Abstract
Background/Objectives
Recurrent fracture risk is high among fragility fracture survivors. Osteoporosis treatment reduces recurrent fractures and consequent morbidity and mortality. To assess uptake of post-fracture care guidelines, we studied osteoporosis care in a national cohort of community-dwelling, Medicare patients with fractures.
Design
Retrospective, observational cohort study.
Setting
Claims based study using U.S. Medicare administrative inpatient, outpatient (2003–2010) and prescription (2006–2010) data.
Participants
Patients 68 years or older who survived at least 12 months after a fracture of the hip, radius or humerus
Measurements
Poisson regression modeled factors, including patient characteristics, co-morbidities and hospital referral region (HRR), associated with bone density testing and/or osteoporosis pharmacotherapy in the 6 months following fracture. Models were repeated for patients with no osteoporosis care observed prior to fracture (“attention naïve”).
Results
Among 61,832 fracture patients, mean age was 80.6; 87.0% were female; 88.5% were white; 2.6% were Black; 62.1% were “attention naïve” at the time of fracture. 21.8% received testing and/or pharmacotherapy in the 6 months following fracture. In adjusted models, factors associated with significantly lower likelihood of receiving this care were: Black race, male sex, and an upper extremity fracture (vs. hip). In models restricted to “attention naïve” patients the same factors were associated with lower RRs of achieving care. Adjusted HRR-level care rates ranged from 14.7%–22.9% (10th to 90th percentile). The proportion receiving care increased from 2006 to 2009.
Conclusion
Post-fracture osteoporosis care was uncommon, particularly among Black and male patients. Care increased over time, but for most a fracture was insufficient to trigger effective secondary prevention, especially for patients without pre-fracture osteoporosis attention. Clinicians and policy makers must consider effective remedies to this persistent care gap.
Keywords: Medicare, Osteoporosis, Fragility Fracture
INTRODUCTION
Fragility fractures associated with osteoporosis confer substantial morbidity, mortality and health care costs on older adults.(1–3)Patients with hip, humerus, or radius fractures have a high risk of recurrent fracture.(4)Patients who sustain a hip fracture have an estimated 5- to 8-fold increased 3-month risk of death.(2)Appropriate attention to osteoporosis can reduce the risk of recurrent fractures and the associated impact on quality of life and longevity.(5, 6)Evidence-based guidelines recommend bone density testing and osteoporosis pharmacotherapy after a fracture.(7, 8)In 2004, the National Committee on Quality Assurance (NCQA) introduced an osteoporosis care quality measure, endorsed by the National Quality Forum (NQF); the measure assesses receipt of appropriate bone density testing and pharmacotherapy within 6 months of a fragility fracture for women over age 67.(9, 10)
Despite the development and dissemination of treatment guidelines and the NCQA quality measure aimed at promoting osteoporosis care in fracture patients, studies have shown persistent low testing and treatment rates.(11–15) Research has also documented treatment disparities by fracture type (hip compared to upper extremity) and among populations typically considered at low risk for osteoporosis, such as Black and male patients.(11, 14–19)
Previous studies of post-fracture care have largely been limited to select patients in a single geographic region or institution, have small study populations, rely on survey methods or assess inpatient treatment only.(13, 16, 17, 20, 21) Many predate the emergence and broad dissemination of pharmacotherapy guidelines and the NCQA quality measure.(11, 14, 16, 17) Research examining guideline concordant, post-fracture osteoporosis management specifically among beneficiaries in Medicare, the largest payer for such care, has likewise been limited. Previously published Medicare studies of osteoporosis care have used managed Medicare plan (Medicare Advantage) data or examined care in a broad group of osteoporosis patients, not specifically the group at highest risk for a fracture: those who have already incurred one.(22–24) Medicare Advantage plans vary and provide care through an integrated delivery model; as a consequence observations may not be generalizable to other Medicare Advantage plans. They are likely even less generalizable to the larger fee-for-service Medicare population for whom care delivery is not necessarily as highly integrated or coordinated.
We used fee-for-service Medicare administrative data to advance the understanding of osteoporosis management through examination of care among a cohort of patients who should likely be the highest priority for attention to osteoporosis, fragility fracture survivors (patients who sustained a fragility fracture and survived at least 12 months). By restricting our cohort to community dwelling patients, continuously insured for inpatient, outpatient and prescription services, we believe this analysis provides a conservative and valid assessment of recent uptake and dissemination of fragility fracture care guidelines and an indication of post-fracture care quality among older adults in the U.S. Understanding factors associated with the likelihood of receiving recommended care will help inform policies and practices aimed at improving care and reducing the burden of osteoporosis in this population of vulnerable older adults.
METHODS
Using Medicare claims data we identified fragility fracture survivors and assessed the use of bone density testing and osteoporosis pharmacotherapy in this cohort after a fracture. We then evaluated factors associated with the likelihood of receiving this care.
Setting and Design
From a 40% random sample of Medicare beneficiaries, we identified U.S. residents age 68 years or older who experienced a fragility fracture between May 1, 2006 and December 31, 2009 and were continuously enrolled in fee-for-service Medicare Part A (insurance for inpatient services) and Part B(insurance for outpatient services) for at least 36 months preceding the index fracture and at least 12 months following the fracture. This 36-month “look back” period was used to distinguish incident, from prevalent fractures and initial from subsequent fractures. The time period was also used to ensure Medicare enrollment in the time before fracture so that we might ascertain pre-fracture bone density testing. This study design resulted in the exclusion of incident fractures occurring between age 65 (Medicare enrollment) and age 68, our earliest entry age. To capture use of osteoporosis pharmacotherapy, we required cohort members to have continuous Medicare Part D (prescription drug insurance)enrollment for at least the 4 months preceding and 12 months following the index fracture and at least one Part D prescription drug fill claim in the 12 months following the fracture.
