Summary
The Rac GTPase regulates Rho signaling in a broad range of physiological settings and in oncogenic transformation [1–3]. Here, we report a novel mechanism by which cross-talk between Rac and Rho GTPases is achieved. Activated Rac1 binds directly to p190B RhoGAP (GTPase activating protein), a major modulator of Rho signaling. p190B co-localizes with constitutively active Rac1 in membrane ruffles. Moreover, activated Rac1 is sufficient to recruit p190B into a detergent-insoluble membrane fraction, which is accompanied by a decrease in GTP-bound RhoA from membranes. p190B is recruited to the plasma membrane in response to integrin engagement [4]. We demonstrate that collagen type-I, a potent inducer of Rac1-dependent cell motility in HeLa cells, counteracts cytoskeletal collapse resulting from overexpression of wild-type p190B but not of a p190B mutant specifically lacking the Rac1-binding sequence. Furthermore, this p190B mutant exhibits dramatically enhanced RhoGAP activity consistent with a model whereby binding of Rac1 relieves autoinhibition of p190B RhoGAP function. Collectively, these observations establish that activated Rac1, through direct interaction with p190B, modulates subcellular RhoGAP localization and activity, thereby providing a novel mechanism for Rac to control Rho signaling in a broad range of physiological processes.
Results
Activated Rac1 binds directly to the "middle domain" in p190B RhoGAP
The Ras superfamily of G proteins mediates virtually all aspects of cell biology [1–3]. Their activity is regulated through the action of guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), and there is accumulating evidence that GEFs and GAPs mediate cross-talk between small GTPases [1]. The p190 RhoGAPs p190A and p190B are widely expressed, potent Rho regulators comprised of an N-terminal GTPase domain, a C-terminal GAP domain separated by a sparingly characterized "middle domain" (p190B-MD; Figure 1A). Rnd proteins, a unique branch of Rho-like G proteins, bind the p190-MD directly to promote RhoGAP activity of p190 molecules [5]. We therefore explored the possibility that other Ras-like G proteins might similarly bind the p190 RhoGAPs to affect Rho function.
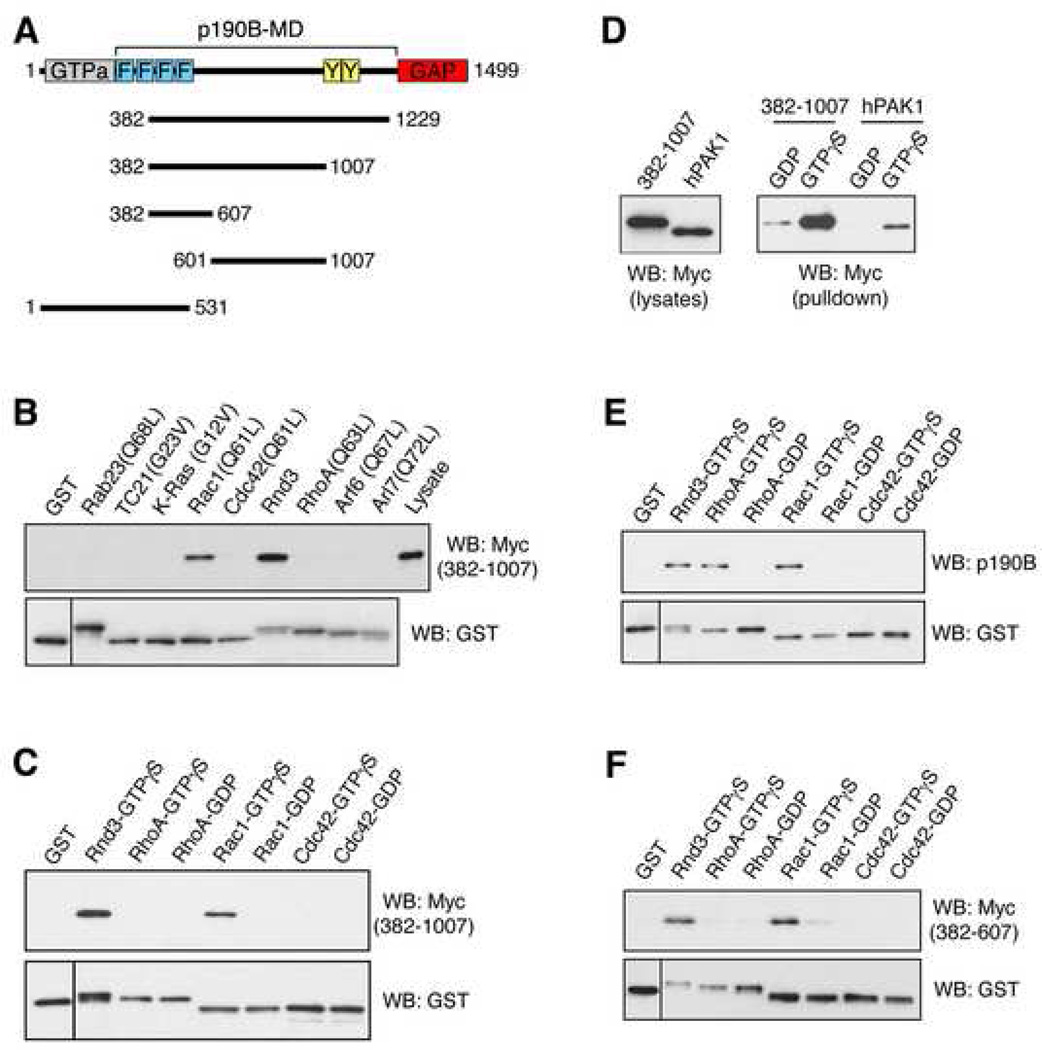
Figure 1.
