Abstract
Researchers have begun to characterize the subtle biological and cognitive processes that precede the clinical onset of Alzheimer disease (AD), and to set the stage for accelerated evaluation of experimental treatments to delay the onset, reduce the risk of or completely prevent clinical decline. Here, we provide an overview of the experimental strategies, and brain imaging and cerebrospinal fluid biomarker measures that are used in early detection and tracking of AD, highlighting at-risk individuals who could be suitable for preclinical monitoring. We discuss how these advances have contributed to reconceptualization of AD as a sequence of biological changes that occur during progression from preclinical AD, to mild cognitive impairment and finally dementia, and we review recently proposed research criteria for preclinical AD. Advances in the study of preclinical AD have driven the recognition that efficacy of at least some AD therapies may depend on initiation of treatment before clinical manifestation of disease, leading to a new era of AD prevention research.
Introduction
Alzheimer disease (AD) is the most common cause of dementia in older people, and takes a devastating toll on patients and families1. Owing to the growing number of people living to older ages, a considerable increase is expected in the number of older adults with AD2–4 unless we can find effective treatments. Concern is increasing that AD treatments in development may need to be started before clinical onset, when extensive evidence of disease pathology already exists, to exert their most profound benefit.5 This concern, together with recent efforts to detect and track cognitive, clinical and biomarker changes associated with the preclinical stages of AD, has contributed to the interest in the evaluation of preclinical AD treatments6–10, which we have previously defined6 as “interventions that are started in the absence of mild cognitive impairment (MCI) or dementia and intended to postpone the onset, reduce the risk of, or completely prevent the clinical stages of AD.”
The pathogenic cascade of AD is thought to begin at least 1–2 decades prior to cognitive impairment, starting with accumulation of the amyloid-β1–42 (Aβ1–42) peptide (the major constituent of neuritic plaques) into oligomeric and fibrillar assemblies. The cascade eventually leads to neuroinflammatory changes, synaptic dysfunction and loss, accumulation and phosphorylation of the microtubule-associated protein tau (the main constituent of neurofibrillary tangles) and, ultimately, to neuronal degeneration11. Research has also suggested that some of these processes can be assessed using brain imaging and fluid biomarkers12, 13. Recent studies, however, have indicated that other changes might precede Aβ accumulation. Such studies found evidence of mitochondrial dysfunction, accumulation tau pathology at young ages14–16, and less temporal cortex grey matter and smaller hippocampi in infants at increased genetic susceptibility for AD, raising the possibility that some changes may be developmental17, perhaps providing a starting point for the cascade noted above.
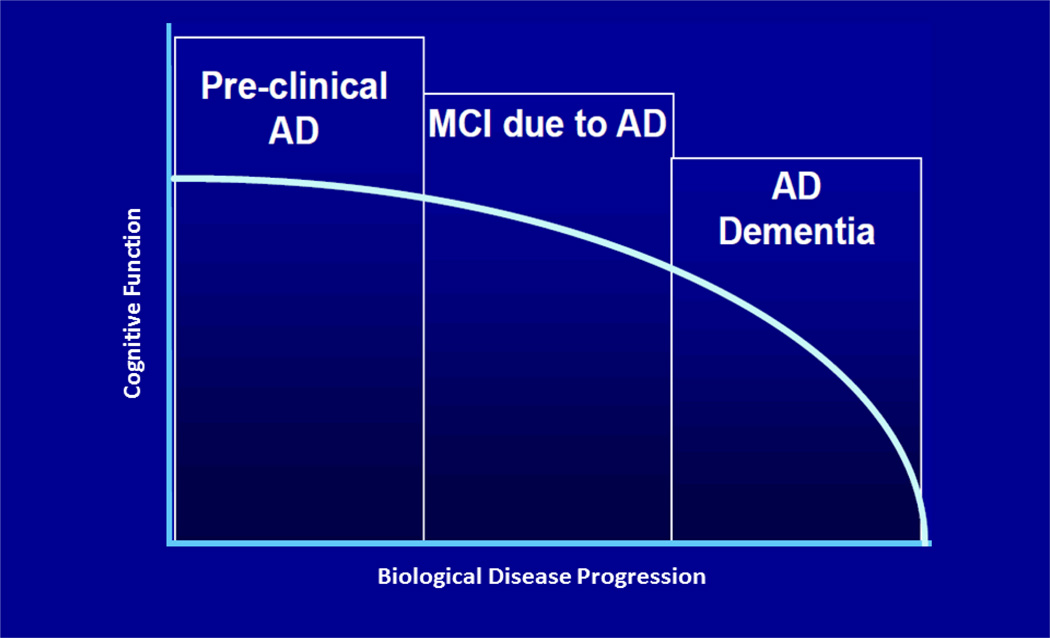
The International Working Group for New Research Criteria for the Diagnosis of AD18 and, more recently, working groups from the National Institute on Aging (NIA) and Alzheimer’s Association (AA) have championed efforts to reconceptualize AD as a progressive sequence of pathophysiological stages, some of which can be assessed using biomarkers, and which roughly correspond to preclinical, mild cognitive impairment (MCI) and dementia stages. The NIA-AA proposed revised criteria for clinical diagnosis of MCI19 and dementia due to AD20, and research criteria were proposed for the preclinical stages of AD21. These provisional, hypothesis-driven research criteria include three staging categories (Table 1) and are intended to provide a common language for researchers, to facilitate comparison of findings from different laboratories, and to help set the stage for evaluation of preclinical AD treatments. Approximately one- third of cognitively normal older adults over the age of 70 have been suggested to meet NIA-AA criteria for preclinical AD (stages 1–3)22. Of these individuals, approximately 10% progress to a diagnosis of MCI or dementia within 1 year, and of those in stage 3, 43% progress to MCI or dementia in this time frame.23
Table 1.
Staging of preclinical AD21
| Stage | Pathological features | Biomarkers | ||
|---|---|---|---|---|
| Amyloid-β (PET or CSF) |
Neurodegeneration (tau, FDG, MRI) |
Cognitive change |
||
| 1 | Asymptomatic amyloidosis | Present | Absent | Absent |
| 2 | Asymptomatic amyloidosis and neurodegenration | Present | Present | Absent |
| 3 | Asymptomatic amyloidosis, neurodegenration and subtle cognitive decline | Present | Present | Positive |
Abbreviations: CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose.
Brain imaging and other biomarker measures have had a considerable impact on the study of AD, and are expected to have an important role in the effort to find effective preclinical AD therapies. In this article, we review well-established cognitive, brain imaging, and fluid biomarkers for preclinical detection and tracking of AD. We also discuss studies in genetic at-risk groups as well as longitudinal studies examining progression to the clinical stages of AD. Finally, we note how these efforts are helping to accelerate evaluation of preclinical AD treatments in cognitively unimpaired individuals who are at increased risk of AD according to genetic or biomarker findings.