Fragility fractures were defined as (1) at least one Current Procedural Terminology (CPT) code for hip fracture repair or (2) at least one International Classification of Diseases, Ninth Edition(ICD-9) diagnosis code for distal radius or proximal humerus fracture plus at least one upper extremity radiography claim within 7 days (plus or minus) of the index ICD-9 diagnosis claim date. (Appendix Table 1) Vertebral fractures were included as a covariate in the analysis (see below), as an indicator of higher fracture risk, but not included as an index fracture for cohort inclusion because the onset of these fractures is difficult to determine using claims data.
Cohort Exclusions
To ensure a relatively homogeneous cohort of patients newly experiencing a fragility fracture, we excluded patients with a non-vertebral fragility fracture in the 36 months preceding the index fracture. Patients were also excluded if (1) they were hospitalized in an acute care facility for more than 90 days of the first 6 months following index fracture, (2) original Medicare eligibility was due to disability or end-stage renal disease, (3) they had one or more diagnosis for cancer (other than non-melanoma skin cancer) at any time in claims records analyzed, (4) they were enrolled in hospice at any point in the observation period, or (5) they did not survive 12 months after the index fracture. Patients were also excluded if any prescriptions were filled at a long term care pharmacy.(25) We excluded these patients to optimize prescription claim capture and to establish a cohort of community dwelling patients most appropriate for osteoporosis care.
Outcomes
The principal outcome was attention to osteoporosis defined as bone density testing and/or receipt of osteoporosis pharmacotherapy within 6 months of an index fracture. This outcome and time frame were based upon the NCQA/NQF quality measure for “Osteoporosis Management in Women who had a Fracture.”(9)We defined receipt of osteoporosis pharmacotherapy(henceforth referred to as pharmacotherapy) as one or more prescription fill(s) for pharmacotherapy appearing in the Part D Prescription Drug Event File (PDE) or a claim for zoledronic acid infusion in the Medicare Part B files. Medications considered pharmacotherapy included oral estrogens, raloxifene, calcitonin, teriparatide, and intravenous and oral bisphosphonates.(Appendix Table 2) We identified bone density testing through Part B claims.(Appendix Table 3) Secondary outcomes included receipt of testing and/or pharmacotherapy within 12 (rather than 6) months of index fracture, pharmacotherapy alone, bone density testing alone, and receipt of both.
Covariates
Using Medicare denominator files and claims data for inpatient and outpatient services, we obtained the following covariates: age (categorized as 68–70, 71–75, 76–80, over 80), race/ethnicity (white, Black, Hispanic, or other), sex, calendar year of fracture, and Part D low income subsidy status, a measure of poverty and indicator of very low prescription cost share.(26) Charlson co-morbidities present in >2% of the cohort were used to capture the burden of co-morbid illness and were recorded if diagnosed once on an inpatient or twice on an outpatient claim during the 36-month look-back period or in the 12 month period following the index fracture.(27) (Appendix Table 4) In the main models, these were categorized as 0, 1, 2, and ≥ 3 co-morbidities. We also identified a diagnosis of vertebral fracture and bone density testing occurring in the 36-months preceding fracture as well as pharmacotherapy preceding the index fracture (defined as Part D fill in the 4 months or Part B record for zoledronic acid infusion in the 12 months before index fracture). Patient ZIP code was used to assign each cohort member to a Dartmouth Atlas of Healthcare hospital referral region (HRR).(28)
Analysis
Poisson regression with robust variance estimation, clustered by HRR, was used to model bone density testing and/or pharmacotherapy in the 6 months following fracture. These models included anatomic location of the index fracture (hip, radius, humerus) and covariates listed above. Poisson models were also used to derive fully adjusted HRR level percent of patients receiving attention to osteoporosis among HRRs with at least 50 cohort members.
Secondary Analyses
In secondary analyses, we repeated the main analyses examining bone density testing and pharmacotherapy independently as outcomes. We also repeated models for the main outcome allowing a 12 month post-fracture period. To assess the impact of individual co-morbidities rather than co-morbidity count categories, we ran models including individual co-morbidities (rather than summary counts). For these models we added to the Charlson co-morbidities two broad mental illness states: depression and serious mental illness (schizophrenia, bipolar and other non-organic psychoses). We also repeated main models separately in the following sub-cohorts: (1) those with no evidence of testing or pharmacotherapy prior to fracture (“attention naïve” sub-cohort) (2) women only (population for the NCQA/NQF quality measure) (3)hip and non-hip fracture type.
RESULTS
We identified 61,832 fracture patients meeting inclusion criteria. Distribution of fracture type was 37.3% hip, 19.8% proximal humerus, and 42.9% distal radius. (Table 1) The mean age was 80.6(standard deviation 7.0); 87.0% of patients were female; 88.5% were white; 2.6% were Black. Overall, 9.6% of the cohort received pharmacotherapy prior to the index fracture and 34.1% received bone density testing in the 36 months before fracture. Prior to fracture, 62.1% had no observed testing or pharmacotherapy; we describe this sub-cohort as “attention naïve.” Compared to the overall cohort, the “attention naïve” patients were more likely to be older, have higher comorbidity counts, be men, and be of non-white race/ethnicity. Characteristics of the women only sub-cohort, analyzed separately due to their specific targeting by the NCQA/NQF quality measure, paralleled the overall cohort which was 87% women.
