Abstract
Studies of adolescent drug use show (1) a pattern in which the use of tobacco precedes the use of other drugs and (2) a positive relationship between adolescent tobacco use and later drug use. These observations have led to the hypothesis that a causal relationship exists between early exposure to nicotine and the later use of hard drugs such as cocaine. Using male C57BL/6J mice, we tested the hypothesis that nicotine exposure in adolescence leads to increased intravenous self-administration (IVSA) of cocaine in adulthood. Using miniature osmotic pumps, we exposed mice and their littermate controls to nicotine (24 mg/kg/day) or vehicle, respectively, over the entire course of adolescence [postnatal days (P) 28–56]. Nicotine exposure was terminated on P56 and mice were not exposed to nicotine again during the experiment. On P73, mice were allowed to acquire cocaine IVSA (1.0 mg/kg/infusion) and a dose–response curve was generated (0.18, 0.32, 0.56, 1.0, 1.8 mg/kg/infusion). Lever pressing during extinction conditions was also evaluated. All mice rapidly learned to lever press for the combination of cocaine infusions and non-drug stimuli. Analysis of the dose–response curve revealed that adolescent nicotine-exposed mice self-administered significantly more (P < 0.05) cocaine than controls at all but the highest dose. No significant differences were observed between adolescent nicotine-exposed and control mice during the acquisition or extinction stages. These results indicate that adolescent nicotine exposure can increase cocaine IVSA in mice, which suggests the possibility of a causal link between adolescent tobacco use and later cocaine use in humans.
Keywords: Cocaine, gateway effect, mouse, nicotine, osmotic pump, self-administration
INTRODUCTION
Nicotine exposure from tobacco use is widespread in the United States, with an estimated 27.7% of Americans 12 or older using some form of tobacco (SAMHSA 2010). Initiation of nicotine use typically begins during adolescence (SAMHSA 2010), a time when the brain is still developing (Casey et al. 2005). During this window of vulnerability, acute insults may alter the normal course of development and, consequently, have lifelong effects (Andersen 2003). Studies of adolescent drug use show that, typically, nicotine and alcohol use precede the use of marijuana, which precedes the use of ‘hard’ drugs such as cocaine (Kandel 1975). Epidemiological studies suggest that the use of nicotine during adolescence also substantially increases the probability that later illicit drug use will occur (Torabi, Bailey & Majd-Jabbari 1993; Merrill et al. 1999; Lai et al. 2000; Hanna et al. 2001; Wagner & Anthony 2002). Observations such as these have inspired the concept of the gateway hypothesis, which proposes that a causal relationship exists between early exposure to drugs (e.g. alcohol, nicotine, marijuana) and the use of hard drugs later in life (Kandel & Yamaguchi 2002).
Although epidemiological studies show a clear relationship between tobacco use during adolescence and later drug use, the causal relationship between the two is still unclear. However, the idea that exposure to nicotine through tobacco use in adolescence directly affects hard drug use later in life is compelling because nicotinic acetylcholine receptors (nAChRs) to which nicotine binds are a critical component in the modulation of brain development during adolescence (Dani & Bertrand 2007). Studies in experimental animals have shown that nicotine exposure during these critical periods can have long-term effects on developing brain regions known to modulate drug use such as the prefrontal cortex, nucleus accumbens and amygdala (reviewed in Slotkin 2004; Dwyer, McQuown & Leslie 2009). Thus, it is not surprising that several studies in rats and mice have shown that exposure to nicotine during adolescence affects measures of drug reward and sensitivity (e.g. Kelley & Middaugh 1999; Collins & Izenwasser 2004; Kelley & Rowan 2004; McQuown, Belluzzi & Leslie 2007; McQuown et al. 2009; Anker & Carroll 2011). These effects, in some cases, persisted long after the termination of nicotine exposure.
In the present study, we specifically tested the hypothesis that adolescent nicotine exposure increases intravenous self-administration (IVSA) of cocaine in adulthood. We based this hypothesis on previous results in C57BL/6J (B6), DBA/2J (D2) and two lines of BXD recombinant inbred mice, which showed that adolescent nicotine exposure dose and genotype dependently attenuated in adult mice the facilitating effects of a challenge injection of cocaine on nucleus accumbens shell dopamine release evoked by repetitive electrical stimulation of dopaminergic axons in the medial forebrain bundle (Dickson et al. 2011). In order to provide the strongest test of our hypothesis, we specifically selected B6 mice for the present study because this mouse line was the least affected by adolescent nicotine treatment (i.e. B6 mice showed the smallest reduction in evoked dopamine release following cocaine challenge). Additionally, B6 mice were selected because (1) the B6 strain readily self-administers cocaine (e.g., Carney et al. 1991; Grahame & Cunningham 1995), and (2) most nAChR subunit null mutants are maintained on a B6 background (reviewed in Fowler, Arends & Kenny 2008).
Using subcutaneously implanted miniature osmotic pumps, we exposed B6 mice to nicotine (24 mg/kg/day) or vehicle throughout adolescence [postnatal days (P) 28–56]. Two weeks following termination of nicotine exposure, mice were implanted with jugular catheters and then allowed to acquire cocaine IVSA (1.0 mg/kg/infusion) after a 3-day recovery period. Following acquisition of cocaine IVSA, the self-administration response was allowed to stabilize at five doses of cocaine to establish a dose–response curve (0.18, 0.32, 0.56, 1.0, 1.8 mg/kg/infusion). Following stabilization on the final cocaine dose, mice were tested during extinction conditions for 6 days.
MATERIALS AND METHODS
The following experiments were approved by the Institutional Animal Care and Use Committee at the University of Memphis and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Efforts were made to reduce the number of animals used, and to minimize animal pain and discomfort.
Animals
Mice were bred in the Department of Psychology at the University of Memphis. All experimental subjects were second- or third-generation offspring of mice originally purchased from the Jackson Laboratory (Bar Harbor, Maine). Twenty male B6 mice (10 litters, two mice per litter) were used to assess the effects of adolescent nicotine exposure on cocaine IVSA. In a second study, three male B6 mice (three litters, one mouse per litter) were used to assess plasma cotinine levels in nicotine-exposed mice at the midpoint of exposure (P42). Throughout the experiment, mice were maintained in a temperature controlled environment (21 ± 1°C) on a 12:12 light : dark cycle (lights on at 8:00 am) and were given free access to food and water.
