Abstract
The nuclear envelope represents a key barrier to successful nonviral transfection and gene therapy both in vitro and in vivo. Although the main purpose of the nuclear envelope is to partition the cell to maintain cytoplasmic components in the cytoplasm and nuclear components, most notably genomic DNA, in the nucleus, this function poses a problem for transfections in which exogenous DNA is delivered into the cytoplasm. After delivery to the cytoplasm, nucleic acids rapidly become complexed with cellular proteins that mediate interactions with the cellular machinery for trafficking. Thus, it is these proteins that, in essence, control the nuclear import of DNA, and we must also understand their activities in cells. In this review, we will discuss the principles of nuclear import of proteins and DNA–protein complexes, as well as the various approaches that investigators have used to improve nuclear targeting of plasmids. These approaches include complexation of plasmids with peptides, native and engineered proteins, ligands and polymers, as well as the inclusion of transcription factor-binding sites for general and cell-specific delivery.
Keywords: Nonviral gene transfer, plasmid, nuclear pore complex, importin, nuclear localization signal, karyopherin
Introduction
Nonviral plasmid-based gene delivery systems show promise for gene therapy because of their ability to be repeatedly administered and their generally good safety profile. However, their greatest limitation has been the reduced levels of gene transfer and expression compared with their viral counterparts. Viruses have had millions of years to develop strategies to circumvent cellular barriers to ensure infection of their target cells. Most of these mechanisms involve designing and incorporating proteins into the virus that help stabilize virus–cell interactions and increase internalization, enhance endosomal escape, promote movement through the cytoplasm to the nuclear envelope, improve nuclear entry in dividing and nondividing cells (often by promoting mitosis) and increase transcription. Nonviral vectors have not had the luxury of evolution to aid their delivery and are thus confronted by each of these barriers.
Although many researchers disagree about the one single ‘rate-limiting’ barrier to efficient gene delivery, it is clear that trafficking across the nuclear envelope is one of the major barriers. Significant progress has been made over the past 20 years to elucidate the mechanisms of nuclear import and export of proteins and RNAs (such as mRNA, tRNA, 5S RNA). Similarly, over the past 10 years, mechanisms for the nuclear import of plasmids have been described, and methods to optimize delivery and expression based on exploitation of these mechanisms have been developed.
It has long been appreciated that the nuclear envelope represents a barrier to efficient gene delivery. Most successful laboratory transfections occur in actively dividing cells. As one of the hallmarks of mitosis is nuclear envelope breakdown, any DNA that has entered the cytoplasm before mitosis would gain access to the nuclear compartment once cells enter the M phase. Indeed, nonviral transfections are cell cycle dependent. This is at least one of the reasons why many primary cells, growth-arrested cells and terminally differentiated cells remain difficult to transfect, and is the reason why a multitude of ‘new and improved’ transfection reagents are constantly being introduced and advertised to the community.
Mario Chapecchi showed almost 30 years ago that when plasmids were microinjected into the cytoplasm of mouse fibroblasts, they largely failed to express. By contrast, when the same plasmids were microinjected into the nuclei of the same cultured cells, between 50 and 100% of the cells showed some level of gene expression. More recently, several groups have quantified levels of gene expression and found that it takes between 30 and 100 times more DNA delivered to the cytoplasm than it does to the nucleus to give the same level of gene expression, even in dividing cells.1 It is estimated that after lipoplex- or polyplex-mediated transfection, between 2000 and 100 000 plasmids are delivered to each cell, depending on the applied dose of DNA.2 Depending on the cell type transfected and the methods used for detection, it is estimated that between 1 and 10% of unmodified plasmids delivered to the cell can then be detected in the nuclear fraction, using quantitative PCR, Southern blot or electron microscopy.2,3 Thus, only a fraction of input DNA reaches the nucleus for gene expression.
Mechanisms of NLS-mediated protein nuclear import have been elucidated
Although plasmids are delivered to cells using a number of carriers, entry of DNA into the cytoplasm is followed by dissociation of DNA from the carriers. This DNA does not stay ‘naked’ or uncomplexed for long and is quickly bound by a number of cellular proteins, cationic peptides and polyamines. Numerous studies that have been discussed below have shown that the subsequent intra-cellular trafficking events are controlled by proteins that are bound to the DNA, and as such, discussion of how proteins are directed into the nucleus is highly relevant for understanding how the DNA moves.
A number of investigators have worked to identify the signals within nuclear proteins that allow their selective accumulation in the nucleus, the receptors for these signals and the mechanisms by which they entered or exited the nucleus. In brief, a protein that is targeted to the nucleus contains a relatively short sequence known as the ‘nuclear localization sequence’ or nuclear localization signal (NLS). Two of the best characterized NLSs are the classical NLS from SV40 Large T-antigen (PKKKRKV) and the bipartite NLS in which the classical NLS is split into two halves (typically KKKX5-20RK). 4 Cargo proteins bearing NLSs consisting of clusters of basic residues are bound and imported by a class of proteins known as karyopherins (importins) that are soluble in the nuclear pore complex (NPC). In the case of the classical NLS system, the NLS of the cargo protein is recognized in the cytoplasm by importin-α, an NLS receptor, which then dimerizes with importin-β to form a nuclear pore targeting complex.4 However, most proteins bind directly to importin-β isoforms for their nuclear translocation, bypassing the need for importin-α (Figure 1). Mammalian cells contain 6 importin-α isoforms and some 20 importin-β isoforms. These different isoforms, either as β-monomers, αβ or ββ-heterodimers, recognize distinct target proteins to facilitate and likely specifically regulate nuclear import. For example, histone proteins are shuttled into the nucleus by importin/importin 7 hetero-dimer, importin 5 and transportin, whereas ribosomal proteins are shuttled into the nucleus by importin, transportin, importin 5, importin 7 and importin 11.