Table 1.
Characteristics of Older Medicare Patients with Fragility Fractures Occurring May 2006 - December 2009: Overall cohort, Attention Naïve Sub-Cohort and Women Sub-Cohort
| Overall | Attention Naive Sub- Cohort | Women Sub-Cohort | ||||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Cohort | 61,832 | 38,376 | 53,814 | |||
| Mean age (SD) | 80.6 (7.0) | 81.3 (7.2) | 80.8 (7.0) | |||
| Age group | ||||||
| 68–70 | 5,978 | 9.7 | 3,481 | 9.1 | 4,916 | 9.1 |
| 71–75 | 11,719 | 19.0 | 6,638 | 17.3 | 10,052 | 18.7 |
| 76–80 | 11,620 | 18.8 | 6,663 | 17.4 | 10,074 | 18.7 |
| >80 | 32,515 | 52.6 | 21,594 | 56.3 | 28,772 | 53.5 |
| Male | 8,018 | 13.0 | 7,314 | 19.1 | ||
| Female | 53,814 | 87.0 | 31,062 | 80.9 | ||
| Race | ||||||
| White | 54,744 | 88.5 | 33,708 | 87.8 | 47,608 | 88.5 |
| Black | 1,618 | 2.6 | 1,233 | 3.2 | 1,455 | 2.7 |
| Hispanic | 3,292 | 5.3 | 2,112 | 5.5 | 2,824 | 5.3 |
| Other | 2,178 | 3.5 | 1,323 | 3.5 | 1,927 | 3.6 |
| Fracture location | ||||||
| Hip | 23,048 | 37.3 | 15,500 | 40.4 | 19,232 | 35.7 |
| Proximal humerus | 12,275 | 19.8 | 7,583 | 19.8 | 10,758 | 20.0 |
| Distal radius | 26,509 | 42.9 | 15,293 | 39.9 | 23,824 | 44.3 |
| Part D low income subsidy | 18,824 | 30.4 | 12,994 | 33.9 | 16,939 | 31.5 |
| Year of fracture | ||||||
| 2006 | 9,722 | 15.7 | 6,249 | 16.3 | 8,499 | 15.8 |
| 2007 | 17,981 | 29.1 | 11,443 | 29.8 | 15,616 | 29.0 |
| 2008 | 17,548 | 28.4 | 10,999 | 28.7 | 15,267 | 28.4 |
| 2009 | 16,581 | 26.8 | 9,685 | 25.2 | 14,432 | 26.8 |
| Charlson co-morbidity counts | ||||||
| 0 | 16,380 | 26.5 | 9,413 | 24.5 | 14759 | 27.4 |
| 1 | 16,551 | 26.8 | 10,061 | 26.2 | 14653 | 27.2 |
| 2 | 12,084 | 19.5 | 7,638 | 19.9 | 10461 | 19.4 |
| ≥3 | 16,817 | 27.2 | 11,264 | 29.4 | 13941 | 25.9 |
| Osteoporosis pharmacotherapy before fracture | 5,941 | 9.6 | 5,853 | 10.9 | ||
| Bone density test in 36 month look-back | 21,074 | 34.1 | 20,410 | 37.9 | ||
| Vertebral fracture in 36 month look-back | 2,354 | 3.8 | 1,138 | 3.0 | 2,100 | 3.9 |
Age is at time of fragility fracture. Race/Ethnicity groups are from Medicare Denominator file. Part D low income subsidy is an indicator of income < 150% of federal poverty level. Charlson co-morbidities from 1987 Journal of Chronic Disease. Osteoporosis pharmacotherapy includes receipt of one or more prescription fills for osteoporosis pharmacotherapy (bisphosphonate, calcitonin, estrogen, estrogen receptor modifier, teriparatide) in the 4 months preceding fracture and/or intravenous zoledronic acid in the 12 months preceding fracture.
We examined the unadjusted prevalence of post-fracture osteoporosis care and found low achievement of care. Overall, 21.8% of the cohort received testing and/or pharmacotherapy in the 6 months following fracture. Among these 7.5% received bone density testing, 11.7% received pharmacotherapy and 2.6% received both. (Appendix Table 5) Greater attention to osteoporosis was seen with the progression of calendar time. Among patients fracturing in 2006, 16.8% received attention to osteoporosis within 6 months. Among those with fractures in 2008 and 2009, the proportion achieving this care was 22.1% and 30.5%, respectively.(Table 2) Among the “attention naïve” sub-cohort, osteoporosis care after fracture was less common:11.8% received testing and/or pharmacotherapy in the 6 months following fracture (7.2% bone density testing, 3.1% pharmacotherapy, and 1.5% both). In the sub-cohort of women, 23.9% received testing and/or pharmacotherapy in the 6 months following fracture.
Table 2.