Measurement of AD biomarkers
To date, the most well-established measurements for detection and tracking of the preclinical and clinical stages of AD include structural (MRI measurements of regional and whole-brain tissue shrinkage, fluorodeoxyglucose (FDG) PET measurements of decline in the regional cerebral metabolic rate for glucose (CMRgl), PET measurements of fibrillar amyloid-β (Aβ) burden, and cerebrospinal fluid (CSF) measures of Aβ1–42 , total tau (t-tau) and phospho-tau (p-tau)24, 25 (Box 1). Other increasingly well-studied AD biomarkers include functional connectivity MRI (fcMRI) and task-related functional MRI. Notably, information provided by these and other biomarker measures depends not only on the modality used, but on the manner in which the data are acquired and analysed.
Box 1. Biomarkers of Alzheimer disease.
| Markers of amyloid-β accumulation |
| Amyloid-β in cerebrospinal fluid |
| PET amyloid imaging using 11C-Pittsburgh compound B or 18F radiotracers to bind to fibrillar amyloid-β |
| Markers of neurodegeneration |
| Tau and phospho-tau in cerebrospinal fluid |
| Markers of neuronal activity |
| Functional MRI measures of task-based neuronal activation, and resting neuronal connectivity |
| Markers of neuronal loss |
| MRI measures of cortical thinning, hippocampal volume, and whole-brain volume |
| Markers of synaptic dysfunction |
| 18F-fluorodeoxyglucose PET |
Structural MRI
Structural MRI has been the most extensively used brain imaging method in the detection and tracking of AD, and shows establishment of brain atrophy at the time of diagnosis dementia due to AD. These measurements also reveal that patients with MCI and dementia due to AD have accelerated rates of atrophy of the hippocampus, entorhinal cortex, regional grey matter, and whole brain26, 27. Many of these measurements correlate with clinical severity28, 29, subsequent clinical decline29, 30, and neuronal loss31. Moreover, these MRI changes are apparent before onset of clinical symptoms, with hippocampal volumes reduced by approximately 10% at least 3 years prior to diagnosis of dementia due to AD, and atrophy beginning at least 5 years prior to the diagnosis27, 32.
FDG PET
AD is associated with preferential CMRgl reductions in the precuneus, posterior cingulate, and parietotemporal cortex, some of which are apparent prior to onset of dementia, and extend to the frontal cortex and whole brain as disease severity progresses33. CMRgl abnormalities could be related to reductions in activity or density of terminal neuronal fields or perisynaptic glial cells34, 35, metabolic dysfunction36, 37, or a combination of these factors. CMRgl reductions are progressive, correlate with clinical severity and are predictive of subsequent clinical decline38.
Fibrillar Aβ PET
PET measurements of fibrillar Aβ deposition could help to advance the study of AD by enabling in vivo measurement of fibrillar amyloid in the brain39. Clinically affected patients with AD show fibrillar Aβ deposition in the precuneus, posterior cingulate, parietal, temporal and frontal cortices, which mostly occurs in early disease stages, with fibrillar Aβ levels likely stabilizing later in the disease40. Cortical fibrillar amyloid seen with PET imaging correlates closely with amyloid pathology at autopsy41, 42.
Functional connectivity MRI
Resting state fcMRI allows characterization of neural network activity when an individual is not completing a task. The default mode network (DMN) represents a cluster of brain regions—predominantly consisting of midline and lateral frontal regions, medial and lateral parietal regions extending into the posterior cingulate–retrosplenial cortex—that have elevated activity in states of relative rest43, 44. Such regions seem to be suppressed during various cognitive activities, including encoding of new memories45, 46. Reduced resting state connectivity47 and alterations in task-induced deactivation responses on functional MRI have been identified in normal ageing48, 49, MCI46, 50 and AD43, 49 compared with younger, healthy controls.
The DMN overlaps anatomically with brain regions that have Aβ deposition51–53, regional atrophy and areas of reduced white matter integrity as measured on MRI54, and reduced CMRgl as measured using FDG PET47. Moreover, the DMN overlaps with brain regions that rely on glucose beyond its usual role, referred to as “aerobic glycolysis” in adequately oxygenated tissue55. Given the spatial distribution of aerobic glycolysis in young adults (age 20–33 years-old) overlaps spatially with PET measurements of fibrillar Aβ deposition, it is suggestive that aerobic glycolysis may have a role in preclinical AD, though the biological processes remain to be clarified.
Cerebrospinal fluid measures
Measurement of CSF Aβ42, particularly when combined with t-tau or p-tau181 measures, is useful for establishment of a diagnosis in people with MCI or very mild dementia, and for prognostication56. Clinically affected patients with AD have abnormally low CSF Aβ42 levels, and elevated p-tau181 and t-tau levels57, 58. The reduction in CSF Aβ42 may seem counterintuitive, but is thought to result from sequestration of Aβ42 in amyloid plaques in the brain56. CSF changes precede clinical onset by over a decade59–61, and are associated with smaller whole-brain volumes in cognitively healthy adults60. Although CSF Aβ42 levels are well-established in detection and differential diagnosis of AD62, this measure is not well correlated with disease duration or clinical severity63. Similarly, elevated t-tau is consistently reported in patients with clinical AD but is not closely associated with severity of dementia56, 64.
Detecting the earliest brain changes
Several AD-associated biomarkers show changes years before onset of symptoms in individuals at increased genetic risk of AD (for example, carriers of the ε4 allele of the apolipoprotein E (APOE) gene65 and individuals with gene mutations that cause early-onset AD59) and those with Down syndrome66, as well as cognitively normal individuals who subsequently progressed to clinical AD67, 68 Considerable research is this area has been done to date, although the need remains for continued cohort studies with large sample sizes, and head to head comparisons of identified biomarkers, in conjunction with development of new biomarkers, to determine the extent to which these measurements, alone or in combination with other factors, predict subsequent rates of clinical decline.