Nicotine exposure
To assess the effects of adolescent nicotine exposure on cocaine IVSA, two randomly selected male mice from each litter were weaned and individually housed on P28. One mouse from each litter was randomly assigned to the nicotine group and the other to the control group. Mice assigned to the nicotine group were implanted with an Alzet (Cupertino, CA) miniature osmotic pump (model 1004), which provided 28 days of continuous subcutaneous delivery of 24 mg/kg/day of nicotine dissolved in sterile water. Mice from the control group were implanted with pumps filled with sterile water. All pumps were implanted on P28 and explanted on P56. Pump implantation and explantation was performed under oxygen/isoflurane anesthesia using sterile surgical techniques. Between P28 and P56 all mice were weighed at the same time daily in order to evaluate the effects of chronic nicotine exposure on body weight.
Plasma cotinine levels
To assess plasma cotinine levels in nicotine-exposed mice, three randomly selected male B6 mice from three different litters were implanted with a nicotine-filled pump on P28 using the protocol detailed above. On P42, trunk blood was collected following decapitation and was immediately centrifuged for 10 minutes. Blood plasma was stored for 7–14 days at −80°C. Plasma cotinine levels were assessed using a commercial cotinine enzyme immunoassay kit (#1124ET) from OraSure Technologies, Inc (Bethlehem, PA) in accordance with the manufacturer’s recommendations. We chose to measure plasma cotinine because it is the preferred method of quantifying nicotine exposure in humans. Thus, measuring plasma cotinine allowed for a direct comparison with human studies. Additionally, plasma cotinine and nicotine have been shown to be very strongly correlated in B6 mice (Marks et al. 2004).
Catheterization surgery
On P70, an indwelling catheter was implanted into the right external jugular vein under oxygen/isoflurane anesthesia using procedures described in detail by Thomsen & Caine (2005, 2007). Briefly, the catheter was inserted 12 mm into the jugular vein and anchored with sutures. The catheter was run subcutaneously to an incision over the skull where a 25-gauge stainless steel catheter access port was secured to the skull with a light-cured resin using procedures described in detail by Groseclose et al. (1998).
Apparatus
IVSA data were collected using 10 Med Associates (St. Albans, VT, USA) operant conditioning chambers (307W) enclosed in sound attenuating cubicles (ENV-022MD). Two retractable response levers (ENV-310W) were mounted on the front wall. A stimulus light (ENV-321W) was mounted above each lever. A house light (ENV-315W) with bulb (CM1829; Chicago Miniature Lighting, LLC, Novi, MI, USA) was centrally mounted on the rear wall. A 25-gauge single-channel stainless steel swivel was mounted to a counterbalanced lever arm attached to the exterior of the chamber. Tubing was used to connect a 20 ml syringe mounted on the infusion pump to the swivel, and to connect the swivel to a catheter port affixed to the mouse’s skull.
Catheter ports were constructed in-house using 25-gauge stainless steel hypodermic tubing (Small Parts; HTX-25T-36-10). Operant conditioning chambers were controlled by a Lafayette Instruments (Lafayette, IN, USA) BNC MKII control unit running Campden BNC Control software (version 1.21).
Experimental procedure
Acquisition of cocaine IVSA
On P73 following 3 days of postsurgical recovery, mice began cocaine IVSA acquisition on a fixed ratio (FR) 1 schedule at a dose of 1.0 mg/kg/infusion. Mice were tested in 2-hour sessions at the same time daily 7 days per week throughout the experiment. Each session began with the illumination of the house light and extension of the two response levers. A left (active) lever press resulted in a cocaine infusion and the illumination of both stimulus lights for 2 seconds. This was followed by a 20-second time-out during which the house light was off and lever presses were recorded but had no consequences. Throughout the entire session, right (inactive) lever presses were recorded but had no consequences. The infusion pump delivered 11.76 µl of cocaine solution per second. Cocaine dose was adjusted to body weight by slightly varying the pump time (infusions lasted approximately 2 seconds). Mice were weighed daily before testing.
Acquisition was defined as five consecutive sessions during which ≥10 infusions occurred. Stabilization was defined as two consecutive sessions during which infusions did not vary by more than 20% and at least 70% of lever presses were on the active lever. Thus, acquisition and stabilization of responding occurred, at the earliest, on the fifth day of IVSA training in some mice.
Generation of the dose–response curve
Following acquisition of cocaine IVSA, the self-administration response was allowed to stabilize at five doses of cocaine to establish a dose–response curve. Doses were presented in the following order: 1.0, 0.56, 1.8, 0.18, 0.32 mg/kg/infusion. Once mice had stabilized at each dose, they were moved to the subsequent dose on the following session.
Extinction of cocaine IVSA
Following stabilization at the final dose of cocaine, responding on the previously active and previously inactive levers was examined during extinction conditions for six daily 2-hour sessions. During extinction sessions, the house light was continuously illuminated, stimulus lights were never illuminated and lever presses had no consequences. Mice were connected to infusion tubing, which was connected to a syringe filled with sterile saline, but the infusion pump was never activated during extinction sessions.
Maintenance of catheter patency
To maintain patency, catheters were flushed before and after each daily testing session with 25 µl of a heparin lock solution (100 U/ml heparin/saline; Hospira, Lake Forest, IL, USA).To forestall bacterial infection, mice were infused (2 µl/g) with an enrofloxacin/saline solution (10 mg/kg) immediately before the heparin flush at the end of each session. Catheters were tested for patency with an infusion (2 µl/g) of a methohexital/saline solution (5 mg/kg) once a complete dose–response curve was established. Rapid loss of muscle tone was interpreted as an indication of patency.
Chemicals
Nicotine hydrogen tartrate salt and cocaine hydrochloride were obtained from Sigma-Aldrich Chemical Co. (St Louis, MO). Methohexital (Brevital®) and enrofloxacin (Baytril®) were obtained from Henry Schein (Melville, NY). Nicotine hydrogen tartrate salt was dissolved in distilled water. Methohexital and cocaine hydrochloride were dissolved in 0.9% saline. All solutions were filtered through 0.22 µm syringe filters. Reported nicotine doses are in freebase form (i.e., adjusted to freebase form using calculations in Matta et al. 2007) and are based on the average weight of B6 mice at P42, the midpoint of nicotine exposure.
Dependent variables
The following dependent variables were collected during each 120 minute cocaine IVSA session: (1) number of infusions, (2) inter-infusion interval and (3) number of active and inactive lever presses.
Mortality and attrition of experimental subjects
Over the 28-day course of nicotine exposure, one nicotine-exposed and one vehicle-exposed mouse were dropped from the study because the implanted pumps became externalized. Additionally, two nicotine-exposed mice and one vehicle-exposed mouse died during the catheter implantation surgery. Finally, one nicotine-exposed and one vehicle-exposed mouse were excluded from the extinction analysis because catheter ports became detached from the skull.