Figure 1.
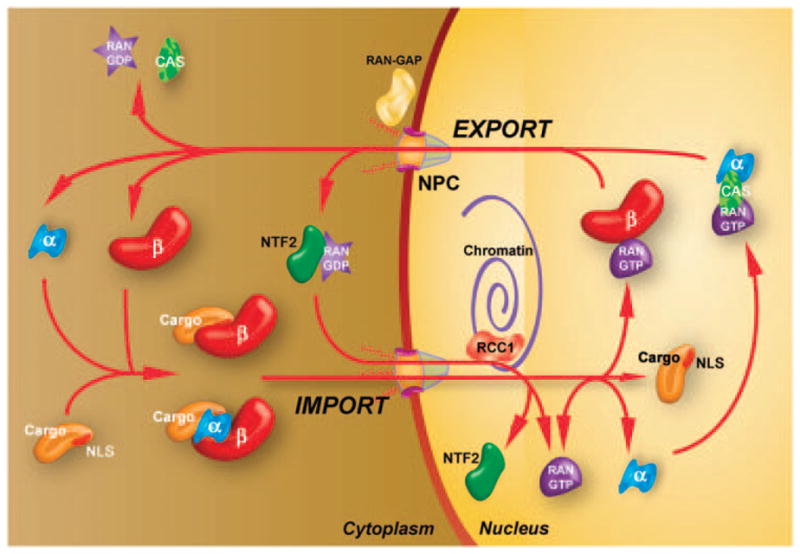
Mechanisms of protein nuclear import. Cargos targeted for nuclear import contain NLSs on their surface which interact with a number of distinct importin-α/β heterodimers or importin-β isoforms directly. These complexes are targeted to and translocated across the nuclear envelope through the NPC. Upon reaching the nucleus, RanGTP binds to importin-β, causing a conformational change that releases the bound cargo. The importins are then recycled to the cytoplasm as a RanGTP–Importin-β complex or, in the case of importin-α, by its export carrier CAS. The complex then dissociates in the cytoplasm after the hydrolysis of RanGTP, which is facilitated by RanGAP at the cytoplasmic face of the NPC. RanGDP is then imported to the nucleus by NTF2 and the guanine exchange factor RCC1 converts it to RanGTP.
Once bound to its karyopherin, the NLS cargo protein targets to the NPC, a large (~125 MDa) macromolecular assembly that perforates the nuclear envelope to allow all macromolecular movement between the cytoplasm and the nucleus. Although the exact mechanism remains controversial, interaction between the importins and a series of phenylalanine-glycine (FG)-repeat proteins that make up the NPC facilitates translocation across the pore.5 Once inside the nucleus, the small guanosine triphosphate (GTP)-binding protein RAN, in its GTP-bound state, recognizes the importin–cargo complex at the nuclear face of the NPC, binds to importin and induces a conformational change in the protein that releases the cargo. After inducing release of the cargo within the nucleus, the RAN–GTP–importin-β complex is transported back into the cytoplasm where the Ran GTPase-activating protein (RanGAP) regenerates RAN-GDP to maintain the RAN gradient. RAN is maintained in its GTP-bound state in the nucleus by the guanine nucleotide exchange factor RCC1 which is bound to chromatin.4 To maintain relative levels of RAN across the nuclear envelope, RAN-GDP is transported into the nucleus by the small protein NTF2 where it is reconverted to the GTP-bound state. The end result is an exquisitely controlled mechanism to localize proteins to their required cellular compartments, all mediated by the NLS.
Transcription factor-binding sites promote DNA nuclear translocation
Although it has been shown that mitosis-associated nuclear envelope breakdown greatly enhances nuclear localization of plasmids and transfection efficiency, this is not a prerequisite. Numerous groups have shown that plasmids can enter the nuclei through NPCs in the absence of cell division, although the efficiency of such transfection is usually much lower than in dividing cells. Moreover, certain DNA sequences can increase this nuclear targeting of plasmids before mitosis. We, along with others, have shown that the nuclear import of plasmid DNA through the NPC is a sequence-specific process, mediated by specific eukaryotic sequence elements.1 When delivered side by side by microinjection into the cytoplasm, plasmids containing as little as 72 bp of the SV40 enhancer target to the nucleus of most cells within several hours, whereas an identical plasmid lacking this 72-bp sequence remains cytoplasmic until cell division (or indefinitely if the cell is nondividing). This sequence, termed the ‘SV40 DNA nuclear targeting sequence (DTS)’, has been shown to mediate plasmid nuclear import in all cells and cell lines tested, including primary endothelial, vascular smooth muscle, airway epithelial, alveolar epithelial cells, and lung and skin fibroblasts, as well as oligodendricytes derived from monkey, rat, mouse, hamster, chicken and human origin. Furthermore, it has been shown that these sequences also greatly enhance nuclear delivery and gene expression in the vasculature, skeletal muscle and the lungs of living animals.1,6,7
The defining feature of the SV40 DTS is that it contains binding sites for a number of ubiquitously expressed mammalian transcription factors (such as AP1, AP2, nuclear factor (NF)-κB, Oct1, TEF-1). As transcription factors function in the nucleus, they contain NLSs for their nuclear importation. Under normal conditions, these factors would be transported into the nucleus after translation or in a regulated manner when signals activate transcription (for example, tumor necrosis factor-α stimulation of NF-κB). In either case, a significant cytoplasmic pool of these factors exists at any given time. When plasmids carrying the SV40 DTS are delivered into the cytoplasm by any method, some of these transcription factors can bind to the DTS, thereby coating a region of the plasmid with NLSs, at least some of which are oriented away from DNA itself (Figure 2). These DNA-bound NLSs can be recognized by importin-β and/or transportin (importin-β2) and transported into the nucleus through the NPC.7–11 Apart from the requirement for the NLS to be spatially accessible to the importins when the transcription factor is bound to the DNA, the binding sites for the transcription factors must also be accessible to the transcription factors for any complex to assemble. This may be important when plasmids are complexed with peptides, polymers and lipids that may remain bound to the DNA even after escape from the endosomes.