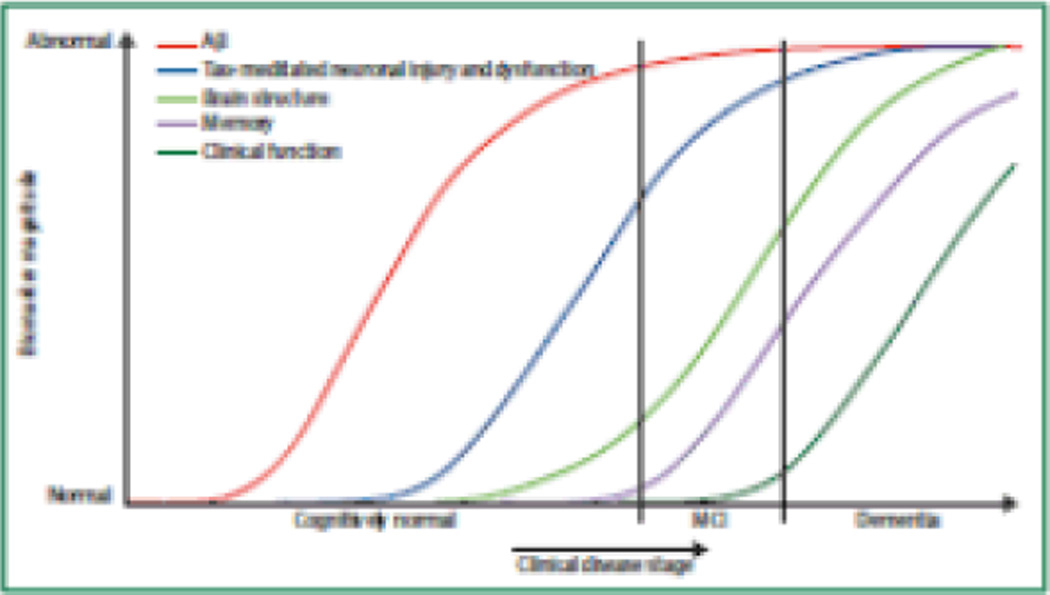
The sequence of biomarker changes
The hypothetical sequence of biomarker changes proposed by Jack and colleagues are thought to begin about 10–20 years prior to clinical onset with biomarker evidence of amyloid plaque deposition (reduced CSF Aβ42 levels and increased fibrillar Aβ PET measurements)12, 13, 59, 61, 69. Other elements of the pathobiological cascade, however, might exist that have yet to be discovered. These changes are probably followed by biomarker evidence of neuronal dysfunction and synaptic loss, such as regional reductions in cerebral glucose metabolism as measured on PET, altered patterns of functional connectivity, alterations in regional brain activity during memory encoding and novel viewing tasks, and reductions in grey matter and cortical thickness as measured on MRI. Biomarker evidence of tau pathology, neurofibrillary tangles, neuronal degeneration, and neuronal loss seem to follow in the sequence of biomarker changes. These changes include elevated CSF t-tau and p-tau levels, and hippocampal atrophy on MRI.
The exact timing of biomarker changes can depend on many factors, including the analytical tools used, the underlying pathobiology, and the age at which participants are studied17. We and others have characterized early biomarker and cognitive changes associated with preclinical AD by studying individuals with different risk of AD on the basis of genetic background, biomarker evidence of AD, or other factors. As part of these studies, the apparent longitudinal trajectory of cognitive and biomarker changes in these at-risk groups was mapped as the individuals progressed to clinical stages of AD or estimated based on years from anticipated age at clinical onset. Certainly there are a number of other risk factors for AD—including but not limited to age, family history, cardiovascular disease and diabetes—that, although important, are beyond the scope of this Review, given its focus on at-risk groups for preclinical treatment trials.
Identification and study of at-risk individuals
Apolipoprotein E
APOE is the major susceptibility gene for late-onset AD. In comparison with individuals with the ε3ε3 genotype, the ε2 allele is associated with decreased risk of late-onset AD and older age at dementia onset. By contrast, each additional copy of the ε4 allele, which is found in about 25% of the population and about 60% of patients with AD dementia, is associated with higher risk of late-onset AD and younger age at dementia onset, and individuals with two copies of this allele have an especially high risk70, 71. The number of other confirmed AD susceptibility genes continues to grow, but these genes are associated with comparatively modest effects on AD risk72–76.
As each APOE genotype is associated with a different level of risk of AD, detection and tracking of cognitive and biomarker changes in individuals with these different genotypes can provide researchers with initial information about which preclinical AD biomarker (baseline measurement or change in measure) or combination of biomarkers is related to subsequent clinical onset, without having to wait several years to obtain such information in unselected populations.
Studies of cognitively unimpaired individuals who carry at least one copy of the APOE ε4 allele show considerable differences in AD biomarkers compared with noncarriers, including MRI-measured accelerated cortical thining77, lower grey matter density78, and accelerated brain atrophy79. Some changes in brain structure are apparent during infancy in ε4 carriers17, although the relationship between such changes and development of AD dementia remains uknown. FDG PET studies of cognitively unimpaired APOE ε4 carriers reported reduced CMRgl in the same posterior cingulate, precuneus, parietal, temporal and frontal regions as in AD dementia80–85, some of which are apparent almost 50 years prior to the expected onset of symptoms86, are progressive87, and are correlated with ε4 allele dose88. Recent evidence suggests that the preclinical hypometabolism in the posterior cingulate precedes hippocampal volume loss associated with APOE ε4 allele dose89, and some findings in cognitively normal older adults (average age 75 years) with greater amyloid deposition and in patients with MCI and Down syndrome, irrespective of APOE, suggest that hypermetabolism may precede metabolic decline in certain brain regions90–92.
A study of adults (49–79 year-old) APOE ε4 carriers reported a pattern of reduced deactivation while performing a semantic categorization task compared with noncarriers, consistent with the DMN, though there was no allele dose effect93. Similarly, relative to age-matched non-carriers, differences in resting state connectivity were detected in both older adult (50–65 year-old)94 and young (20–35-year-old)95 APOE ε4 carriers.
Amyloid PET studies of cognitively unimpaired adult APOE ε4 carriers found substantial fibrillar Aβ deposition in brain regions affected by AD pathology, including frontal, temporal, posterior cingulate–precuneus, and parietal regions compared with noncarriers69, 96–101. Fibrillar Aβ deposition is correlated with ε4 allele dose96, is apparent approximately 10–15 years prior to estimated onset of AD dementia, and might be associated with greater cognitive impairment in ε4 carriers97, 102, 103. Differences in CSF measures of Aβ and tau have been reported, with APOE ε4 carriers having reduced Aβ4285, 101, 104–106, elevated Aβ40/Aβ42 ratios107, and higher t-tau and p-tau181106, 108, 109 compared with noncarriers.
In addition to tracking biomarker changes in cognitively unimpaired APOE ε4 carriers, we and others have also examined the cognitive differences between carriers and noncarriers. Differences have not been consistently identified in early-life110 but, starting in late-middle age, decline in long-term recall memory performance is more prominent in APOE ε4 carriers111–114 and is associated with ε4 allele dose115, 116, despite having no apparent clinical symptoms.
Autosomal dominant Alzheimer disease
More than 200 mutations of the presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) genes have been shown to cause autosomal dominant AD (ADAD)117. As carriers of the genes will almost certainly develop AD, they provide a unique group in which to characterize the trajectory of preclinical AD changes in relationship to their family’s estimated age at clinical onset118. ADAD differs from the more common, late-onset form of AD in several respects—for example, by a generally younger age at clinical onset and overproduction rather than reduced clearance of Aβ1–42119, 120, though the question of overproduction versus clearance is still under study121. The two forms of AD do, however, have common features, particularly in regard to clinical phenotype122, 123. Investigation of ADAD, therefore, provides another approach to preclinical study of AD.