RESULTS
Effect of adolescent nicotine exposure on body weight
Body weights of mice were recorded daily beginning immediately prior to pump implantation on P28 and ending just prior to pump explantation on P56 (data not shown). For each mouse, daily body weights were averaged to provide a weekly body weight for each of the 4 weeks of exposure (week 1: P28–P34; week 2: P35–P41; week 3: P42–P48, week 4: P49–P55). In order to investigate possible effects of adolescent nicotine exposure on weight gain across the 28-day exposure period, we performed a repeated measures analysis of variance (ANOVA) using week (1–4) as the within-subjects factor and nicotine exposure (nicotine or vehicle) as the between-subjects factor. Results indicated that mice in both groups gained weight throughout nicotine exposure [week: F (3, 11) = 267.96, P < 0.05]. Post hoc analyses indicated that, as a group, body weights of all mice increased significantly (P < 0.05) each week relative to the previous week (week 1: M = 15.89, SD = 1.23; week 2: M = 19.23, SD = 1.07; week 3: M = 20.91, SD = 1.08; week 4: M = 22.21, SD = 1.10). There was no significant main effect of nicotine exposure or week × nicotine exposure interaction.
Plasma cotinine levels
Plasma cotinine levels were assessed in three mice at P42, the midpoint of nicotine exposure. The mean plasma cotinine level of these mice was 62.02 ng/ml (SD = 0.32).
Effect of adolescent nicotine exposure on acquisition of cocaine IVSA
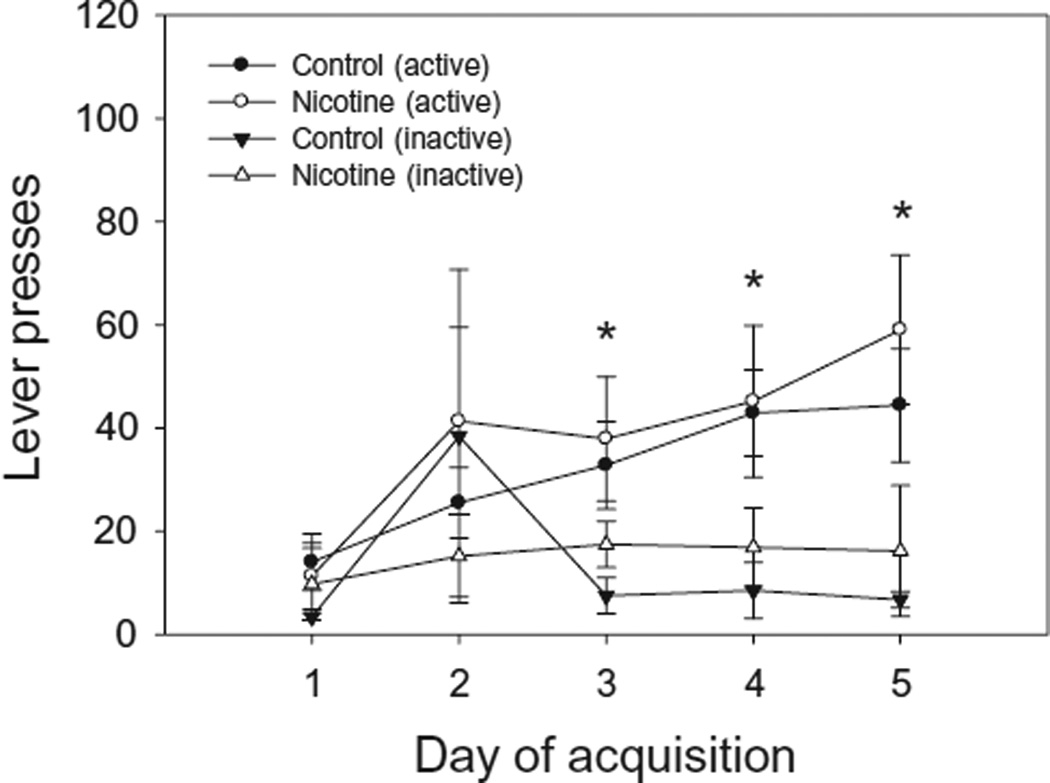
To examine the effect of adolescent nicotine exposure on the acquisition of cocaine IVSA (Fig. 1), we performed a repeated measures ANOVA using number of lever presses as the dependent factor, day (1–5) and side (active or inactive) as within-subjects factors and nicotine exposure (nicotine or vehicle) as the between-subjects factor. Days of acquisition were restricted to five because two of the seven nicotine-exposed mice (28.6%) and two of the eight controls (25.0%) reached the acquisition criterion on day 5. Repeated measures ANOVA revealed a significant day × side interaction [F (4, 10) = 5.75, P < 0.05] as well as significant main effects of day [F (4, 10) = 7.06, P < 0.05] and side [F (1, 13) = 27.69, P < 0.05]. Post hoc tests indicated that the number of presses on the active lever increased across the first 5 days of acquisition, with the number of active presses on days 3–5 being significantly greater (P < 0.05) than the number on day 1. Additionally, the number of inactive lever presses did not differ significantly across days, and the number of active lever presses was significantly greater (P < 0.05) than the number of inactive lever presses on days 3–5. This indicates that all mice, as a group, were pressing the active lever for the combination of cocaine and cocaine-paired stimuli by day 3 of the acquisition stage. Repeated measures ANOVA indicated that mean days to acquire cocaine self-administration (i.e. five consecutive days of ≥10 infusions) did not differ significantly between nicotine-exposed (M = 7.00, SD = 2.24) and control mice (M = 7.00, SD = 2.98). Thus, regardless of nicotine exposure group, all mice that entered the study acquired cocaine IVSA, and the rate at which the two groups acquired did not differ.
Figure 1.