Unadjusted Prevalence of Bone Density Testing and/or Osteoporosis Pharmacotherapy in the Six Months Following Fragility Fracture: Overall cohort, Attention Naïve Sub-Cohort and Women Only Sub-Cohort
| Overall Cohort | Attention Naive Sub-Cohort | Women Sub- cohort | ||
|---|---|---|---|---|
| N | 13,452 | 4,534 | 12,879 | |
| Percent Receiving Testing and/or Pharmacotherapy within 6 months of Fracture | ||||
| Overall | 21.8 | 11.8 | 23.9 | |
| Age group | 68–70 | 23.3 | 14.0 | 26.9 |
| 71–75 | 24.7 | 14.4 | 27.7 | |
| 76–80 | 23.6 | 13.5 | 25.8 | |
| >80 | 19.8 | 10.2 | 21.4 | |
| Sex | Male | 7.2 | 5.7 | |
| Female | 23.9 | 13.3 | ||
| Race | White | 21.9 | 11.9 | 24.1 |
| Black | 13.2 | 8.6 | 14.4 | |
| Hispanic | 19.7 | 11.9 | 21.8 | |
| Other | 26.9 | 13.4 | 29.1 | |
| Fracture location | Hip | 21.1 | 12.8 | 23.5 |
| Proximal Humerus | 19.6 | 9.8 | 21.6 | |
| Distal radius | 23.4 | 11.9 | 25.3 | |
| Part D low income subsidy | No | 23.1 | 12.5 | 25.7 |
| Yes | 18.7 | 10.4 | 20.0 | |
| Year of fracture | 2006 | 16.8 | 10.3 | 18.4 |
| 2007 | 16.0 | 10.1 | 17.5 | |
| 2008 | 22.1 | 13.3 | 24.3 | |
| 2009 | 30.5 | 13.1 | 33.8 | |
| Charlson co-morbidity counts | 0 | 25.2 | 13.4 | 27.2 |
| 1 | 23.1 | 12.9 | 25.0 | |
| 2 | 21.3 | 11.8 | 23.4 | |
| ≥3 | 17.5 | 9.5 | 19.8 | |
| Bone density test in 36 month look- back | No | 16.2 | 18.5 | |
| Yes | 32.4 | 32.9 | ||
| Osteoporosis pharmacotherapy before fracture | No | 14.8 | 16.2 | |
| Yes | 87.3 | 87.4 | ||
| Vertebral fracture in 36 month look- back | No | 21.5 | 11.8 | 23.7 |
| Yes | 28.3 | 14.1 | 30.1 | |
Age is at time of fragility fracture. Race/Ethnicity groups are from Medicare Denominator file. Part D low income subsidy is an indicator of income < 150% of federal poverty level. Charlson co-morbidities from 1987 Journal of Chronic Disease. Osteoporosis pharmacotherapy includes receipt of one or more prescription fills for osteoporosis pharmacotherapy (bisphosphonate, calcitonin, estrogen, estrogen receptor modifier, teriparatide) in the 4 months preceding fracture and/or intravenous zoledronic acid in the 12 months preceding fracture.
Models fully adjusted for patient characteristics and co-morbidity count categories were run on the main outcome (testing and/or pharmacotherapy within 6 months)for the overall cohort and separately for “attention naïve” patients and women (Table 3). Factors most strongly associated with a lower likelihood of attention to osteoporosis in the full cohort were: age over 80 (vs. 68–70), relative risk (RR)0.83 (95% CI 0.78, 0.88), male sex RR 0.45 (95% CI 0.41, 0.49), Black race (vs. white) RR 0.80 (95% CI 0.70, 0.92). Compared to patients with a hip fracture, patients with humerus fractures RR 0.85 (95% CI 0.82, 0.90) and radius fractures RR 0.94 (95% CI 0.91, 0.97) were less likely to receive osteoporosis care. Greater likelihood of post-fracture attention to osteoporosis was seen among those with testing RR 1.29 (95% CI 1.25, 1.34) or pharmacotherapy RR 4.84 (95% CI 4.65, 5.02) prior to fracture.
Table 3.
Fully Adjusted Poisson Models: Bone Density Testing and/or Osteoporosis Pharmacotherapy in the 6 Months Following Fragility Fracture: Overall cohort, Attention Naïve Sub-Cohort and Women Only Sub-Cohort
| Overall cohort | Attention Naive Sub-Cohort | Women Sub-cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N= 61,832 | N = 38,376 | N = 53,814 | ||||||||
| aRR | 95% CI | aRR | 95% CI | aRR | 95% CI | |||||
| Age group | 68–70 | referent | referent | referent | ||||||
| 71–75 | 1.0 | 0.95 | 1.08 | 0.99 | 0.89 | 1.1 | 1.01 | 0.96 | 1.06 | |
| 76–80 | 0.97 | 0.91 | 1.03 | 0.89 | 0.81 | 1.0 | 0.96 | 0.91 | 1.01 | |
| >80 | 0.83 | 0.78 | 0.88 | 0.63 | 0.57 | 0.7 | 0.83 | 0.79 | 0.87 | |
| Sex | Male | 0.45 | 0.41 | 0.49 | 0.39 | 0.36 | 0.4 | |||
| Female | referent | referent | ||||||||
| Race | White | referent | referent | referent | ||||||
| Black | 0.80 | 0.70 | 0.92 | 0.80 | 0.67 | 1.0 | 0.82 | 0.74 | 0.91 | |
| Hispanic | 1.05 | 0.96 | 1.14 | 1.12 | 0.99 | 1.3 | 1.04 | 0.98 | 1.1 | |