Autosomal dominant versus sporadic AD
Findings from biomarker studies of cognitively unimpaired ADAD mutation carriers are generally consistent with those from cognitively unimpaired APOE ε4 carriers, although the exact timing and patterns (for example, fibrillar Aβ deposition) can differ. Comparison between ADAD and groups who are genetically at-risk of sporadic AD—in this case, APOE ε4 carriers—is important for determination of how findings from trials in ADAD carriers relate to sporadic AD, given the planned preclinical treatment trials, discussed below.
Cognitively unimpaired, young adult ADAD mutation carriers can have reduction in grey matter volume as measured by voxel-based techniques124, 125 in the same brain regions preferentially affected by AD, even before CSF or PET evidence of Aβ42 deposition61, with changes in hippocampal volume apparent approximately 15 years before expected symptom onset59, 126 that continue to decline over time127. Research by the Dominantly Inherited Alzheimer Network (DIAN) will be crucial in teasing apart the timing and trajectory of MRI changes, although to date it has only reported findings in regard to hippocampal volume59. Studies in ADAD mutation carriers have also reported CMRgl reductions in the posterior cingulate, precuneus, parietal, and temporal cortex at least 10 years prior to expected symptom onset59, 128–130.
Findings in amyloid PET studies to determine the pattern and timing of preclinical fibrillar Aβ deposition are generally similar in ADAD mutation carriers and APOE ε4 carriers, with deposition apparent approximately 10 years prior to the expected age at clinical onset59, 61. Some studies, however, have reported preferential deposition in the striatum in at least certain ADAD mutations131, 132. A notable difference, highlighted by data from DIAN, is that in clinically affected ADAD mutation carriers, fibrillar Aβ deposition may continue to rise after clinical onset of AD. Conversely, this finding has not been replicated in the PSEN1 E280A kindred61, perhaps owing to the difference in fibrillar Aβ patterns observed with different ADAD mutations. In cognitively unimpaired ADAD mutation carriers, the direction of CSF Aβ differences between carriers and noncarriers seems to depend on the age of participants, though the assay and batching of samples likely also play an important role. For example, in a recent study by our group, young adult PSEN1 E280A mutation carriers had significantly higher CSF Aβ42 levels and significantly lower CSF t-tau/Aβ42 and p-tau/Aβ42 ratios compared with kindred non-carriers125, in contrast to most findings reported in older preclinical individuals and in the clinical stages of late-onset AD and autosomal dominant AD63, 133. Findings from the DIAN study, which involved a larger number individuals of different mutations at different ages, has suggested that CSF Aβ42 levels begin to decline 25 years before their estimated age at clinical onset. The researchers did not, however, detect differences in CSF, plasma, or brain imaging measures between the 13 carriers and 13 non-carriers who were studied more than 20 years before their estimated age at clinical onset, perhaps owing to the small sample size59. Similar to findings in APOE ε4 carriers, cognitive decline—including changes in memory, visuospatial and executive function—was reported in ADAD mutation carriers despite ongoing normal clinical status134–138.
Other at-risk individuals
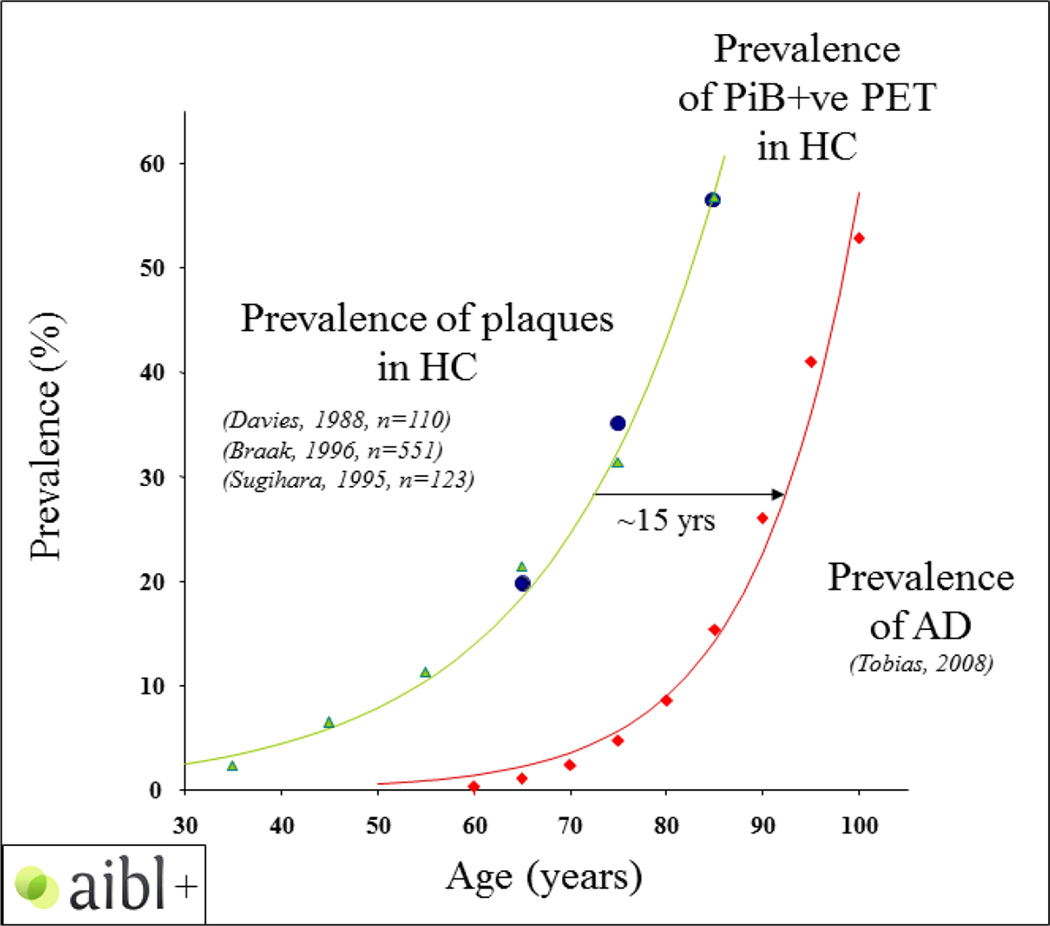
Individuals with biomarker evidence of AD pathology but no clinical symptoms represent another group in which to track the trajectory of preclinical AD. Amyloid PET studies suggested that approximately one-third of cognitively unimpaired older adults have significant fibrillar Aβ deposition, which is consistent with intermediate or high likelihood of pathological AD69, 98, 139–141, with most of the rise in deposition occurring during the preclinical stage of AD142. Notably, most studies report that cognitive function is normal or only mildly affected in older individuals with PET evidence of Aβ deposition98, 143–145, and that Aβ deposition could be more closely associated with longitudinal cognitive decline in older adults, particularly in regard to episodic memory146–149.