Acquisition of intravenous cocaine self-administration in adult mice exposed to nicotine (n = 7) or vehicle (n = 8) during adolescence. Both nicotine- and vehicle-exposed mice learned to discriminate between the active and inactive lever by the third day of the acquisition stage. This is indicated by a significantly greater number of active relative to inactive lever presses on days 3 through 5 compared to day 1 (*P < 0.05). Inactive lever presses did not differ significantly across days. Performance of nicotine- and vehicle-exposed mice did not differ significantly during the acquisition stage
Effects of adolescent nicotine exposure on the cocaine dose–response curve
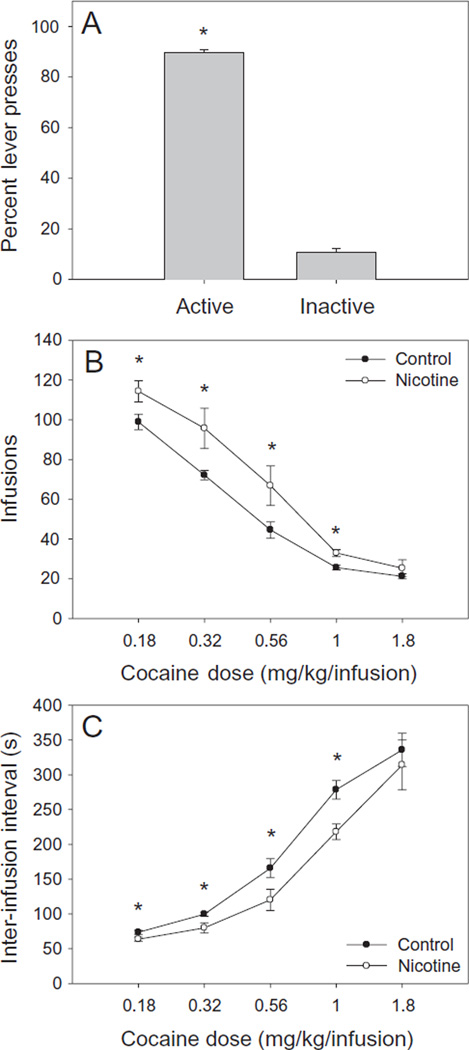
To determine if mice continued to discriminate between the active and inactive levers during the generation of the dose–response curve, we performed a repeated measures ANOVA using percent of active lever presses as the dependent factor, cocaine dose (0.18, 0.32, 0.56, 1.0, 1.8 mg/kg/infusion) as the within-subjects factor, and nicotine exposure (nicotine or vehicle) as the between-subjects factor. Repeated measures ANOVA indicated that there was no significant main effect of nicotine exposure or cocaine dose, and there was no significant interaction between the two factors. Because there was no effect of nicotine exposure or cocaine dose on percent of active lever presses, we collapsed across these factors and compared the percent of active lever presses to the percent of inactive lever presses. As shown in Fig. 2a, percent of active lever presses was significantly and substantially greater than the percent of inactive lever presses, t(28) = 36.97, P < 0.001.
Figure 2.
Intravenous cocaine self-administration performance of adult mice exposed to nicotine (n = 7) or vehicle (n = 8) during adolescence. (a)The percentage of active lever presses was substantially and significantly greater (P < 0.05) than inactive lever presses in both nicotine- and vehicle-exposed mice. There was no significant effect of nicotine treatment or cocaine dose. (b) Adolescent nicotine-exposed mice self-administered significantly more cocaine (*P < 0.05) than vehicle-exposed controls at all but the highest cocaine dose. (c) Inter-infusion intervals of nicotine-exposed mice were significantly shorter (*P < 0.05) than those of vehicle-exposed controls at all but the highest cocaine dose
To examine the effect of adolescent nicotine exposure on the number of infusions at each cocaine dose (Fig. 2b), we performed a repeated measures ANOVA using number of cocaine infusions as the dependent factor, cocaine dose as the within-subjects factor and nicotine exposure as the between-subjects factor. Repeated measures ANOVA indicated a significant interaction of cocaine dose and nicotine exposure [F (4, 10) = 3.71, P < 0.05], and significant main effects of cocaine dose [F (4, 10) = 148.81, P < 0.05] and nicotine exposure [F (1, 13) = 6.53, P < 0.05]. As shown in Fig. 2b, post hoc tests indicated that adult mice exposed to nicotine during adolescence self-administered significantly more cocaine (P < 0.05) relative to control mice at all but the highest dose of cocaine (1.8 mg/kg/infusion). Additionally, post hoc tests indicated that the number of self-infusions differed significantly at each dose in nicotine-exposed mice. In control mice, the number of self-infusions differed significantly at all doses with the exception that the 1.0 mg/kg/infusion dose did not differ significantly from the 0.56 and 1.8 mg/kg/infusion doses.
In order to determine the effect of adolescent nicotine exposure on inter-infusion interval (Fig. 2c), we performed a repeated measures ANOVA using inter-infusion interval as the dependent factor, cocaine dose as the within-subjects factor and nicotine exposure as the between-subjects factor. Repeated measures ANOVA revealed a significant interaction of cocaine dose and nicotine exposure [F (4, 10) = 4.18, P < 0.05], and a significant main effect of cocaine dose [F (4, 10) = 150.48, P < 0.05]. The main effect of nicotine exposure approached but did not reach significance [F (1, 13) = 4.11, P = 0.064]. A repeated measures ANOVA using time-out responses on the active lever as the dependent factor, cocaine dose as the within-subjects factor, and nicotine exposure as the between-subjects factor revealed a significant effect of cocaine dose [F (1, 13) = 7.37, P < 0.05], but not of nicotine exposure or the interaction between cocaine dose and nicotine exposure.
