lead ions. Further investigations are being carried out to investigate these possibilities.
Acknowledgements
We thank Jill Sinagra and Christy Gianios, Jr. for administrative and technical support on this project. We also thank Dr. Steve Patierno for the BJ cells. This work was funded by the National Institute of Environmental Health Sciences grant number ES016893 (J.P.W.) and the Maine Center for Toxicology and Environmental Health. Work was conducted under NMFS permit #1008-1637-00 (J.PW.).
Footnotes
This paper is based on a presentation given at the 5th Aquatic Annual Models of Human Disease conference: hosted by Oregon State University and Texas State University-San Marcos, and convened at Corvallis, OR, USA September 20-22, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Chromium. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2008. [Google Scholar]
- Bodannes RS, Chan PC. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979;105:195–196. doi: 10.1016/0014-5793(79)80609-2. [DOI] [PubMed] [Google Scholar]
- Geisler CD, Schmidt D. An overview of chromium in the marine environment. Dt. Hydrogr. Z. 1991;44:185–196. [Google Scholar]
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP., Sr. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2005;203:167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Kaczmarek M, Timofeeva OA, Karaczyn A, Malyguine A, Kasprzak KS, Salnikow K. The role of ascorbate in the modulation of HIF-1alpha protein and HIF-dependent transcription by chromium(VI) and nickel(II) Free Radic. Biol. Med. 2007;42:1246–57. doi: 10.1016/j.freeradbiomed.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus SD, Rolland RM. The urban whale: North Atlantic right whales at the crossroads. Harvard University Press; 2007. [Google Scholar]
- Leroi AM, Koufopanou V, Burt A. Cancer selection. Nat. Rev. Cancer. 2003;3:226–231. doi: 10.1038/nrc1016. [DOI] [PubMed] [Google Scholar]
- Li Chen T, Wise SS, Kraus S, Shaffiey F, Grau M, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T, Wise JP., Sr. Particulate hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and skin fibroblasts. Environ. Mol. Mutagen. 2009a;50:387–393. doi: 10.1002/em.20471. [DOI] [PubMed] [Google Scholar]
- Li Chen T, Wise SS, Homes A, Shaffiey F, Wise JP, Jr., Thompson WD, Kraus S, Wise JP., Sr. Cytotoxicity and gentoxicity of hexavalent chromium in human and North Atlantic right whale (Eubalaena glacialis) lung cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009b;150:487–494. doi: 10.1016/j.cbpc.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak OV, Kubrak OI, Mykorak MZ, Storey KB, Lushchak VI. The effect of potassium dichromate on free radical processes in goldfish: possible protective role of glutathione. Aquat. Toxicol. 2008;87:108–114. doi: 10.1016/j.aquatox.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Martineau D, Lemberger K, Dallaire A, Labelle P, Lipscomb TP, Michel P, Mikaelian I. Cancer in wildlife, a case study: beluga from the St. Lawrence Estuary, Québec, Canada. Environ. Health Perspect. 2002;110:285–292. doi: 10.1289/ehp.02110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Shantha TR, Bourne GH. Histochemical detection of L-gulonolactone: phenazine methosulfate oxidoreductase activity in several mammals with special reference to synthesis of Vitamin C in primates. Histochemie. 1969;18:293–301. doi: 10.1007/BF00279880. [DOI] [PubMed] [Google Scholar]
- Neff JM. Chromium in the Ocean. In: Neff JM, editor. Bioaccumulation in marine organisms: effect of contaminants from oil well produced water. Elsevier Ltd; 2002. pp. 131–143. [Google Scholar]
- Newman SJ, Smith SA. Marine mammal neoplasia. Vet. Pathol. 2006;43:865–880. doi: 10.1354/vp.43-6-865. [DOI] [PubMed] [Google Scholar]
- Pettine M, Millero FJ. Chromium speciation in seawater: the probable role of hydrogen peroxide. Limnol. Oceanogr. 1990;35:730–736. [Google Scholar]
- Pulido MD, Parrish AR. Metal-induced apoptosis: mechanisms. Review. Mutat. Res. 2003;533:227–241. doi: 10.1016/j.mrfmmm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide. SAS Institute Inc.; Cary, NC: 2008. [Google Scholar]
- Singh J, Pritchard DA, Carlisle DL, Mclean JA, Montaser A, Orenstein JM, Patierno SR. Internalization of carcinogenic lead chromate particles by cultured normal human lung epithelial cells: Formation of intracellular lead-inclusion bodies and induction of apoptosis. Toxicol. Appl. Pharmacol. 1999;161:240–248. doi: 10.1006/taap.1999.8816. [DOI] [PubMed] [Google Scholar]
- Sugiyama M. Role of physiological antioxidants in chromium (VI)-induced cellular injury. Free Radic. Biol. Med. 1992;12:397–407. doi: 10.1016/0891-5849(92)90089-y. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- Wise SS, Holmes AL, Wise JP., Sr. Hexavalent chromium-induced DNA damage and repair mechanisms. Rev. Environ. Health. 2008;23(1):39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- Wise SS, Shaffiey F, LaCerte C, Goertz CE, Dunn JL, Gulland FM, Aboueissa A-M, Zheng T, Wise JP., Sr. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to Steller sea lion lung cells. Aquat. Toxicol. 2009;91(4):329–335. doi: 10.1016/j.aquatox.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat. Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise S.S, Kraus, S., Shaffiey F, Grau M, Li Chen T, Perkins C, Thompson WD, Zheng T, Zhang Y, Romano T, O’Hara T. Hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and testes fibroblasts. Mutat. Res. 2008;650:30–38. doi: 10.1016/j.mrgentox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Payne R, Wise SS, LaCerte C, Wise J, Gianios C, Jr., Thompson WD, Perkins C, Zheng T, Zhu C, Benedict L, Kerr I. A global assessment of chromium pollution using sperm whales (Physeter macrocephalus) as an indicator species. Chemosphere. 2009;75:1461–1467. doi: 10.1016/j.chemosphere.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise SS, Holmes AL, Lacerte C, Shaffiey F, Aboueissa A-M. The cytotoxicity and genotoxicity of hexavalent chromium in Stellar sea lion lung fibroblasts compared to human lung fibroblasts. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010;152:91–98. doi: 10.1016/j.cbpc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise JP, Sr., Wise SS, LaCerte C, Wise JP, Jr., Aboueissa A-M. The genotoxicity of particulate and soluble chromate in sperm whale (Physeter macrocephalus) skin fibroblasts. Environ. Mol. Mutagen. 2011;52:43–49. doi: 10.1002/em.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylitalo GM, Stein JE, Hom T, Johnson LL, Tilbury KL, Hall AJ, Rowles T, Greig D, Lowenstine LJ, Gulland FMD. The role of organochlorines in cancer-associated mortality in California sea lions (Zalophus californianus) Mar. Pollut. Bull. 2005;50:30–39. doi: 10.1016/j.marpolbul.2004.08.005. [DOI] [PubMed] [Google Scholar]