| Other | 1.13 | 1.04 | 1.24 | 1.08 | 0.9 | 1.3 | 1.11 | 1.04 | 1.19 | |
| Fracture location | Hip | referent | referent | referent | ||||||
| Proximal Humerus | 0.85 | 0.82 | 0.9 | 0.66 | 0.61 | 0.7 | 0.87 | 0.84 | 0.9 | |
| Distal Radius | 0.94 | 0.91 | 0.97 | 0.77 | 0.72 | 0.8 | 0.96 | 0.93 | 0.99 | |
| Part D low income subsidy | No | referent | referent | referent | ||||||
| Yes | 0.90 | 0.86 | 0.94 | 0.81 | 0.76 | 0.9 | 0.90 | 0.87 | 0.93 | |
| Year of Fracture | 2006 | referent | referent | referent | ||||||
| 2007 | 0.98 | 0.92 | 1.04 | 0.97 | 0.89 | 1.1 | 0.97 | 0.93 | 1.02 | |
| 2008 | 1.37 | 1.29 | 1.45 | 1.28 | 1.17 | 1.4 | 1.37 | 1.31 | 1.44 | |
| 2009 | 1.29 | 1.22 | 1.36 | 1.28 | 1.17 | 1.4 | 1.29 | 1.23 | 1.35 | |
| Charlson co-morbidity counts | 0 | referent | referent | referent | ||||||
| 1 | 0.96 | 0.95 | 0.97 | 0.97 | 0.91 | 1 | 0.97 | 0.94 | 1 | |
| 2 | 1.00 | 1.00 | 1.00 | 0.91 | 0.84 | 1 | 0.94 | 0.90 | 0.97 | |
| ≥3 | 1.00 | 1.00 | 1.00 | 0.76 | 0.70 | 0.8 | 0.84 | 0.81 | 0.88 | |
| Bone density test in 36 month look-back | Yes | 1.29 | 1.25 | 1.34 | 1.27 | 1.23 | 1.31 | |||
| Osteoporosis pharmacotherapy before fracture | Yes | 4.84 | 4.65 | 5.02 | 4.79 | 4.64 | 4.93 | |||
| Vertebral fracture in 36 month look-back | Yes | 1.16 | 1.07 | 1.25 | 1.28 | 1.11 | 1.5 | 1.15 | 1.08 | 1.22 |
Age is at time of fragility fracture. Race/Ethnicity groups are from Medicare Denominator file. Part D low income subsidy is an indicator of income < 150% of federal poverty level. Osteoporosis pharmacotherapy includes receipt of one or more prescription fills for osteoporosis pharmacotherapy (bisphosphonate, calcitonin, estrogen, estrogen receptor modifier, teriparatide) in the 4 months preceding fracture and/or intravenous zoledronic acid in the 12 months preceding fracture. Poisson models included variables listed as well as Charlson co-morbidities present in > 2% of the cohort (diabetes, cerebrovascular disease, lung disease, liver disease, renal disease, peptic ulcer disease, congestive heart failure, dementia) and co-morbidities selected for clinical importance in osteoporosis and/or medication adherence: depression.
Fully adjusted models including only the “attention naïve” sub-cohort paralleled those of the full cohort but with stronger estimates: age over 80 (vs. 68–70) RR 0.63 (95% CI 0.57, 0.70), male sex RR 0.39 (95% CI 0.36, 0.40), Black race (vs. white) RR 0.80 (95% CI 0.67, 1.0), humerus or radius fracture (vs. hip) RR 0.66 (95% CI 0.61, 0.70) and RR 0.77 (95% CI 0.72, 0.80), respectively. In the models restricted to women, lower likelihoods of attention to osteoporosis were associated with age over 80 (vs. 68–70) RR 0.83 (95% CI 0.79, 0.86) and Black race (vs. white) RR 0.82 (95% CI 0.74, 0.91).
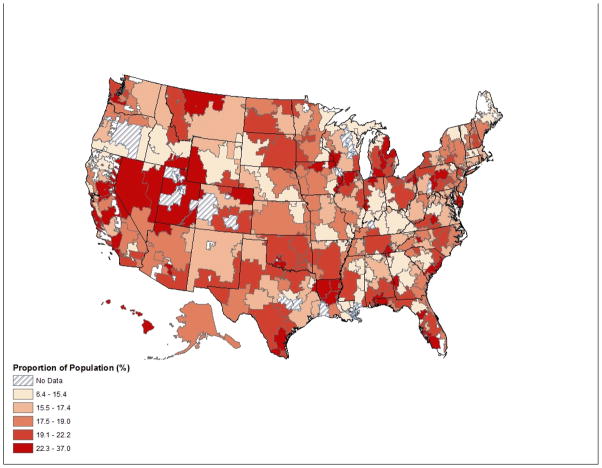
Region of residence was associated with substantial differences in osteoporosis care. In the 285 (of 306) HRRs with 50 or more cohort members, the unadjusted median estimate for post-fracture attention to osteoporosis was 21.5%. In adjusted analyses, the likelihood of testing and/or pharmacotherapy ranged from 6.4% to 37.0%; the 10th to 90th percentile range was 14.7% to 22.9%. See Figure 1 for HRR map of care distribution.
Figure 1.
Proportion of Cohort Receiving Bone Density Testing and/or Osteoporosis Pharmacotherapy Within 6 Months of Fracture by Dartmouth Atlas of Health Care Hospital Referral Region (HRR)
Secondary analyses
When we allowed 12 months to achieve the primary outcome, 28.4% of patients in the overall cohort received testing and/or pharmacotherapy. Characteristics associated with achieving this outcome in 12 months paralleled the main, 6-month models. (Appendix Table 6) In models including individual comorbidities rather than co-morbidity count categories, only dementia, congestive heart failure (CHF) and rheumatoid arthritis (RA) were associated with a statistically significant differences in likelihood of attention to osteoporosis with a RR 0.89 (95% CI 0.83–0.94) for dementia, a RR 0.92 (95% CI 0.87–0.96) for CHF, and a RR of 1.2 (95% CI 1.13–1.30) for RA.