Predicting clinical progression
Retrospective and longitudinal studies have been helpful for tracking of changes that occur from preclinical AD to AD dementia. For example, retrospective analyses of individuals who eventually progressed to AD dementia have generally reported decline in memory—particularly episodic, semantic and working memory—to be a defining feature of preclinical AD,150, 151, with the rate of cognitive decline and affected domains greatly accelerating 5–6 years prior to diagnosis of dementia152. Importantly, cognitive decline in older age may be specific to those who progress to MCI or AD dementia and might not be an inevitable part of ageing per se152, supporting the utility of cognition as a predictive marker of clinical progression. We and others have been particularly interested in determining the optimal combination of cognitive assessments for tracking cognitive decline prior to clinical progression of AD153–155.
Non-biomarker-enriched populations
AD biomarkers could be useful for prediction of clinical AD progression in populations who are not selected on the basis of AD biomarker profiles. For example, people with MCI who subsequently progress to probable AD dementia show significantly greater declines in CMRgl (measured on FDG PET) in AD-related brain regions than do individuals with MCI who remain stable during the same time interval156, 157. MRI-measured reductions in hippocampal and entorhinal cortex volume parallel very early memory decline and are associated with subsequent progression to MCI or AD dementia30, 158, 159.
Functional connectivity MRI could also be useful in predicting conversion from MCI to AD dementia160, 161. Increased activity in “task positive” networks (as opposed to brain networks that deactivate during tasks such, as the DMN) in patients with MCI or AD dementia have been interpreted as attempts at compensation, although this hypothesis remains to be demonstrated conclusively. Alternative explanations include dedifferentiation of cortical function and aberrant excitation—a finding that has also been seen in animal models of AD162. In addition, lifelong patterns of increased brain activity might themselves predispose an individual to Aβ deposition163. The latter hypothesis is intriguing, particularly given that Aβ deposition, as measured by amyloid PET, is associated with longitudinal cognitive decline in some normal adults and with progression to AD dementia68, 98. As clinical progression occurs, however, Aβ accumulation slows98, 159 and probably plateaus by the time of diagnosis of AD dementia164. Similar to functional MRI, elevated ratios of CSF tau/Aβ42 and p-tau/Aβ42 are predictive of subsequent clinical progression in preclinical AD or MCI to AD dementia63, 165. Together, positivity for PET and CSF measures of Aβ seem to confer a threefold to fivefold higher likelihood of progression from preclinical AD or MCI to AD dementia166–171.
Biomarker-enriched populations
Several studies have examined clinical outcomes in individuals with biomarker evidence of AD pathology. Multiple positive AD biomarkers might have additive predictive value. For instance, in people with MCI, having abnormal CSF t-tau and p-tau concentrations and hippocampal atrophy predicted time to AD dementia172. Similarly, lower CSF Aβ42 concentration, hypometabolism as measured on FDG PET, and hippocampal atrophy were associated with a faster time to AD dementia in people with MCI173, supporting the hypothetical dynamic biomarker model discussed previously12, 13. Moreover, in the latter study, people with MCI who were positive for all of the three AD biomarkers consistently progressed to AD dementia during a 3-year period, whereas those with no positive biomarkers were unlikely to progress. These findings in MCI are supported by findings in cognitively normal individuals in which abnormal amyloid levels on PET imaging and CSF biomarkers, when examined together, are associated with faster time to cognitive impairment, whereas no differences were identified in the predictive value of individual biomarkers174.
Preclinical AD populations
In preclinical AD populations, high Aβ levels on PET imaging correlates with decreased performance on episodic memory and language assessments148 and increased hippocampal atrophy rate175 over 18 months. Additional follow-up is needed to assess the predictive value of high abnormal amyloid levels on PET imaging in cognitively healthy individuals for progression to MCI or AD dementia.
An important related issue is determination of the cut-off value that defines ‘amyloid positivity’. A level could be selected that is consistent with an intermediate to high likelihood of AD pathology, or one that signifies the presence of any Aβ above that observed in low-risk (young APOE ε4 noncarriers) individuals176. The optimal approach probably depends on the question being explored. An intermediate value between these two cut-offs could be a suitable approach for tracking change over time—something that is particularly important as the field begins preclinical AD treatment trials in biomarker-enriched populations—but researchers will need to ensure that this cut-off is associated with a high likelihood of progression to AD.
Needs, challenges and opportunities
Biomarkers of preclinical-treatment response
As growing evidence from natural history studies indicates that brain imaging and other biomarker measurements begin to change years before clinical symptoms emerge, it is plausible that these measures could have a role in evaluation of preclinical AD treatments. However, as we enter this era in AD prevention research and treatment trials, it is important to examine how biomarkers behave in response to treatment, irrespective of what is suggested by longitudinal data in observational studies. Prominent examples of unexpected biomarker responses to experimental treatment include MRI-measured brain shrinkage in response to the anti-Aβ vaccination AN-1792 (despite possible cognitive benefit on a subset of memory measures)177 and in response to the passive Aβ immunotherapy bapineuzumab. Crucially, therefore, trials should incorporate all the established AD biomarker measures to determine how they behave in response to treatment.
Refining and expanding biomarker knowledge
Observational longitudinal cohort studies stand to make important contributions to the field of preclinical AD biomarkers. For example, they are needed to improve our understanding of the trajectory of biomarker changes, enabling determination of the accuracy of prevailing hypotheses regarding the sequence of biomarker changes, and identification of which biomarkers, alone or in combination, predict subsequent clinical course. Additionally, new biomarkers are needed to detect other aspects of disease pathology and process and, if developed, could help in evaluation of potential treatments throughout the disease spectrum. Examples of needed biomarkers include those for assessment of oligomeric Aβ species, tau burden, and neuroinflammation, and more-specific measures of synaptic density.
Preclinical treatment trials
A number of preclinical treatment trials are in the planning stages or are already under way in several at-risk populations of cognitively unimpaired individuals—namely, individuals with biomarker evidence of Aβ as measured by amyloid PET, individuals who carry ADAD mutations, those who are homozygous for the APOE ε4 allele, and individuals with variable-length polymorphisms in TOMM40. Although observational studies conducted to date have been valuable in preparing researchers for preclinical treatment trials, an important point to consider is that prevalence estimates of factors such as amyloid burden in older adults, which are derived from population-based studies, might not be observed in clinical trials owing to recruitment biases.
Over the next several years, the field will certainly see more trials as a result of initiatives including, but not limited to, the National Alzheimer’s Project Act, the French Alzheimer Plan, and Alzheimer Europe. These prevention trials, which will embed currently available AD biomarkers among sensitive composite cognitive test scores, are designed to show that the treatment effects on biomarker measures are reasonably likely to predict clinical benefit, with the intent that one or more of these biomarkers may receive regulatory agency qualification as a surrogate end point for use in preclinical AD treatment trials5–7. In some cases, all of the data and biological samples will be made available to the scientific community following trial completion, with the aim of accelerating development of new biomarkers and sensitive data analysis methodologies. Moreover, these trials should provide a better test of the amyloid hypothesis than do trials in AD dementia or MCI.