Effects of adolescent nicotine exposure on extinction of cocaine IVSA
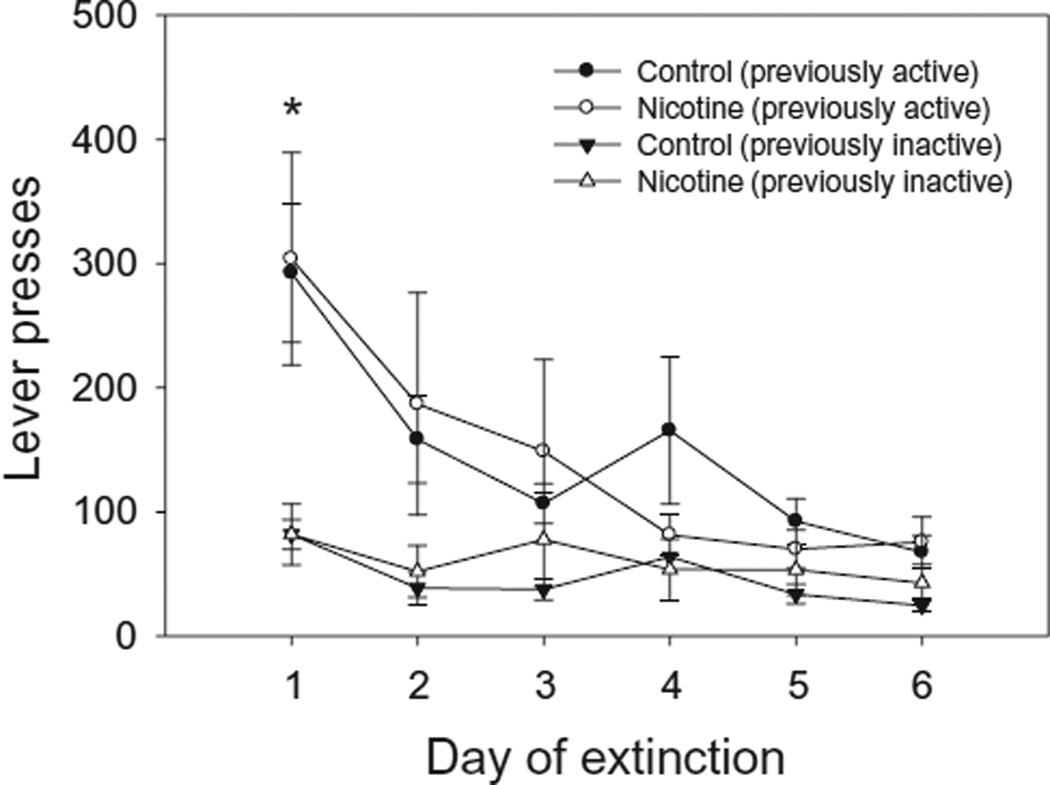
In order to examine the effect of adolescent nicotine exposure on the extinction of cocaine IVSA (Fig. 3), we performed a repeated measures ANOVA using lever presses as the dependent factor, day (1–6) and side (active or inactive) as within-subjects factors and nicotine exposure (nicotine or vehicle) as the between-subjects factor. Repeated measures ANOVA did not indicate a main effect of nicotine exposure, and no interaction which included nicotine exposure as a factor was significant. However, repeated measures ANOVA revealed a significant day × side interaction [F(5, 7) = 4.75,P < 0.05] as well as a significant main effect of day [F (5, 7) = 5.49, P < 0.05] and side [F (1, 11) = 20.54, P < 0.05]. Post hoc tests indicated that regardless of exposure group, the number of presses on the previously active lever was significantly greater (P < 0.05) than those on the previously inactive lever on all 6 days of extinction, and all mice pressed significantly more (P < 0.05) on the first day of extinction than on days 2–6, which did not differ significantly.
Figure 3.
Extinction of cocaine self-administration in adult mice exposed to nicotine (n = 6) or vehicle (n = 7) during adolescence. The number of previously active lever presses was significantly greater (*P < 0.05) on the first day of extinction relative to all other days, which did not differ significantly. Additionally, on all 6 days of extinction, the number of lever presses on the previously active lever was significantly greater (P < 0.05) than the number on the previously inactive lever. Performance of adolescent nicotine- and vehicle-exposed mice did not differ significantly during the extinction stage
DISCUSSION
In the present study, B6 mice were exposed to either nicotine (24 mg/kg/day) or vehicle during adolescence from P28 to P56 using subcutaneously implanted miniature osmotic pumps. As in a previous study (Dickson et al. 2011), this dose and method of nicotine exposure did not affect weight gain during the exposure period relative to vehicle-exposed controls. Plasma cotinine levels in nicotine-exposed mice, assessed at the midpoint of nicotine exposure, were comparable to plasma cotinine levels in human adolescent smokers (Rubinstein et al. 2007). Following 17 nicotine-free days, mice were tested on a cocaine IVSA paradigm during adulthood. Adolescent nicotine-exposed and control mice learned to lever press for the combination of cocaine infusions and drug-paired stimuli by the third day of the acquisition stage, as evidenced by the significantly greater number of active relative to inactive lever presses on days 3–5 of the acquisition stage (Fig. 1). Once cocaine IVSA had been acquired (i.e. five consecutive sessions during which ≥10 infusions occurred), the self-administration response was allowed to stabilize on five different doses of cocaine (0.18, 0.32, 0.56, 1.0, 1.8 mg/kg/infusion). Relative to control mice, nicotine-exposed mice self-administered significantly more cocaine at all but the highest cocaine dose. Following stabilization on the final cocaine dose, saline was substituted for cocaine and all mice were tested during extinction conditions for 6 days. No differences were detected between adolescent nicotine-exposed and control mice during extinction.
Nicotine dose and exposure method: relevance to human smoking
Several methods of nicotine exposure are routinely used in animal research including miniature osmotic pumps (present study), repeated daily injections and exposure via the drinking water. There are advantages and disadvantages to the use of each method (reviewed in Matta et al. 2007). In the present study, we chose to use osmotic pumps instead of other methods because the use of osmotic pumps (1) allows precise control over nicotine dose compared to exposure via the drinking water and (2) exposes the animal to less stress across adolescence compared to repeated daily injections. The reduction of stress during adolescent nicotine exposure was necessary in the present study given (1) the well-established relationship between exposure to chronic stress during adolescence and subsequent drug abuse (reviewed in Sinha 2001; Enoch 2011) and (2) the recent report showing that chronic stress and chronic nicotine counteract each other’s effects on cocaine-dependent dopamine release in the striatum (Salas & De Biasi 2008). The effect of stress on striatal dopamine release is an important consideration because of the well-established relationship between striatal dopamine and cocaine self-administration (e.g. Dalley et al. 2007; Belin & Everitt 2008).
Regarding the dose of nicotine used in the present study, the assay of plasma cotinine levels revealed that the 24 mg/kg/day nicotine dose, although relatively high compared to other species, resulted in plasma cotinine levels in B6 mice which are quite similar to those observed in adolescent human smokers (Rubinstein et al. 2007). This finding is consistent with the relatively rapid nicotine metabolism in the mouse species compared to other animals (Petersen, Norris & Thompson 1984), a phenomenon that necessitates higher nicotine dosages in the mouse to achieve blood plasma nicotine levels which are comparable to those observed in human smokers (Matta et al. 2007).
It is important to note that although we have reduced the potential impact of stress by using osmotic pumps instead of daily subcutaneous injections, the use of osmotic pumps may still represent a stressor in B6 mice. Thus, in the present study, it is possible that the effects of stress resulting from the use of osmotic pumps increased self-administration of cocaine in all mice or modulated the effect of adolescent nicotine exposure on adult cocaine IVSA in nicotine-exposed mice specifically. Additionally, it should be remembered that osmotic pumps deliver a constant dose of nicotine (similar to a nicotine patch), while the pattern of nicotine use in human adolescents is intermittent. Thus, it is possible that these two modes of nicotine administration result in unique neuroplastic changes in the brain, each having a distinct effect on adult cocaine use. Finally, it should be noted that the experimental design used in the present study introduced a period of nicotine abstinence immediately following adolescent nicotine exposure which continued through cocaine IVSA testing in adulthood. The purpose of this design was to assess the effects of nicotine exposure restricted to adolescence. However, in human populations, nicotine use frequently occurs simultaneously with cocaine use. Thus, it is possible that the effect of adolescent nicotine exposure on subsequent cocaine use in the absence of nicotine, as seen in the currently study, differs from what is frequently seen in human populations in which cocaine use occurs concurrently with nicotine use.