DISCUSSION
The vast majority of this large cohort of older, community dwelling fragility fracture survivors did not receive attention to osteoporosis following their fracture events. The prevalence of treatment was even lower in select subpopulations. These low levels of care reflect current management of patients insured for services and prescriptions, and these patterns occurred in the context of ample evidence, treatment guidelines, and quality measures emerging steadily since 2000.(8, 9, 29) The proportion of patients achieving guideline concordant care in this study is similar to that reported in earlier publications on distinct populations.(11, 12, 16, 30)While it is encouraging that osteoporosis care quality improved modestly over our study period, the overall proportion remains remarkably low.
Attention to osteoporosis was especially low among Black patients and men. These disparities have been documented by others.(14, 16–18, 31) Our findings provide a national and recent depiction of care for male and Black fracture survivors. Although male and Black patients have a lower risk of developing osteoporosis, hip fractures in male and Black patients are associated with a higher mortality rate compared to females and white patients, respectively.(32, 33)As most clinical research trials for osteoporosis have focused on white women, there is limited evidence for the efficacy and effectiveness of pharmacotherapy in osteoporotic male and Black patients. Studies show treatment of osteoporotic males and Black patients improves markers of bone turnover, reduces vertebral fractures and that testing is cost-effective.(34–37) Furthermore, once a fragility fracture occurs, patients are considered osteoporotic and should be considered for treatment regardless of their predisposing risk factors, gender or race.(7, 8, 38) We found “attention naïve” patients were the least likely to achieve care after a fracture. This suggests that some combination of clinician and patient factors results in inattention to osteoporosis among patients at risk before a fracture occurs. More importantly, for most the approach does not change even after a fracture is sustained. That this pattern of inattention to osteoporosis emerged from this insured cohort demonstrates that insurance coverage alone is not sufficient to achieve quality care and raises questions about other factors influencing the persistent post-fracture care gap.
Compared to hip fracture patients, we found humerus and radius fracture patients less likely to receive attention to osteoporosis. Although the morbidity and mortality associated with upper extremity fractures is lower than that of hip fractures, one fragility fracture significantly increases the risk for a future fracture and is a better predictor of future fractures than low bone density.(19, 39, 40) An upper extremity fragility fracture should be recognized as a herald for risk of a more debilitating event, a hip fracture, and thus should prompt heightened attention to osteoporosis.
That region of residence is associated with substantially different care patterns suggests local patient, clinician and system characteristics are important determinants of osteoporosis care quality. Examination of outliers (both high and low) could reveal factors associated with lesser and greater attention to osteoporosis. This could help inform policies and identify opportunities for targeted interventions.
Care gaps in secondary prevention have been observed among older adults for other conditions. Studies reveal low use of beta-blockers and statins among patients over 65 after hospitalization for acute coronary events; patients over the age of 80 are even less likely to receive this evidence-based care.(41, 42) The pattern, recapitulated by this and other osteoporosis care studies, suggests the need for broad physician and patient education as well as policy interventions targeting secondary prevention among high-risk older patients.
Although this study identified patient characteristics and regions associated with lower attention to osteoporosis after a fracture, the determinants of the observed care are imperfectly understood. Likely many factors influence these care gaps including: clinician knowledge about the benefits of osteoporosis treatment in selected populations such as men and Black patients, lack of care continuity between the orthopedists treating a fracture and primary care physicians who generally manage bone density testing and pharmacotherapy, burdensome patient cost-share, competing co-morbidities, and patient preferences.(11, 43–45)Clinician and patient concerns for severe adverse effects of bisphosphonates such as atypical femur fractures, osteonecrosis of the jaw, esophageal cancer and fracture non-union may contribute to treatment gaps in current practice.(46, 47) As these issues began to emerge near the end of or after our study period, they likely do not explain much of the low treatment prevalence we observed.
Limitations
Our study has important limitations. This claims-based analysis contains no information on patient preferences or physician orders for testing or pharmacotherapy that were not fulfilled. Our data include no information on calcium and vitamin D use as these products are typically obtained over-the-counter. We thus cannot assess the use of this necessary but insufficient component of osteoporosis management.(7, 8, 38) Such information would permit a more complete understanding of care but would not change our findings or conclusions. Our measured outcomes do not include pharmacotherapy such as intravenous zoledronic acid infusion or bone density testing received during an inpatient stay. We believe such treatment rare but these missing data could result in an underestimate of outcomes.
The retrospective study included data beginning in May 2006, the first year of full Part D drug program implementation. We use the first months of 2006 to assess pre-fracture pharmacotherapy, but we lack information on oral pharmacotherapy prior to 2006. Similarly we look back only 36 months for previous bone density testing. Some patients may have received bone density testing and/or pharmacotherapy well before their observed fracture. Although the optimal duration of pharmacotherapy with bisphosphonates remains controversial, recent recommendations suggest three to five years of bisphosphonate use for average risk patients.(48) Some patients may have received such a course of pharmacotherapy prior to the observed index fracture, however patients who sustain a fragility fracture are no longer considered average risk and resumption of osteoporosis pharmacotherapy should be considered. (8, 38)
CONCLUSIONS
We found attention to osteoporosis after a fragility fracture uncommon in this large population of older U.S. Medicare beneficiaries. We also found care disparities among Black and male patients, among upper extremity fracture patients and in certain geographic regions. While the optimal rate of osteoporosis testing and pharmacotherapy after a fracture in a population of older adults is not known, it is certainly less than 100%. Some of our observed treatment gap is likely appropriate, perhaps reflecting patient preferences or prioritization of competing morbidities.(45) A personalized approach to care is best, but with more than 70% of patients receiving no pharmacotherapy or testing for osteoporosis following a 2009 fracture, we believe these care patterns are not fully explained by a patient centered approach. Many patients are likely unknowingly missing a potentially beneficial secondary prevention opportunity.