Conclusions
The pathogenic cascade of AD is thought to begin at least 10–20 years prior to cognitive impairment, and AD biomarkers have played a crucial role in the detection and tracking of the preclinical and clinical stages of AD. As we begin this era of AD prevention research, biomarkers and sensitive cognitive measures are poised to continue to make important contributions. For instance, AD biomarkers, alone or in combination, could provide both scientific advances and regulatory approval for treatments under “Accelerated Approval provisions” or under the standard approval process if the biomarker has been validated to predict clinical benefit. Although there is no guarantee that treatments in the development pipeline will be effective, interest is growing in evaluation of these treatments in the preclinical stage of AD. Given the potential benefits to society if an effective AD or preclinical AD treatment is found, researchers and other involved parties should have a sense of urgency. Moreover, this enthusiasm needs to be shared with the general public, informing them how to volunteer in prevention-focused research, given the likelihood that for every prevention trial, thousands of individuals will need to be screened in order to find enough eligible participants. With these factors in mind, we will be better prepared to deal with the complexities and uncertainties that lay ahead.
Figure 1.
Reconceptualizing Alzheimer’s Disease
Figure 3.
Hypothetical dynamic biomarkers of the AD pathological cascade12
Figure 4.
Temporal link between amyloid deposition and onset of AD dementia
With permission from Chris Rowe, reprinted from69
Key points.
The pathogenic cascade of Alzheimer disease (AD) is thought to begin at least 1–2 decades prior to cognitive impairment
Disappointing results of several AD drugs in late-stage development have suggested the need for early therapeutic intervention, calling for development of biomarkers and sensitive cognitive measures for preclinical disease. The better established measurements for detection and tracking of preclinical and clinical stages of AD include MRI, fluorodeoxyglucose PET, amyloid PET, and cerebrospinal fluid measures of Aβ42, total tau, and phospho-tau
Individuals at genetic risk of AD can provide insights into cognitive and biomarker changes that precede clinical manifestation of AD, and are suitable candidates for ongoing monitoring and early-intervention strategies
We are entering an era of AD prevention research, with a number of preclinical AD treatment trials in the planning stages or under way for several at-risk, cognitively unimpaired populations
Acknowledgements
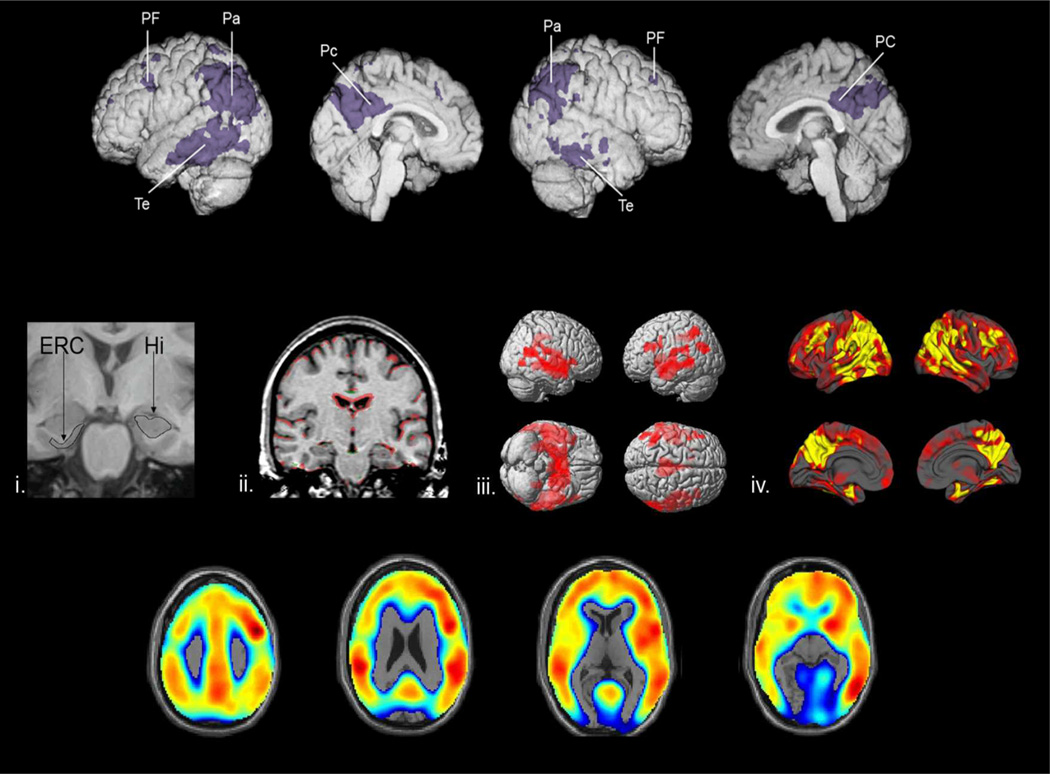
This article was supported by grants from the National Institute on Aging (R01AG031581 and P30AG19610 to EMR, RF1AG041705 to EMR, PNT and FL), the National Institute of Neurological Disorders and Stroke (F31-NS078786 to YTQ), Colciencias (1115-493-26133, 1115-545-31651 and 1115-519-29028 FL), the Banner Alzheimer’s Foundation, and the state of Arizona. We thank Drs. Nick Fox, Chris Rowe, and Michael Weiner and their colleagues for permission to use their images in Figure 2. We thank our valued research for their invaluable dedication and inspiration.
Figure 2.