Figure 2.
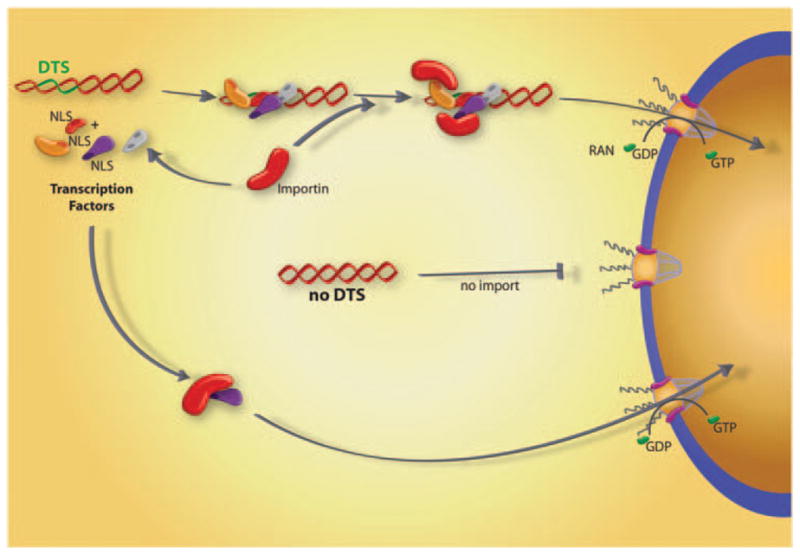
Protein-mediated plasmid nuclear import. Transcription factors and other nuclear proteins normally enter the nucleus through interactions between their NLSs and importin family members. However, if plasmids containing certain sequences that act as scaffolds for transcription factors and other DNA binding proteins (termed ‘DTS’, or DNA nuclear targeting sequences) are deposited into the cytoplasm during transfection, they can form complexes with these proteins, thereby attaching NLSs to the DNA. Some, but not all, of these NLSs may be in a conformation able to interact with importins for transport of the DNA–protein complex into the nucleus through the nuclear pore complex.
As the function of the DTS is mediated by binding of NLS-containing transcription factors, it would seem that any eukaryotic promoter or enhancer could function similarly for DNA nuclear import. Surprisingly, this is not the case, and although half a dozen or so DTSs have been identified, most promoters and enhancers, including the cytomegalovirus immediate early promoter/ enhancer, the Herpes TK promoter and the Rous sarcoma virus long terminal repeat (RSV LTR), have no import activity. The likely explanation for this is that transcription factors bound to these promoters may not present their NLSs in an orientation that is accessible to the importins.
Several other DNA sequences that can drive nuclear import in the absence of cell division have been identified. Ziv Reich and colleagues have shown that plasmids containing multiple NF-κB-binding sites demonstrate a 12-fold enhanced expression in calcium phosphate-transfected HeLa, Hek-293, Hep G2 and U373 cells that could be further increased when the cells were treated with tumor necrosis factor-α, an NF-κB activator. By tracking fluorescently labeled versions of these plasmids, they concluded that the increased levels of gene transfer were due to more efficient transfer across the nuclear envelope. In addition to enhancement of nuclear localization of these plasmids by tumor necrosis factor-α activation of NF-κB, a recent paper has shown that amphiphilic block copolymers used for gene transfer, such as Pluronics, can also activate NF-κB to increase nuclear import of NF-κB-binding site containing plasmids.12 More recent work using fluorescence resonance energy transfer (FRET) to measure nuclear import, has shown that roughly 60 times more plasmids enter the nucleus when multiple NF-κB-binding sites are present.13 Several cell-specific DTSs have also been identified (see below). A major strength of many of these DTSs is that endogenously expressed proteins are used to coat transfected plasmid vectors with NLSs required for import. This means that as long as plasmids are delivered to the cytoplasm, protein complexes can form using normal cellular proteins, obviating the need for supplying DNA-binding, nuclear targeting proteins or peptides in trans.