Research into determinants of and solutions for care gaps is needed. At the clinical level, care pathways, standard order sets and prompts from computerized order entry systems, shared-decision making resources, and improved collaboration between orthopedists and primary care clinicians are logical targets of interventions to improve care for fragility fracture patients. At the health policy level, linking osteoporosis care quality measures to financial incentives, for example, through the Centers for Medicare and Medicaid Services(CMS) Accountable Care Organizations program may be an effective way to advance improvements in post-fracture osteoporosis care.
Supplementary Material
Acknowledgments
Funding: National Institutes on Aging K23AG035030 (Morden) and P01 AG019783 Robert Wood Johnson Foundation Dartmouth Atlas Project National Institute of Arthritis and Musculoskeletal and Skin Diseases P60AR062799
The authors thank Harold C. Sox Jr., MD for his assistance and critical review of this manuscript.
This work was funded through the following grants:
National Institutes on Aging K23AG035030 (Morden) and P01 AG019783
Robert Wood Johnson Foundation Dartmouth Atlas Project
National Institute of Arthritis and Musculoskeletal and Skin Diseases P60AR062799
Sponsor’s Role: The grant sponsors had no input on the design, methods, analysis or preparation of this paper.
Footnotes
Author Contributions: All of the listed authors participated in the study concept and design, data analysis, interpretation of data, and preparation of the manuscript.
Conflict of Interest: Stephen Liu has received training grant funding from the Department of Health and Human Services, the University of Colorado and the Hartford Foundation for projects not related to this study or paper. The grant sponsors had no input on the design, methods, analysis or preparation of this paper. Stephen Liu is a consultant for the Oak Group which produces the Managed Care Appropriateness Program (MCAP) Clinical Review Criteria. This consulting work was not related to the design, methods, analysis or preparation of this paper. All other authors have no conflict of interest nor any financial support to report.
References
- 1.Tosteson AN, Gottlieb DJ, Radley DC, et al. Excess mortality following hip fracture: The role of underlying health status. Osteoporos Int. 2007;18:1463–1472. doi: 10.1007/s00198-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haentjens P, Magaziner J, Colon-Emeric CS, et al. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–389. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budhia S, Mikyas Y, Tang M, et al. Osteoporotic fractures: A systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30:147–170. doi: 10.2165/11596880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Colon-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: Data from two longitudinal studies. Osteoporos Int. 2003;14:879–883. doi: 10.1007/s00198-003-1460-x. [DOI] [PubMed] [Google Scholar]
- 5.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357 doi: 10.1056/NEJMoa074941. nihpa40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLean C, Newberry S, Maglione M, et al. Systematic review: Comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 7.Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation; 2010. Internet. Available from: www.nof.org/sites/default/files/pdfs/NOF_ClinicianGuide2009_v7.pdf. [Google Scholar]
- 8.Qaseem A, Snow V, Shekelle P, et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: A clinical practice guideline from the american college of physicians. Ann Intern Med. 2008;149:404–415. [PubMed] [Google Scholar]
- 9.National Quality Forum. Osteoporosis management in women who had a fracture. STEWARD: National Committee for Quality Assurance. Internet cited October 22, 2012]. Available from: http://www.qualityforum.org/QPS/0053.
- 10.The National Committee for Quality Assurance. That state of health care quality. Washington, D.C: The National Committee for Quality Assurance; 2004. Internet. cited December 21, 2012]. Available from: www.ncqa.org/communications/SOMC/SOHC2004.pdf. [Google Scholar]
- 11.Elliot-Gibson V, Bogoch ER, Jamal SA, et al. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: A systematic review. Osteoporos Int. 2004;15:767–778. doi: 10.1007/s00198-004-1675-5. [DOI] [PubMed] [Google Scholar]
- 12.Giangregorio L, Papaioannou A, Cranney A, et al. Fragility fractures and the osteoporosis care gap: An international phenomenon. Semin Arthritis Rheum. 2006;35:293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Jennings LA, Auerbach AD, Maselli J, et al. Missed opportunities for osteoporosis treatment in patients hospitalized for hip fracture. J Am Geriatr Soc. 2010;58:650–657. doi: 10.1111/j.1532-5415.2010.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris CA, Cabral D, Cheng H, et al. Patterns of bone mineral density testing: Current guidelines, testing rates, and interventions. J Gen Intern Med. 2004;19:783–790. doi: 10.1111/j.1525-1497.2004.30240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papaioannou A, Kennedy CC, Ioannidis G, et al. The osteoporosis care gap in men with fragility fractures: The Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2008;19:581–587. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuner JM, Zhang X, Sparapani R, et al. Racial and socioeconomic disparities in bone density testing before and after hip fracture. J Gen Intern Med. 2007;22:1239–245. doi: 10.1007/s11606-007-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiebzak GM, Beinart GA, Perser K, et al. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med. 2002;162:2217–2222. doi: 10.1001/archinte.162.19.2217. [DOI] [PubMed] [Google Scholar]
- 18.Feldstein AC, Nichols G, Orwoll E, et al. The near absence of osteoporosis treatment in older men with fractures. Osteoporos Int. 2005;16:953–962. doi: 10.1007/s00198-005-1950-0. [DOI] [PubMed] [Google Scholar]
- 19.Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27:2039–2046. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 20.Vanasse A, Dagenais P, Niyonsenga T, et al. Bone mineral density measurement and osteoporosis treatment after a fragility fracture in older adults: Regional variation and determinants of use in quebec. BMC Musculoskelet Disord. 2005;6:33. doi: 10.1186/1471-2474-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenspan SL, Wyman A, Hooven FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60:455–461. doi: 10.1111/j.1532-5415.2011.03854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Viswanathan HN, Ward MA, et al. Patterns of osteoporosis treatment change and treatment discontinuation among commercial and Medicare advantage prescription drug members in a national health plan. J Eval Clin Pract. 2013;19:50–59. doi: 10.1111/j.1365-2753.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- 23.Halpern R, Becker L, Iqbal SU, et al. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: A retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17:25–39. doi: 10.18553/jmcp.2011.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conwell LJ, Esposito D, Garavaglia S, et al. Out-of-pocket drug costs and drug utilization patterns of postmenopausal Medicare beneficiaries with osteoporosis. Am J Geriatr Pharmacother. 2011;9:241–249. doi: 10.1016/j.amjopharm.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 25.ResDAC Research Data Assistance Center. primary dispenser type code. 2012 [Internet]. Available from: http://www.resdac.org/cms-data/variables/Primary-Dispenser-Type-Code.