Well Established Brain Imaging Techniques in the Detection and Tracking of AD
Reference List
- 1.Alzheimer's Association 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: The 90+study. Ann. Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookmeyer R, et al. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2011;7(1):61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis. Assoc. Disord. 2001;15:169–173. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Reiman EM, Langbaum JBS. Brain imaging in the evaluation of putative Alzheimer's disease slowing, risk-reducing and prevention therapies. In: Jagust WJ, D'Esposito M, editors. Imaging the Aging Brain. New York: Oxford University Press; 2009. pp. 319–350. [Google Scholar]
- 6.Reiman EM, Langbaum JBS, Tariot PN. Alzheimer's Prevention Initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomarkers in Medicine. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiman EM, et al. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;25:293–301. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman RJ, et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res. Ther. 2011;2:35. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aisen PS, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Draft guidance. U.S Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2013. Guidance for industry Alzheimer's disease: Developing drugs for the treatment of early stage disease. [Google Scholar]
- 11.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valla J, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer's susceptibility gene. J. Alzheimers. Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Del TK. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 16.Elobeid A, Soininen H, Alafuzoff I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 2012;123:97–104. doi: 10.1007/s00401-011-0906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knickmeyer RC, et al. Common Variants in Psychiatric Risk Genes Predict Brain Structure at Birth. Cereb. Cortex. 2013 doi: 10.1093/cercor/bhs401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois B, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 19.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann GM, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knopman DS, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiman EM, Jagust WJ. Brain imaging in the study of Alzheimer's disease. Neuroimage. 2012;61:505–516. doi: 10.1016/j.neuroimage.2011.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Leon MJ, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y. Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson BC, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 27.Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chetelat G, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 31.McGeer PL, et al. Comparison of PET, MRI, and CT with pathology in a proven case of Alzheimer's disease. Neurology. 1986;36:1569–1574. doi: 10.1212/wnl.36.12.1569. [DOI] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology. 2008;70:1740–1752. doi: 10.1212/01.wnl.0000281688.77598.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langbaum JBS, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45:1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz WJ, et al. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science. 1979;205:723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]
- 35.Meguro K, et al. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET. Implications for Alzheimer's disease. Brain. 1999;122(Pt 8):1519–1531. doi: 10.1093/brain/122.8.1519. [DOI] [PubMed] [Google Scholar]
- 36.Magistretti PJ, Pellerin L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb. Cortex. 1996;6:50–61. doi: 10.1093/cercor/6.1.50. [DOI] [PubMed] [Google Scholar]
- 37.Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J. Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman DH, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. Journal of the American Medical Association. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 39.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 40.Weiner MW, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8:S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark CM, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. Journal of the American Medical Association. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark CM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 43.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Raichle ME, et al. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pihlajamaki M, DePeau KM, Blacker D, Sperling RA. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. American Journal of Geraitric Psychiatry. 2008;16:283–292. doi: 10.1097/JGP.0b013e318162a0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorg C, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lustig C, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedden T, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drzezga A, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlassenko AG, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holtzman DM. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol. Aging. 2011;32(Suppl 1):S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thal LJ, et al. The Role of Biomarkers in Clinical Trials for Alzheimer Disease. Alzheimer Dis Assoc Disord. 2006;20:6–15. doi: 10.1097/01.wad.0000191420.61260.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fagan AM, et al. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 59.Bateman RJ, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagan AM, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleisher AS, et al. Florbetapir PET analysis of amyloid-b deposition in presenilin 1 E280A autosomal-dominant Alzheimer's disease kindred: a cross-sectional study. Lancet Neurol. 2012;11:1057–1065. doi: 10.1016/S1474-4422(12)70227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sunderland T, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 63.Fagan AM, et al. Cerebrospinal fluid tau/b-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 64.Sunderland T, et al. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol Psychiatry. 1999;46:750–755. doi: 10.1016/s0006-3223(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 65.Reiman EM, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the e4 allele for apolipoprotein E. N. Engl. Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 66.Beacher F, et al. Brain anatomy and ageing in non-demented adults with Down's syndrome: an in vivo MRI study. Psychol. Med. 2009:1–9. doi: 10.1017/S0033291709990985. [DOI] [PubMed] [Google Scholar]
- 67.Jack CR, Jr, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris JC, et al. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rowe CC, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 71.Saunders AM, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 72.Roses AD, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics. J. 2010;10:375–384. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerreiro R, et al. TREM2 variants in Alzheimer's disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonsson T, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Espeseth T, et al. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol. Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 78.Wishart HA, et al. Regional brain atrophy in cognitively intact adults with a single APOE e4 allele. Neurology. 2006;67:1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- 79.Chen K, et al. Correlations between apolipoprotein E e4 gene dose and whole brain atrophy rates. Am. J Psychiatry. 2007;164:916–921. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- 80.Reiman EM, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 81.Small GW, et al. Early detection of Alzheimer's disease by combining apolipoprotein E and neuroimaging. Ann N. Y. Acad. Sci. 1996;802:70–78. doi: 10.1111/j.1749-6632.1996.tb32600.x. [DOI] [PubMed] [Google Scholar]
- 82.de Leon MJ, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc. Natl. Acad. Sci. U. S. A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langbaum JB, et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch. Neurol. 2010;67:462–468. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Small GW, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo RY, et al. Longitudinal change of biomarkers in cognitive decline. Arch Neurol. 2011;68:1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc. Natl. Acad. Sci. U. S. A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reiman EM, et al. Declining brain activity in cognitively normal apolipoprotein E e4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reiman EM, et al. Correlations between apolipoprotein E e4 gene dose and brain-imaging measurements of regional hypometabolism. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Protas HD, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle age persons at three levels of genetic risk for Alzheimer's disease. Arch Neurol. doi: 10.1001/2013.jamaneurol.286. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen AD, et al. Basal Cerebral Metabolism May Modulate the Cognitive Effects of A{beta} in Mild Cognitive Impairment: An Example of Brain Reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haier RJ, et al. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology. 2003;61:1673–1679. doi: 10.1212/01.wnl.0000098935.36984.25. [DOI] [PubMed] [Google Scholar]
- 92.Oh H, Habeck C, Madison C, Jagust W. Covarying alterations in Ab deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Human Brain Mapping. doi: 10.1002/hbm.22173. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Persson J, et al. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia. 2008;46:1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 94.Fleisher AS, et al. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage. 2009;47:1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filippini N, et al. Distinct patterns of brain activity in young carriers of the APOE-{varepsilon}4 allele. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reiman EM, et al. Fibrillar amyloid-b burden in cognitively normal people at three levels of genetic risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pike KE, et al. Cognition and beta-amyloid in preclinical Alzheimer's disease: data from the AIBL study. Neuropsychologia. 2011;49:2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 98.Villemagne VL, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mielke MM, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fleisher AS, et al. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol. Aging. 2013;34:1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 101.Morris JC, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kantarci K, et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78:232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim YY, et al. Abeta amyloid, cognition, and APOE genotype in healthy older adults. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Peskind ER, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- 105.Popp J, et al. Cerebrospinal fluid markers for Alzheimer's disease over the lifespan: effects of age and the APOEepsilon4 genotype. J. Alzheimers Dis. 2010;22:459–468. doi: 10.3233/JAD-2010-100561. [DOI] [PubMed] [Google Scholar]