Cell-specific transcription factors drive cell-specific DNA nuclear entry
In the search for additional DTSs, several DNA sequences were identified that promoted plasmid nuclear import in specific cell types. Expression of cell-specific promoters is restricted to specific cell types because of the presence of a unique set of transcription factors present in those cells only. Combinations of binding sites for these factors in a given promoter and the presence or absence of these factors control whether the promoter is active in a specific cell type. On the basis of this, we reasoned that by screening promoters that are transcriptionally active only in a desired cell type, it could be possible to pull out DNA sequences (that is, from cell-specific promoters) that also function for cell-specific nuclear import (Figure 3). To date, such sequences that act in osteoblasts (DD Strong, TA Linkhart and DA Dean, unpublished data), endothelial cells, alveolar type II epithelial cells and smooth muscle cells have been identified.1,6–8 The best studied of these is the smooth muscle-specific DTS in which as little as 176 bp of the smooth muscle γ-actin (SMGA) promoter can drive nuclear import of plasmids in airway or vascular smooth muscle cells but not in other cell types, both in cultured cells and in vivo.
Figure 3.
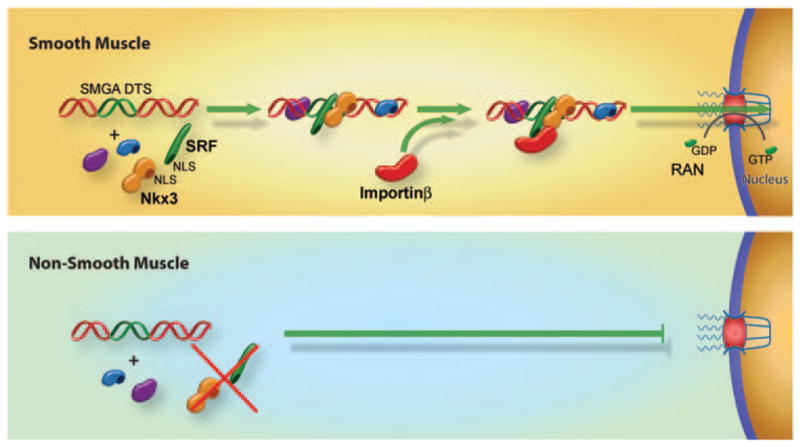
Cell-specific plasmid nuclear import. Certain DNA nuclear targeting sequences have been shown to act in cell-restricted manners. In the case of the smooth muscle γ-actin promoter, which acts as a smooth muscle cell DTS, it has been shown that two key factors that are coexpressed in smooth muscle, SRF and Nkx3, form complexes with the plasmid leading to an importin-recognizable complex that can be localized to the nucleus. By contrast, in nonsmooth muscle cells that do not express one or the other of these factors, an importin-binding complex is not formed leading to greatly reduced nuclear import.
It has been shown that two transcription factors that are preferentially coexpressed in smooth muscle, Nkx3.1/3.2 and SRF, are both necessary and sufficient for DNA nuclear uptake in these cells.8 When the binding sites for these factors were mutated within the SMGA promoter, plasmids containing the mutant DTS remained in the cytoplasm of microinjected cells. Similarly, when Nkx3.1/3.2 and SRF were silenced in smooth muscle cells through the use of small interfering RNA, nuclear import of plasmids carrying the wild-type SMGA promoter was abolished, again showing that these factors are necessary for DNA nuclear import.8 Sufficiency of these two transcription factors alone was shown by expressing the factors in bacteria, complexing the purified proteins with SMGA DTS plasmids before cytoplasmic microinjection and obtaining nuclear import in nonsmooth muscle cells that do not normally express these factors.8 Interestingly, complexation of SMGA DTS plasmids with these two proteins resulted in greatly increased rates of plasmid nuclear import in nonsmooth muscle cells, compared with import of uncomplexed DNA in smooth muscle cells, suggesting that this approach may provide a new method for enhanced transfection of any cell type. As the minimal SMGA promoter identified to date is just 176 bp, it could be incorporated into any plasmid, and then complexed with these two recombinant proteins for enhanced nuclear import and transfection.
Proteomics approaches have been used successfully to study DNA nuclear entry
Several recent studies have used proteomics approaches to begin to identify the constituents of the protein–DNA complexes that form once plasmids enter the cell and which may have a role in the intracellular trafficking and nuclear import of the plasmids. In one study, a permeabilized cell assay was used to reconstitute plasmid nuclear import with fluorescent plasmids and cytoplasmic extracts.10 These extracts that support DNA nuclear import were passed over a plasmid affinity column composed of supercoiled plasmids containing the ubiquitously active SV40 DTS, immobilized on a Sepharose column through the use of a triplex-forming peptide nucleic acid clamp. The depleted extracts collected from the column flow-through failed to support nuclear import, but when the DNA-binding proteins were eluted from the column and added to the depleted extracts, nuclear import was restored. The eluted proteins from the most active fractions were then subjected to two-dimensional SDS-PAGE and many were identified by mass spectrometry. Of the proteins identified, there were a number of bona fide-specific DNA-binding proteins (including histone H2B), chaperones, cytoskeletal proteins and the small GTPase RAN. Two novel DNA-binding proteins were identified and tested, along with histone H2B, for their ability to support DNA nuclear import in a reconstituted system. When added along with importins-α and -β, RAN, and an energy-regenerating system, histone H2B and NM23-H2 (a ubiquitous nucleoside diphosphate kinase found to stimulate c-myc transcription) stimulated DNA nuclear import over sixfold above the importins alone and to the same level as unfractionated cell extracts. The third protein, the homeobox transcription factor Chx10, also stimulated nuclear import in transfected cells when its binding site was included on a plasmid. Taken together, these results suggest that at least several of the proteins identified can indeed support and enhance plasmid nuclear import.