- 26.Creation of new race-ethnicity codes and socioeconomic status (SES) indicators for Medicare beneficiaries. U.S. Department of Health & Human Resources; 2008. [Internet]. Available from: http://www.ahrq.gov/qual/medicareindicators/medicareindicators2.htm. [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–378. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.The Dartmouth Atlas of Health Care. The Dartmouth Institute for Health Policy and Clinical Practice. Center for Health Policy Research; 2008. [Internet]. Available from: http://www.dartmouthatlas.org/atlases/atlas_series.shtm. [Google Scholar]
- 29.Colon-Emeric C, Yballe L, Sloane R, et al. Expert physician recommendations and current practice patterns for evaluating and treating men with osteoporotic hip fracture. J Am Geriatr Soc. 2000;48:1261–1263. doi: 10.1111/j.1532-5415.2000.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 30.Solomon DH, Finkelstein JS, Katz JN, et al. Underuse of osteoporosis medications in elderly patients with fractures. Am J Med. 2003;115:398–400. doi: 10.1016/s0002-9343(03)00357-7. [DOI] [PubMed] [Google Scholar]
- 31.Papaioannou A, Kennedy CC, Ioannidis G, et al. The osteoporosis care gap in men with fragility fractures: The canadian multicentre osteoporosis study. Osteoporos Int. 2008;19:581–587. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penrod JD, Litke A, Hawkes WG, et al. The association of race, gender, and comorbidity with mortality and function after hip fracture. J Gerontol A Biol Sci Med Sci. 2008;63:867–872. doi: 10.1093/gerona/63.8.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsen L, Sogaard AJ, Meyer HE, et al. Survival after hip fracture: Short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 34.Bell NH, Bilezikian JP, Bone HG, 3rd, et al. Alendronate increases bone mass and reduces bone markers in postmenopausal african-american women. J Clin Endocrinol Metab. 2002;87:2792–2797. doi: 10.1210/jcem.87.6.8575. [DOI] [PubMed] [Google Scholar]
- 35.Sawka AM, Papaioannou A, Adachi JD, et al. Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord. 2005;6:39. doi: 10.1186/1471-2474-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298:629–367. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 37.Boonen S, Reginster J, Kaufman J, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012:1714–1723. doi: 10.1056/NEJMoa1204061. 11/01; 2012/11;367. [DOI] [PubMed] [Google Scholar]
- 38.The care of patients with fragility fractures. British Orthopaedic Association; 2007. Internet. Available from: www.fractures.com/pdf/BOA-BGS-Blue-Book.pdf. [Google Scholar]
- 39.Browner WS, Pressman AR, Nevitt MC, et al. Mortality following fractures in older women. the study of osteoporotic fractures. Arch Intern Med. 1996;156:1521–1525. [PubMed] [Google Scholar]
- 40.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Lee HY, Cooke CE, Robertson TA. Use of secondary prevention drug therapy in patients with acute coronary syndrome after hospital discharge. J Manag Care Pharm. 2008;14:271–280. doi: 10.18553/jmcp.2008.14.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krumholz HM, Radford MJ, Wang Y, et al. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National cooperative cardiovascular project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 43.Haaland DA, Cohen DR, Kennedy CC, et al. Closing the osteoporosis care gap: Increased osteoporosis awareness among geriatrics and rehabilitation teams. BMC Geriatr. 2009;9:28. doi: 10.1186/1471-2318-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabenda V, Vanoverloop J, Fabri V, et al. Low incidence of anti-osteoporosis treatment after hip fracture. J Bone Joint Surg Am. 2008;90:2142–2148. doi: 10.2106/JBJS.G.00864. [DOI] [PubMed] [Google Scholar]
- 45.Guiding principles for the care of older adults with multimorbidity: An approach for clinicians. American geriatrics society expert panel on the care of older adults with multimorbidity. J Am Geriatr Soc. 2012;60:E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khosla S, Bilezikian JP, Dempster DW, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012;97:2272–2278. doi: 10.1210/jc.2012-1027. [DOI] [PubMed] [Google Scholar]
- 47.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2013;360:89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 48.Whitaker M, Guo J, Kehoe T, et al. Bisphosphonates for osteoporosis — where do we go from here? N Engl J Med. 2012;366:2048–2051. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.