- 106.Kester MI, et al. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1353–1358. doi: 10.1212/WNL.0b013e3181bd8271. [DOI] [PubMed] [Google Scholar]
- 107.Fagan AM, et al. Differences in the Abeta40/Abeta42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann. Neurol. 2000;48:201–210. [PubMed] [Google Scholar]
- 108.Glodzik-Sobanska L, et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol Aging. 2009;30:672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mosconi L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ihle A, Bunce D, Kliegel M. APOE epsilon4 and cognitive function in early life: a meta-analysis. Neuropsychology. 2012;26:267–277. doi: 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- 111.Baxter LC, Caselli RJ, Johnson SC, Reiman E, Osborne D. Apolipoprotein E e4 affects new learning in cognitively normal individuals at risk for Alzheimer's disease. Neurobiol. Aging. 2003;24:947–952. doi: 10.1016/s0197-4580(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 112.Caselli RJ, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 113.Lind J, et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci. Lett. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 114.Caselli RJ, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007;64:1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 115.Caselli RJ, et al. Longitudinal modeling of age-related memory decline and the APOE e4 effect. N. Engl. J. Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caselli RJ, et al. Longitudinal modeling of frontal cognition in APOE e4 homozygotes, heterozygotes, and noncarriers. Neurology. 2011;76:1383–1388. doi: 10.1212/WNL.0b013e3182167147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alzheimer's Disease and Frontotemporal Dementia Mutation Database. 2013 [Google Scholar]
- 118.Campion D, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cirrito JR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl. Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 122.Godbolt AK, et al. Sporadic and familial dementia with ubiquitin-positive tau-negative inclusions: clinical features of one histopathological abnormality underlying frontotemporal lobar degeneration. Arch Neurol. 2005;62:1097–1101. doi: 10.1001/archneur.62.7.1097. [DOI] [PubMed] [Google Scholar]
- 123.Lleo A, Berezovska O, Growdon JH, Hyman BT. Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS-1 mutations. Am. J. Geriatr. Psychiatry. 2004;12:146–156. doi: 10.1097/00019442-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 124.Quiroz Y, et al. Cortical signature of Alzheimer's disease-related thinning in presymptomatic presenilin-1 mutation carriers. Alzheimers Dement. 2011;7:S220. [Google Scholar]
- 125.Reiman EM, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012 doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fox NC, Warrington EK, Stevens JM, Rossor MN. Atrophy of the hippocampal formation in early familial Alzheimer's disease. A longitudinal MRI study of at-risk members of a family with an amyloid precursor protein 717Val-Gly mutation. Ann. N. Y. Acad. Sci. 1996;777:226–232. doi: 10.1111/j.1749-6632.1996.tb34423.x. [DOI] [PubMed] [Google Scholar]
- 127.Fox NC, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 128.Kennedy AM, et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer's disease. Neurosci. Lett. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- 129.Mosconi L, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- 130.Scholl M, et al. Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol Aging. 2011;32:1388–1399. doi: 10.1016/j.neurobiolaging.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 131.Klunk WE, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Villemagne VL, et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009;66:1537–1544. doi: 10.1001/archneurol.2009.285. [DOI] [PubMed] [Google Scholar]
- 133.Ringman JM, et al. Cerebrospinal fluid biomarkers and proximity to diagnosis in preclinical familial Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33:1–5. doi: 10.1159/000335729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parra MA, et al. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133:2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- 135.Arango-Lasprilla JC, Cuetos F, Valencia C, Uribe C, Lopera F. Cognitive changes in the preclinical phase of familial Alzheimer's disease. J Clin. Exp. Neuropsychol. 2007;29:892–900. doi: 10.1080/13803390601174151. [DOI] [PubMed] [Google Scholar]
- 136.Newman SK, Warrington EK, Kennedy AM, Rossor MN. The earliest cognitive change in a person with familial Alzheimer's disease: presymptomatic neuropsychological features in a pedigree with familial Alzheimer's disease confirmed at necropsy. J. Neurol Neurosurg. Psychiatry. 1994;57:967–972. doi: 10.1136/jnnp.57.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ringman JM, et al. Neuropsychological function in nondemented carriers of presenilin-1 mutations. Neurology. 2005;65:552–558. doi: 10.1212/01.wnl.0000172919.50001.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Acosta-Baena N, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 139.Pike KE, et al. b-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 140.Johnson KA, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mintun MA, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 142.Vlassenko AG, et al. Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011;70:857–861. doi: 10.1002/ana.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sperling RA, et al. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol. Aging. 2013;34:822–831. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aizenstein HJ, et al. Frequent Amyloid Deposition Without Significant Cognitive Impairment Among the Elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rentz DM, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann. Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Resnick SM, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ellis KA, et al. Decline in Cognitive Function over 18 Months in Healthy Older Adults with High Amyloid-beta. J. Alzheimers Dis. 2013 doi: 10.3233/JAD-122170. [DOI] [PubMed] [Google Scholar]
- 149.Lim YY, et al. Rapid Decline in Episodic Memory in Healthy Older Adults with High Amyloid-beta. J. Alzheimers Dis. 2013;33:675–679. doi: 10.3233/JAD-2012-121516. [DOI] [PubMed] [Google Scholar]
- 150.Elias MF, et al. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 151.Saxton J, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 152.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive Decline in Prodromal Alzheimer Disease and Mild Cognitive Impairment. Archives of Neurology. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sperling R, Donohue M, Aisen P. The A4 trial: Anti-amyloid treatment of asymptomatic Alzheimer's disease. Alzheimers Dement. 2012;8:425–426. [Google Scholar]
- 154.Langbaum JB, et al. Composite cognitive endpoints with improved power to detect presymptomatic Alzheimer's disease treatment effects in APOE4 carriers: Findings from the Alzheimer's prevention initiative. Alzheimers Dement. 2011;7:S502. [Google Scholar]
- 155.Ayutyanont N, et al. Composite cognitive endpoints with improved power to detect presymptomatic Alzheimer's disease treatment effects: Findings in the Colombian kindred with the E280A Presenilin 1 mutation and the Alzheimer's Prevention Initiative. Alzheimers Dement. 2011;7:S608. [Google Scholar]
- 156.Mosconi L, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 157.Drzezga A, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 158.de Leon MJ, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 159.Jack CR, Jr, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dickerson BC, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Celone KA, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jagust WJ, Mormino EC. Lifespan brain activity, beta-amyloid, and Alzheimer's disease. Trends Cogn Sci. 2011;15:520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klunk WE, Mathis CA, Price JC, Lopresti BJ, DeKosky ST. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006;129:2805–2807. doi: 10.1093/brain/awl281. [DOI] [PubMed] [Google Scholar]
- 165.Li G, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 166.Forsberg A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 167.Mattsson N, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 168.Visser PJ, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet. Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 169.Wolk DA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vemuri P, et al. MRI and CSF biomarkers in normal, MCI, AD subjects: Diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vemuri P, et al. MRI and CSF biomarkers in normal MCI, and AD subjects: Predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van Rossum IA, et al. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology. 2012;79:1809–1816. doi: 10.1212/WNL.0b013e3182704056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prestia A, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80:1048–1056. doi: 10.1212/WNL.0b013e3182872830. [DOI] [PubMed] [Google Scholar]
- 174.Roe CM, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013 doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Andrews KA, et al. Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials. PLoS. One. 2013;8:e58816. doi: 10.1371/journal.pone.0058816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fleisher AS, et al. Using Positron Emission Tomography and Florbetapir F 18 to Image Cortical Amyloid in Patients With Mild Cognitive Impairment or Dementia Due to Alzheimer Disease. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 177.Fox NC, et al. Effects of Ab immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]