Effects of adolescent nicotine exposure on the acquisition of cocaine IVSA
There were no significant differences in the acquisition of responding for cocaine between the two exposure groups. This result supports the hypothesis that exposure to nicotine during adolescence does not influence the propensity to acquire cocaine IVSA in B6 mice, and is in agreement with a previous study in rats (Anker & Carroll 2011). It should be noted that the present results contrast with an earlier study in which nicotine-exposed adolescent rats acquired cocaine IVSA more rapidly than saline-exposed adolescent controls and saline- and nicotine-exposed adults (McQuown et al. 2007). One caveat to this study, however, is that the acquisition of cocaine IVSA began the day following termination of nicotine exposure. Thus, rats acquired cocaine IVSA during early withdrawal from nicotine. This is important because it has been shown that nicotine withdrawal temporarily reduces nucleus accumbens dopamine levels in rats and decreases brain reward function in rats and mice (reviewed in O’Dell & Khroyan 2009). Additionally, it has recently been shown in B6 mice that dopamine dynamics in the nucleus accumbens are temporarily disrupted during nicotine withdrawal (Zhang et al. 2012). These changes, although temporary, persisted for between 5 and 10 days depending on the length of nicotine exposure. Thus, it is possible that the increased cocaine intake in nicotine-exposed relative to saline-exposed adolescent rats in McQuown et al. (2007) resulted from transient changes in nucleus accumbens dopamine dynamics and reward threshold resulting from nicotine withdrawal. The fact that acquisition of cocaine IVSA in nicotine- and saline-exposed adult rats did not differ may be due to differences in the rewarding and aversive effects of nicotine between adolescents and adults (reviewed in O’Dell & Khroyan 2009).
A second consideration that should be taken into account when comparing the results of the present study with those reported by McQuown et al. (2007) is that genetic background strongly modulates the degree to which adolescent nicotine exposure affects nucleus accumbens dopamine dynamics in response to cocaine challenge (Dickson et al. 2011). Thus, it is possible that differences in genetic background (possibly combined with species differences) between the B6 mice in the present study and the rats used in McQuown et al. (2007) contributed to different findings during the acquisition stage.
Effects of adolescent nicotine exposure on cocaine efficacy
Following acquisition of cocaine IVSA, adult mice that had been exposed to nicotine throughout adolescence self-administered more cocaine at significantly shorter inter-infusion intervals at all but the highest dose compared to vehicle-exposed controls. In conjunction with our previous study showing a dose- and genotype-dependent reduction in nucleus accumbens dopamine response following a cocaine challenge in adolescent nicotine-exposed adult mice (Dickson et al. 2011), these results support the hypothesis that nicotine exposure during adolescence results in a long-lasting reduction in efficacy of cocaine which leads to a compensatory increase in cocaine IVSA. The implication of this finding is that exposure to nicotine during adolescence results in a long-term risk of significantly increased cocaine use over the lifetime of an individual, resulting in two primary liabilities. First, the use of cocaine and other drugs results in neural adaptations that are hypothesized to contribute to addiction (reviewed in Robinson & Kolb 2004). Thus, reduced efficacy of cocaine as a result of adolescent smoking may predispose individuals to consume more cocaine, directly resulting in neural adaptations which increase the probability of engaging in addictive behaviors, resulting in even greater cocaine use. Second, the risk of cocaine-related cardiovascular complications such as myocardial infarction and aortic ruptures rises substantially with the use of cocaine (reviewed in Maraj, Figueredo & Lynn Morris 2010). For example, the risk of acute myocardial infarction has been shown to increase 24-fold in the hour following cocaine use (Mittleman et al. 1999). Thus, in addition to increases in addictive behaviors, increased cocaine use also raises the risk of cardiovascular complications.
It is also worthwhile to note that increases in impulsivity resulting from adolescent nicotine exposure may play a role in the increased cocaine IVSA observed in the present study. This idea is consistent with the findings that (1) rats exposed to nicotine during adolescence show long-term increases in impulsivity (Counotte et al. 2009), and (2) trait-impulsive rats show a vertical shift in the cocaine IVSA dose–response curve relative to non-impulsive rats (Dalley et al. 2007). Interestingly, like the adolescent nicotine-exposed mice in the present study, trait-impulsive rats do not show facilitated acquisition relative to controls.
Finally, regarding the present study, it is important to consider that in addition to an increase in active lever pressing in nicotine-exposed mice relative to controls, non-active lever pressing also increased. This is consistent with a general increase in activity which is a consequence of the relatively greater cocaine consumption in nicotine-exposed mice. However, it remains possible that this also reflects, at least to some degree, a general increase in activity resulting from adolescent nicotine exposure.
Effects of adolescent nicotine exposure on extinction of cocaine responding
Following the generation of a dose–response curve, mice were tested for 6 days during extinction conditions. Consistent with previous studies in the B6 strain (e.g. Thomsen & Caine 2011), mice showed an initial burst of responding during the first day followed by decreased responding during subsequent days. There was, however, no observed effect of adolescent nicotine exposure on rate of extinction, suggesting that the mechanisms responsible for extinction of an operant response motivated by cocaine reward were not affected in adult mice exposed to nicotine during adolescence. A qualification to this conclusion is that during the extinction stage, multiple stimuli which had previously been paired with delivery of cocaine (i.e. illumination of stimulus lights, activation of infusion pump and infusion of solution) were no longer presented. This is an important consideration because stimuli which have been paired with delivery of cocaine have control over responding in ways which are dissociable from the control over behavior motivated by the drug itself (Panlilio, Weiss & Schindler 1996; Schenk & Partridge 2001; Saunders & Robinson 2010; Millan, Marchant & McNally 2011). With regard to this, Anker & Carroll (2011) recently reported that adolescent nicotine exposure sensitized cue-induced reinstatement of cocaine seeking in rats bred for high and low saccharin intake. Thus, it remains possible that adolescent nicotine exposure could affect extinction or cue-induced reinstatement of cocaine IVSA in B6 mice by altering neural substrates which mediate the association between drugs and drug cues.