A second study used a similar plasmid affinity chromatography approach, but immobilized either a plasmid that supports smooth muscle cell-specific DNA nuclear import because of the presence of a 404-bp SMGA promoter fragment or an identical plasmid that lacks this 404-bp sequence and is not imported into the nucleus in microinjected cells.9 Smooth muscle cell cytoplasmic extracts were passed over the columns, and eluates were subjected to liquid chromatography-mass spectrometry. A total of 274 unique proteins were identified in the pTOPO-SMGA DTS eluates, whereas only 41 unique proteins were identified in the plasmid lacking the SMGA DTS. Of these 41, only 3 appeared to be DNA-binding proteins; the others were primarily cell adhesion or cell structure proteins. None of the 274 proteins that eluted from the SMGA DTS column were found in the eluate from the empty plasmid and of the eluted proteins, 40 proteins had a role in DNA/RNA processing and/or transcription, 6 were involved in nucleocytoplasmic trafficking (including RAN, importin-β and importin 7), 14 were involved in cytoplasmic transport and 17 were chaperones. To determine whether any of these had a role in plasmid nuclear import in cells or were required for DNA nuclear import, smooth muscle cells were electroporated with plasmids carrying no DTS, the SMGA DTS or the SV40 DTS, and at later times the cells were cross-linked and the plasmids and bound proteins were precipitated.9 Levels of several proteins involved in NLS-mediated nuclear import were determined by western blot and it was found that whereas importin-β, importin 7 and RAN formed complexes with the two DTS-containing plasmids, they did not interact with the plasmid lacking a DTS. Furthermore, importin-α, which was not identified by mass spectrometry in the column eluates, was not detected in any of the pull-downs, confirming the specificity of the affinity column approach. One interesting finding was that the complexes appeared to form at different times after transfection: all proteins were detected in complexes with the SV40 DTS plasmid 60 min after electroporation, but they were not detected until 4 h after transfection in the SMGA DTS-transfected cells. These times are very similar to the times required for these plasmids to show nuclear import after cytoplasmic injection.8 Finally, cells were transfected with small interfering RNAs against several of these proteins to examine whether any of them were required for DNA nuclear import. When importin-β was knocked down in smooth muscle cells, nuclear import of plasmids carrying either the SMGA or the SV40 DTS was abolished, whereas knockdown of importin 7 had little effect (knockdown of importin-α was lethal and could not be evaluated).9 These results suggest that not only is importin-β part of the DNA–protein complexes that form during transfection in cells but it is also required for DNA nuclear localization, at least in smooth muscle cells.
NLS peptides complexed with plasmids may enhance DNA nuclear translocation
Perhaps the most common approach to try to increase nuclear localization of DNA has been to complex synthetic or naturally occurring NLS peptides with the delivered DNA (Figure 4). These approaches are based on the assumption that coating DNA with NLS peptides will drive nuclear import of the DNA using the importin pathway. Numerous studies have complexed classical, bipartite and noncanonical NLS peptides to DNA by electrostatic interactions, random covalent conjugation to DNA, covalent attachment to unique sites within the DNA and conjugation through peptide nucleic acid clamps. Although in some cases, transfection efficiency in cultured cells has increased by modest or highly significant levels, in others it has not. The most consistent results have used NLS peptides conjugated to DNA hairpins on linear DNAs. Several studies have shown that when used in vivo, DNAs with conjugated NLS peptides give greater gene expression in injected mouse muscle and have increased cytotoxic T lymphocyte and antibody responses against the expressed antigens.14 However, it should be pointed out that expression and immunogenicity of the NLS-DNA conjugates were compared with DNAs with no attached peptide instead of a peptide that has no nuclear import activity. Although it is intuitive that inclusion of NLS peptides should increase nuclear trafficking and subsequent gene expression, this is not necessarily the case, and the reasons as to why complexation with NLS peptides may work in one case but not in another is unclear. However, as in the case for the ability of certain transcription factors to mediate nuclear import, it is likely that the three-dimensional structure of the peptide–DNA complex is equally important in this case. Many of the NLS peptides that are complexed with DNA may be ‘buried’ and inaccessible or ‘invisible’ to the importins when bound at the same time to DNA. Thus, the manner by which the peptides are complexed with DNA and/or how many peptides are in the complex may also control activity. For example, electrostatic interaction involves multiple NLS peptides compared with covalent attachment, which usually involves fewer NLS peptides (Figure 4). The number of NLS peptides is likely to have a bearing on whether the determining factor on nuclear import is pDNA condensation and/or availability for binding by the importins. Unfortunately, there is no one-to-one relationship between the number of peptides added to the DNA and the ability to enhance nuclear localization, using any attachment method when multiple papers are examined. What is clear is that most of the studies that have reported positive effects resulting from inclusion of NLS peptides have not evaluated nuclear import per se, but rather have used gene expression as a readout. Thus, it is possible that the increased transfection and gene expression seen when plasmids are complexed with NLS peptides may result from factors other than increased DNA nuclear import, for example, increased compaction by cationic peptides.
Figure 4.