Possible effects of nicotine withdrawal on cocaine IVSA
It is important to note that the short-term effects of nicotine withdrawal on nucleus accumbens dopamine levels and reward may be an important contributor to nicotine’s gateway effect (Zhang et al. 2012). However, in order to fully characterize the gateway effect of nicotine, it is necessary to dissociate, with respect to their effects on psychostimulant use, the transient effects of nicotine withdrawal from the persistent effects of adolescent nicotine exposure. Therefore, in the present study, we specifically allowed for an extended period of time between the last nicotine exposure day and the beginning of cocaine IVSA (17 days) to exclude the possibility that transient effects of nicotine withdrawal could contribute to any observed effects on the acquisition, maintenance or extinction of cocaine IVSA. We reasoned that 17 nicotine-free days was sufficient to control for short-term effects of nicotine withdrawal due to a previous report (Damaj, Kao & Martin 2003) in which affective and somatic effects of spontaneous nicotine withdrawal in mice subsided 4 days following the termination of nicotine treatment (24 mg/kg/day via osmotic pump for 2 weeks). However, it remains possible that relatively long-term effects of nicotine withdrawal (e.g. neurobiological changes due to stress experienced during nicotine withdrawal) could have affected cocaine IVSA in the present study.
Possible effects of non-drug stimuli on lever pressing behavior
It should be noted that animals have been shown to respond purely for visual and auditory stimuli in operant conditioning tasks (Kish 1955; Marx, Henderson & Roberts 1955; Blatter & Schultz 2006; Cain, Green & Bardo 2006; Olsen & Winder 2009). However, whether animals will do this and, if so, the degree to which they will respond is largely dependent on the characteristics of the stimuli. In a recent study, Olsen & Winder (2012) investigated the effects of stimulus dynamics on the self-administration of non-drug stimuli in B6 mice. These authors reported that although B6 mice will lever press for flashing lights of varied frequencies and durations using an FR 1 schedule, they will not lever press for the illumination of briefly presented, non-flashing stimulus lights (i.e. the same type used in the present study). Thus, it seems unlikely that active lever pressing in the present study was performed to self-administer non-drug stimuli. Most importantly, the fact that active lever pressing changed significantly with cocaine dose despite the fact that non-drug stimuli did not vary across doses strongly supports the hypothesis that active lever presses represent responding for cocaine, not responding for non-drug stimuli. However, we cannot completely exclude the possibility that the increased active lever pressing observed in nicotine-exposed mice relative to controls was to some degree due to an increased motivation to self-administer non-drug stimuli rather than an increased motivation to self-administer cocaine.
Neurobiological mechanisms
Nicotine-induced upregulation in nAChRs is a well-known consequence of nicotine exposure in rats (Schwartz & Kellar 1983, 1985; Webster et al. 1999), mice (Marks, Burch & Collins 1983; Marks et al. 1992) and humans (Perry et al. 1999). Because nAChRs are expressed in regions of the brain known to modulate drug reward, differences in the degree of nicotine-induced upregulation of nAChRs between adolescents and adults in these areas could partly explain the gateway effect of nicotine observed in the current study (i.e. cocaine sensitization following adolescent nicotine exposure). Consistent with this idea, Trauth et al. (1999) assessed nAChR binding in the midbrain, cerebral cortex and hippocampus and found more widespread and persistent nAChR upregulation in adolescent compared to adult nicotine-exposed rats.
It is also possible that mechanisms other than upregulation in nAChRs are involved in the gateway effect of nicotine. For example, mid-adolescent nicotine exposure has been shown to reduce (and adult exposure to enhance or not affect, respectively) striatal and hippocampal levels of the GluR2/3 subunit of the AMPA glutamate receptor in outbred CD-1 mice when measured 2 months following termination of nicotine exposure (Adriani et al. 2004). These authors suggested that AMPA receptors may affect drug reward by modulating glutamatergic control of synaptic plasticity in the striatum. Collins et al. (2004) have shown that nicotine exposure in periadolescent (but not adult) rats resulted in an increase in dopamine transporter densities in the rostral caudate putamen and nucleus accumbens. These differential effects of adolescent and adult nicotine exposure on nAChRs, AMPA receptors and dopamine transporter densities may provide neurobiological mechanisms for the gateway effect of nicotine observed in the present study.
In addition to adolescent nicotine exposure, it should be noted that nicotine exposure both before and after adolescence may contribute to the gateway effect of nicotine. For example, Novak, Seeman & Le Foll (2010) have reported an increase in dopamine D2High receptor levels following nicotine exposure in adult rats, and Horger, Giles & Schenk (1992) have reported facilitated acquisition of a low dose of cocaine immediately following 9 days of nicotine pre-exposure in adult rats. Additionally, prenatal nicotine exposure has been shown to increase self-administration of a high dose of cocaine (500 µg/kg per injection) in adolescent rats (Franke et al. 2008).
Finally, it should be noted that the use of the term ‘gateway effect’ is typically reserved for human populations. Specifically, this term is used to refer to the phenomenon in which the use of one drug increases the probability of the subsequent use of another drug. With respect to causality, one possibility is that use of the first drug causes sensitization to the second drug. In animal studies, this phenomenon is typically called cross-sensitization. However, in addition to cross-sensitization, other factors likely contribute to the gateway effect in human populations. Specifically, in addition to prior drug use, multiple factors including sex (Bobzean et al. 2010; Vansickel, Stoops & Rush 2010), social status (Morgan et al. 2002), socioeconomic status (Lemstra et al. 2008; Humensky 2010) and stress (Enoch 2011) have been shown to influence the use of addictive drugs. Importantly, some of these factors (e.g. stress; Salas & De Biasi 2008) likely modulate the causal relationship between nicotine exposure and later drug use reported here.
CONCLUSION
In the present study, we have shown in B6 mice that chronic nicotine exposure across adolescence results in elevated intravenous self-administration of cocaine without affecting the rate of acquisition or extinction. Cotinine levels in nicotine-exposed mice were comparable to those in adolescent humans. The observed effects of adolescent nicotine exposure on adult cocaine IVSA were not due to transient changes in reward function or dopamine dynamics which occur during nicotine withdrawal because a 17-day washout period occurred between the last day of nicotine exposure and the first day of cocaine IVSA. Using a similar nicotine exposure protocol in B6, D2 and two BXD recombinant inbred lines, we have previously shown that adolescent nicotine exposure causes persistent dose- and genotype-dependent changes in dopamine functional dynamics in the nucleus accumbens shell (Dickson et al. 2011). Together, these two studies support (1) a causal relationship between adolescent nicotine exposure and later cocaine use and (2) a biological mechanism underlying this effect.