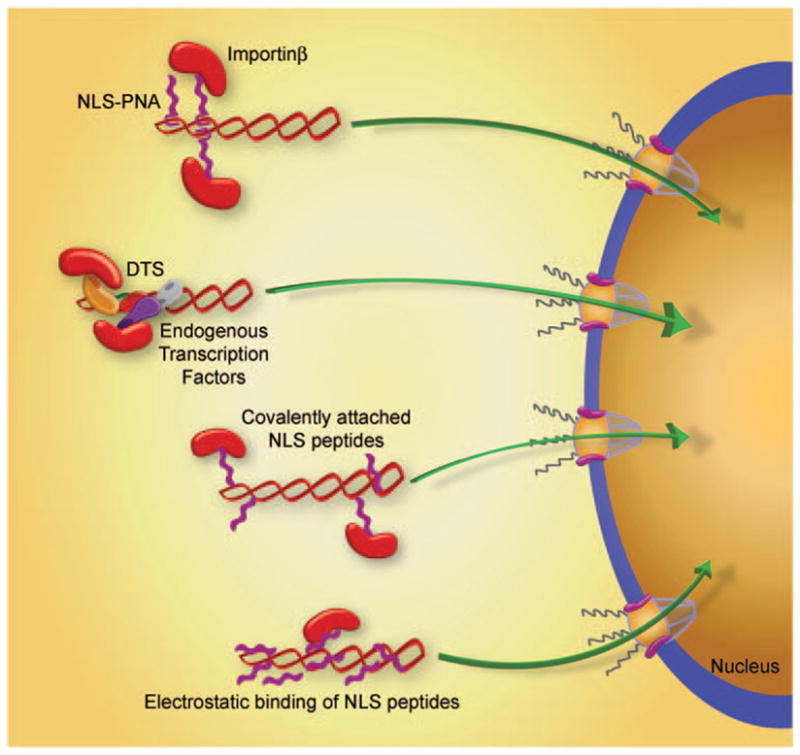
Methods to enhance plasmid nuclear import. A number of different approaches have been developed to promote recognition of plasmids by importin family members to increase nuclear import. These include peptide-nucleic acid clamp-conjugated NLS peptides bound to DNA, sequence-specific DNA binding proteins bound to DNA, NLS peptides covalently attached to DNA and NLS peptides electrostatically bound to DNA.
A complementary approach to using synthetic NLS peptides alone to enhance nuclear import of DNA is to create multidomain peptides that contain combinations of different functional motifs. One recent example fused a protein translocation domain from the HIV (human immunodeficiency virus) tat protein and a DNA interaction domain from Mu transposase with an NLS. Not only did this multidomain peptide show greater transfection in the presence of lipid than did simple polylysine-condensing peptides but the peptide also lead to increased nuclear localization of DNA complexes.15 However, as for NLS peptides alone, other studies have suggested that multidomain peptides, such as a bacterially derived cell-penetrating peptide fused to an NLS, give no increased nuclear localization.16 Thus, the jury remains out.
Although the addition of NLS peptides may or may not increase nuclear localization of plasmids, their use in vivo could potentially result in undesired effects, such as immunogenic responses. One of the main drawbacks to viral vectors has long been their propensity to induce inflammatory and immunological responses limiting subsequent administrations in vivo. By contrast, one of the advantages of nonviral DNA-based vectors is that they do not elicit antibody or cytotoxic T lymphocyte responses against the DNA itself, allowing multiple repeat administrations for sustained gene delivery. As peptides are routinely used to generate immune responses, their inclusion with plasmids for in vivo delivery could in fact make them more virus like and cause humoral responses to be generated. In this case, there would be a trade-off between enhanced gene delivery due to peptide activity, and reduced ability to readminister peptide–DNA complexes in the future.
Nuclear proteins complexed with plasmids facilitate DNA nuclear entry
For an NLS peptide to act as a nuclear localization sequence, it must be free to interact with an importin family member. As most of these sequences are highly basic, they may have a greater tendency to interact with the negatively charged DNA than to protrude from the DNA to bind to the importin(s). Thus, free peptides, or even those bound by some type of covalent or nucleic acid clamp mechanism, may not be accessible to the importins. By contrast, the location of the NLS within a protein is conformationally locked into place on the surface of the protein. If the DNA-binding domain of the protein is spatially distinct (that is, on the other side of the protein) from the NLS, this should result in the presentation of the NLS to importins away from the DNA. By contrast, if the NLSs were overlapping with the DNA-binding domain, as in the case of most zinc-finger transcription factors, the NLS may be unable to function as such when the protein was bound to DNA. Consequently, it may be more advantageous to use proteins as opposed to peptides to try to stimulate nuclear delivery of plasmids. Recently it was shown that when plasmids containing tandem NF-κB-binding sites were complexed in vitro with the p50 subunit of NF-κB, they trafficked through the cytoplasm faster and localized to the nucleus to a greater extent than did uncomplexed plasmids after microinjection.13 Similarly, preformed complexes of bacterially expressed SRF and Nkx3.2 with plasmids containing binding sites for these factors showed greatly enhanced nuclear localization in all cell types.8
Small molecule ligands bound to DNA can increase nuclear entry
Another intriguing method that several groups have developed to increase nuclear targeting of DNA involves attaching small ligands to plasmids that allow binding to importins and other proteins that can chaperone the DNA into the nucleus. For example, several different groups have biotinylated plasmids and then reacted them with various streptavidin-protein conjugates to stimulate nuclear import, including importin-β. The problem with this approach is that as the degree of plasmid labeling increased, the transcriptional activity of the plasmid decreased so that any benefit received from increased nuclear localization of the plasmid was offset by decreased transcription of the DNA. This appears to be a common problem of labeled DNA. When covalent methods are used to attach ligands (for example, fluorophores, biotin, etc.) to plasmids, there is no control over where the attachments are made. As the human nature is to attach as many ligands as possible to the DNA (‘if one is good, more is better’), this can result in a plasmid that has so many labels on it that it cannot be transcribed efficiently because of these DNA adducts. Furthermore, it has also been shown that when DNA is fluorescently labeled using similar chemical methods, it becomes inert and behaves more like a large dextran than as a DNA molecule with regard to intracellular trafficking. A different approach has been to attach dexamethasone, the ligand for the glucocorticoid receptor, to plasmid constructs or to the polyplex carrier.17 Upon binding of dexamethasone, the glucacorticoid receptor undergoes a conformational change resulting in exposure of its NLS and nuclear localization. By modifying the polyplex carriers, either polyamidoamine (PAMAM) dendrimers or polyethyleneimine (PEI), the 20- to 40-fold increased levels of expression appear to be correlated with levels of nuclear localization of the complexes.17
Nanoparticles and polymers may provide alternative routes to the nucleus
Most of the discussion on DNA nuclear import has been related to naked DNA. Multiple groups have shown that upon entry into the cell, most polymers and lipids dissociate from the plasmids, at least partially, before nuclear entry. However, there are several examples of complexes that may not dissociate in the cytoplasm and may in fact use alternative routes for nuclear entry. When DNA and PEI were directly labeled, researchers found that in contrast to what is typically seen with liposome complexes, PEI and DNA remained complexed in the cell and appeared to localize to the nucleus coordinately.13 Electron microscopy studies have suggested that the PEI–DNA complexes can enter the nuclei as crystalline arrays, possibly by novel, unknown mechanisms. However, recent work using FRET and plasmids that bind to NF-κB, has suggested that the nuclear import of PEI–DNA complexes occurs through the NPC, although the manner by which cellular NF-κB and importins bind to plasmids complexed with PEI remains unclear.13 All of these studies also found that at least some of the plasmids that entered the nucleus appeared to still be complexed with PEI, which could account for the fact that PEI-compacted or PEI-complexed DNA may not be as transcriptionally active as DNA in the absence of PEI, as has been observed by others.2,3 Another example of a DNA-polyplex that may behave differently than most is the PEGylated-polylysine/lipid/DNA nanoparticles (approximately 10–20 nm in diameter by up to 100 nm in length, depending on counterion) developed by Pam Davis and colleagues. When terminally differentiated human neuroblastoma SY5Y cells, HuH-7 human hepatoma cells or 16HBEo cells (a human bronchial epithelial cell line) are transfected with these nanoparticles, the particles rapidly enter the cytoplasm, appear in the nucleus within 15 min and then concentrate in the nucleolus.18 This nuclear targeting and import appears to be size dependent, as the nuclear import and subsequent gene expression decreased as the minor diameter of the particles (controlled by the size of the complexed plasmid) exceeded 25 nm (>10 kbp plasmids). These particles do not appear to be taken up by receptor-mediated endocytosis to any degree, but rather bind to a nucleolar protein, nucleolin, that can also exist on the cell surface.18 Whether these particles enter the nucleus through the NPC and if so, in concert with what proteins, remains to be seen. However, the speed with which the particles enter the nucleus suggests that characterization of this pathway could lead to approaches to enhance plasmid nuclear uptake.
Modulation of the NPC may aid nuclear delivery of DNA
One intriguing approach to increase nuclear transport of transfected DNA relies not on modification of the delivered DNA but of the NPC itself. It is believed that the central channel of the NPC, which is composed up of multiple FG-repeat nucleoporins, is hydrophobic and restricts translocation of hydrophilic proteins other than importins and their bound cargoes. The amphipathic molecule TCHD (trans-cyclohexane-1,2-diol) is believed to reversibly disrupt hydrophobic interactions within the central NPC channel, thereby allowing translocation of substrates across the nuclear envelope without the aid of the importins. When cells were treated with TCHD and microinjected with labeled plasmids, increased nuclear localization of the plasmids was observed, as was increased gene expression after transfection with naked DNA, liposomes or PEI.19 Although this approach to enhance nuclear localization of plasmids requires just the addition of a transiently acting and reversible drug, manipulation of the gating activities of the NPC may have serious unforeseen effects on the cell itself, as such modulation of pore permeability could affect partitioning of any and all proteins within the cell as well as cell viability. Although A549 cells showed no decrease in viability after a 1-h incubation with up to 3% (w/vol) TCHD, Vero cells showed a 50% decrease at the same concentration.19 As such, it may not be a useful approach to increase transfection efficiency in animal models.
Prospects
Despite the fact that most investigators agree that the nuclear envelope represents a major barrier to successful transfection and gene therapy, the majority of studies to date have focused on attempting to increase nuclear localization empirically rather than mechanistically. However, a number of studies have focused on elucidating pathways by which plasmids, either alone or in complex with various lipids and polymers, traffic to enter the nucleus. With the characterization of these pathways, we may be able to develop rational approaches to overcome the barrier presented by the nuclear envelope, leading to increased transfection.
Perhaps the most exciting area of research that is now beginning to be applied to the study of plasmid nuclear import is proteomics. Over the next few years, researchers should be able to identify the individual components of DNA–protein complexes as they traffic through cells. The initial studies published in this past year using cell-free systems are a good start, but the analysis of proteins that bind to and mediate nuclear import of plasmids during transfections inside cells and within tissues in animals will be crucial in understanding how intra-cellular trafficking really occurs. These studies will be coupled with the ability to overexpress individual proteins, modulate their activity and silence their expression with RNA interference approaches. By identifying the proteins that mediate this trafficking, we should be able to enhance movement to increase gene delivery.