Funding and Acknowledgements
This project was made possible by a Dunavant Professorship awarded to GM. The authors gratefully acknowledge Dr. William (Tripp) C. Griffin, III, for expert advice on mouse jugular catheterization surgery and Dr. Helen Sable for assistance with the plasma cotinine assay. For assistance with data collection and mouse breeding, the authors gratefully acknowledge Erin Clardy.
Footnotes
Conflicts of Interest: None.
Authors Contribution
PD, GM and CB were responsible for the study concept and design. PD and MM were responsible for the acquisition of animal data. PD and GM performed the data analysis and interpretation. PD drafted the manuscript. GM, CB, TR and MM provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29:869–878. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Adolescent nicotine exposure sensitizes cue-induced reinstatement of cocaine seeking in rats bred for high and low saccharin intake. Drug Alcohol Depend. 2011;118:68–72. doi: 10.1016/j.drugalcdep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Blatter K, Schultz W. Rewarding properties of visual stimuli. Exp Brain Res. 2006;168:541–546. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, Dennis TS, Addison BD, Perrotti LI. Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res Bull. 2010;83:331–336. doi: 10.1016/j.brainresbull.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Processes. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991;2:477–480. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development. Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dickson PE, Rogers TD, Lester DB, Miller MM, Matta SG, Chesler EJ, Goldowitz D, Blaha CD, Mittleman G. Genotype-dependent effects of adolescent nicotine exposure on dopamine functional dynamics in the nucleus accumbens shell in male and female mice: a potential mechanism underlying the gateway effect of nicotine. Psychopharmacology. 2011;215:631–642. doi: 10.1007/s00213-010-2159-2. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur J Neurosci. 2008;27:2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Genetic differences in intravenous cocaine self-administration between C57BL/6J and DBA/2J mice. Psychopharmacology. 1995;122:281–291. doi: 10.1007/BF02246549. [DOI] [PubMed] [Google Scholar]
- Groseclose CH, Draughn RA, Tyor WR, Sallee FR, Middaugh LD. Long-term intracranial cannula stabilization in mice with light cured resin composites. J Neurosci Methods. 1998;79:31–36. doi: 10.1016/s0165-0270(97)00158-1. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Yi HY, Dufour MC, Whitmore CC. The relationship of early-onset regular smoking to alcohol use, depression, illicit drug use, and other risky behaviors during early adolescence: results from the youth supplement to the third national health and nutrition examination survey. J Subst Abuse. 2001;13:265–282. doi: 10.1016/s0899-3289(01)00077-3. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Humensky JL. Are adolescents with high socioeconomic status more likely to engage in alcohol and illicit drug use in early adulthood? Subst Abuse Treat Prev Policy. 2010;5:19. doi: 10.1186/1747-597X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K. Stages of drug involvement in the US population. In: Kandel DB, editor. Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. New York: Cambridge University Press; 2002. pp. 65–89. [Google Scholar]
- Kelley BM, Middaugh LD. Periadolescent nicotine exposure reduces cocaine reward in adult mice. JAddict Dis. 1999;18:27–39. doi: 10.1300/J069v18n03_04. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. Int J Dev Neurosci. 2004;22:339–348. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kish GB. Learning when the onset of illumination is used as reinforcing stimulus. J Comp Physiol Psychol. 1955;48:261–264. doi: 10.1037/h0040782. [DOI] [PubMed] [Google Scholar]
- Lai S, Lai H, Page JB, McCoy CB. The association between cigarette smoking and drug abuse in the United States. J Addict Dis. 2000;19:11–24. doi: 10.1300/J069v19n04_02. [DOI] [PubMed] [Google Scholar]
- Lemstra M, Bennett NR, Neudorf C, Kunst A, Nannapaneni U, Warren LM, Kershaw T, Scott CR. A meta-analysis of marijuana and alcohol use by socio-economic status in adolescents aged 10–15 years. Can J Public Health. 2008;99:172–177. doi: 10.1007/BF03405467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marx MH, Henderson RL, Roberts CL. Positive reinforcement of the bar-pressing response by a light stimulus following dark operant pretests with no after effect. J Comp Physiol Psychol. 1955;48:73–76. doi: 10.1037/h0045062. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Dao JM, Belluzzi JD, Leslie FM. Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology. 2009;207:143–152. doi: 10.1007/s00213-009-1642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JC, Kleber HD, Shwartz M, Liu H, Lewis SR. Cigarettes, alcohol, marijuana, other risk behaviors, and American youth. Drug Alcohol Depend. 1999;56:205–212. doi: 10.1016/s0376-8716(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Mintzer D, Maclure M, Tofler GH, Sherwood JB, Muller JE. Triggering of myocardial infarction by cocaine. Circulation. 1999;99:2737–2741. doi: 10.1161/01.cir.99.21.2737. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Novak G, Seeman P, Le Foll B. Exposure to nicotine produces an increase in dopamine D2(High) receptors: a possible mechanism for dopamine hypersensitivity. Int J Neurosci. 2010;120:691–697. doi: 10.3109/00207454.2010.513462. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci Lett. 2012;511:8–11. doi: 10.1016/j.neulet.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Thompson PJ, Benowitz NL, Shiffman S, Moscicki AB. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine Tob Res. 2007;9:129–135. doi: 10.1080/14622200601078517. [DOI] [PubMed] [Google Scholar]
- Salas R, De Biasi M. Opposing actions of chronic stress and chronic nicotine on striatal function in mice. Neurosci Lett. 2008;440:32–34. doi: 10.1016/j.neulet.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Office of Applied Studies NSH-A. Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. HHS Publication No. SMA 10-4586 Findings (ed) [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology. 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005;Chapter 9(Unit 9):20. doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behav Genet. 2007;37:101–118. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav Pharmacol. 2011;22:239–247. doi: 10.1097/FBP.0b013e328345f8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabi MR, Bailey WJ, Majd-Jabbari M. Cigarette smoking as a predictor of alcohol and other drug use by children and adolescents: evidence of the ‘gateway drug effect. J Sch Health. 1993;63:302–306. doi: 10.1111/j.1746-1561.1993.tb06150.x. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Rush CR. Human sex differences in d-amphetamine self-administration. Addiction. 2010;105:727–731. doi: 10.1111/j.1360-0443.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Webster JC, Francis MM, Porter JK, Robinson G, Stokes C, Horenstein B, Papke RL. Antagonist activities of mecamylamine and nicotine show reciprocal dependence on beta subunit sequence in the second transmembrane domain. Br J Pharmacol. 1999;127:1337–1348. doi: 10.1038/sj.bjp.0702686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry. 2012;71:184–191. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]