Apart from understanding how nuclear import occurs in cultured cells, we must also understand how much of an impact optimizing the pathways for intracellular trafficking and nuclear import have on gene delivery in animal models. One way to accomplish this is to evaluate trafficking events in tissues themselves. The advent of whole animal luminescence and fluorescence imaging has made people realize that these types of experiments can be carried out in animals, but the resolution of current instruments is still far from being able to study any trafficking event at the intracellular or even single cell level. With this said, there are a number of investigators using confocal and two photon imaging systems to study various tissues in living animals. We, along with others, have begun to develop imaging approaches to study trafficking events within cells in the living animal. The next 2 years should see advances in this area so that we can ask the question of whether the increased gene expression we see when plasmids are believed to be targeted to and into the nucleus are actually in fact due to increased nuclear localization. Although the assumption has always been that increased nuclear import is desired, whether nuclear import is a rate-limiting barrier in vivo remains an unresolved question.
In brief.
| Progress | Prospects |
|---|---|
|
|
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv Drug Deliv Rev. 2009;61:603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RN, van der Aa MA, Macaraeg N, Lee AP, Szoka FC., Jr Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J Control Release. 2009;135:166–174. doi: 10.1016/j.jconrel.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glover DJ, Leyton DL, Moseley GW, Jans DA. The efficiency of nuclear plasmid DNA delivery is a critical determinant of transgene expression at the single cell level. J Gene Med. 2010;12:77–85. doi: 10.1002/jgm.1406. [DOI] [PubMed] [Google Scholar]
- 4.McLane LM, Corbett AH. Nuclear localization signals and human disease. IUBMB Life. 2009;61:697–706. doi: 10.1002/iub.194. [DOI] [PubMed] [Google Scholar]
- 5.Brohawn SG, Partridge JR, Whittle JR, Schwartz TU. The nuclear pore complex has entered the atomic age. Structure. 2009;17:1156–1168. doi: 10.1016/j.str.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young JL, Zimmer WE, Dean DA. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp Biol Med. 2008;233:840–848. doi: 10.3181/0712-RM-331. [DOI] [PubMed] [Google Scholar]
- 7.Degiulio JV, Kaufman CD, Dean DA. The SP-C promoter facilitates alveolar type II epithelial cell-specific plasmid nuclear import and gene expression. Gene Therapy. 2010 doi: 10.1038/gt.2009.166. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AM, Dean DA. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Therapy. 2008;15:1107–1115. doi: 10.1038/gt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AM, Munkonge FM, Alton EW, Dean DA. Identification of protein cofactors necessary for sequence-specific plasmid DNA nuclear import. Mol Ther. 2009;17:1897–1903. doi: 10.1038/mt.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munkonge FM, Amin V, Hyde SC, Green AM, Pringle IA, Gill DR, et al. Identification and functional characterisation of cytoplasmic determinants of plasmid DNA nuclear import. J Biol Chem. 2009;284:26978–26987. doi: 10.1074/jbc.M109.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachish-Zalait A, Lau CK, Fichtman B, Zimmerman E, Harel A, Gaylord MR, et al. Transportin mediates nuclear entry of DNA in vertebrate systems. Traffic. 2009;10:1414–1428. doi: 10.1111/j.1600-0854.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Sahay G, Sriadibhatla S, Kabanov AV. Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA. Bioconjug Chem. 2008;19:1987–1994. doi: 10.1021/bc800144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breuzard G, Tertil M, Goncalves C, Cheradame H, Geguan P, Pichon C, et al. Nuclear delivery of NFiB-assisted DNA/polymer complexes: plasmid DNA quantitation by confocal laser scanning microscopy and evidence of nuclear polyplexes by FRET imaging. Nucleic Acids Res. 2008;36:e71. doi: 10.1093/nar/gkn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi Y, Jeon YH, Kang JH, Chung JK, Schmidt M, Kim AC. MIDGE/hNIS vaccination generates antigen-associated CD8+ IFN-gamma+ T cells and enhances protective antitumor immunity. Int J Cancer. 2007;120:1942–1950. doi: 10.1002/ijc.22567. [DOI] [PubMed] [Google Scholar]
- 15.Xavier J, Singh S, Dean DA, Rao NM, Gopal V. Designed multi-domain protein as a carrier of nucleic acids into cells. J Control Release. 2009;133:154–160. doi: 10.1016/j.jconrel.2008.09.090. [DOI] [PubMed] [Google Scholar]
- 16.Duvshani-Eshet M, Keren H, Oz S, Radzishevsky IS, Mor A, Machluf M. Effect of peptides bearing nuclear localization signals on therapeutic ultrasound mediated gene delivery. J Gene Med. 2008;10:1150–1159. doi: 10.1002/jgm.1235. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Kim HA, Bae YM, Choi JS, Lee M. Dexamethasone-conjugated polyethylenimine as an efficient gene carrier with an anti-apoptotic effect to cardiomyocytes. J Gene Med. 2009;11:515–522. doi: 10.1002/jgm.1320. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008;16:333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke RE, Lucas B, Demeester J, De Smedt SC, Sanders NN. Nuclear accumulation of plasmid DNA can be enhanced by non-selective gating of the nuclear pore. Nucleic Acids Res. 2007;35 :e86. doi: 10.1093/nar/gkm440. [DOI] [PMC free article] [PubMed] [Google Scholar]