GTP-bound Rac1 interacts directly with a p190B region that minimally consists of aa382–607. (A) Cartoon of p190B and derivatives used in Figure 1. Numbers refer to amino acids. Known domains in p190B are highlighted in color: N-terminal GTPase domain (grey); C-terminal GAP domain (red); FF domains (blue); RasGAP-binding domain in p190A labeled "Y" (yellow); the function this region in p190B is unknown. (B) Activated Rac1(Q61L) interacts with aa387–1007 of p190B. Aliquots of lysate from Cos7 cells expressing Myc-tagged aa382–1007 of p190B were subjected to GST pulldown assays with activated forms of Ras-like G proteins fused to GST and immobilized on glutathionesepharose beads as previously described [5]. The aa382–1007 fragment is precipitated not only by GST-bound Rnd3 as previously reported [5], but also by GST-bound Rac1(Q61L), in contrast to the other constitutively active Ras-like G proteins tested. (C) The interaction between Rac1 and aa382–1007 of p190B is GTP-dependent and direct. A pulldown assay was conducted with GTPγS- or GDP-loaded wild-type forms of GST-bound RhoA, Rac1, and Cdc42 and recombinant C-terminal FLAG-epitope tagged aa382–1007 of p190B (Figure S2). (D) Comparison by GST-pulldown assay demonstrates that the interaction of activated Rac1 with the aa382–1007 of p190B is as robust as the interaction of activated Rac with wild-type PAK1. (E) GTP-bound Rac1, Rnd3 and RhoA, but not GTP-bound Cdc42, interacts with endogenous p190B from lysates of NIH3T3 cells. Note that, in contrast to GTP-bound Rac1 and Rnd3, the interaction between RhoA-GTP and p190B is mediated solely by the GAP domain [5]. (F) Both activated Rac1 and Rnd3 interact with aa382–607 of p190B in a GST-pulldown assay. All data presented in this work are representative of at least three independent experiments.
Ras-like G proteins have been grouped into nine distinct classes according to their effect on cell morphology and the actin cytoskeleton [6]. We selected one member of each class (Rab23, TC21, K-Ras, Rac1, Cdc42, Rnd3, RhoA, Arf6, and Arl7) and tested their interaction with the p190B-MD. We generated GST-fusion proteins immobilized on glutathione-sepharose beads of constitutively activated derivatives of each protein except for Rnd3, which is constitutively GTP-bound. We conducted pull-down assays from lysates of Cos7 cells expressing a p190B-MD construct (aa382–1229; Figure 1A). Strikingly, in addition to Rnd3, constitutively activated Rac1, but none of the other small G proteins tested, also binds efficiently to p190B-MD (data not shown). Similar results were obtained using a smaller p190B fragment comprised of amino acids 382–1007 (aa382–1007; Figures 1A and 1B). The fact that the interaction between Rac1 and p190B is easily detected in these assays indicates an affinity constant in the low micromolar range.
We then examined the role of nucleotide status on the Rac1-p190B interaction. Rac1 loaded with GTPγS, but not with GDP, exhibits robust binding to aa382–1007 of p190B (Figure 1C). In contrast, no binding to Cdc42 or RhoA was observed. These results were obtained both with aa382–1007 from lysates of transfected Cos7 cells (data not shown), as well as with recombinant aa382–1007 (Figures 1C and S1). These data demonstrate that Rac1 binds directly to the p190B-MD in a GTP-dependent manner. We further compared the interaction of activated Rac1 with aa382–1007 of p190B to that of the Rac effector PAK1 [7]. In pulldown assays from lysates of Cos7 cells transfected with constructs encoding wild-type PAK1 (~62 kD) or aa382–1007 of p190B (~71 kD), GTPγS-bound Rac1 bound aa382–1007 of p190B as effectively as wild-type PAK1 (Figure 1D). We also determined that Rac1 binds endogenous p190B from lysates of NIH 3T3, MDCK, and HeLa cells (Figure 1E, and data not shown). We were unable to detect a GTP-dependent association between activated Rac1 and the middle domain of p190A (data not shown). However, the interaction between Rnd proteins and p190 molecules is significantly more robust for p190B than for p190A [5]. Hence, we cannot exclude that Rac1 also binds to the middle domain of p190A.
We further determined that the aa382–607 region of p190B is required and at least partially sufficient for interacting with activated Rac1, as well as with Rnd3 (Figures S2A and 1F). However, the aa382–607 fragment of p190B yielded interactions that were less robust than those observed with the aa382–1007 fragment (Figure S2A). Moreover, the aa382–607 fragment is less soluble/stable, thus rendering a direct comparison difficult. We therefore cannot rule out that additional motifs within the aa607–1007 sequence stabilize the interaction with GTP-bound Rac1. The aa382–607 fragment includes the third and the fourth FF domain of p190B (Figure 1A). FF domains are protein-protein interaction domains [8] and thus might mediate the interaction of p190B-MD with GTP-bound Rac1. However, we were unable to detect a GTP-dependent association of Rac1 with an N-terminal fragment of p190B containing all four FF domains of p190B (1–531; Figure 1A and Figure S2B), indicating that the region distal to the fourth FF domain is required for the interaction of GTP-bound Rac1 with p190B-MD.
An intact effector domain of Rac1 is required for the interaction with p190B-MD
Differential functions of effector domain mutants of Ras-like G proteins have been utilized to assign cellular responses to select effector molecules and downstream signaling pathways. In activated Rac1, the F37A mutation is defective for membrane ruffling but does not abrogate the interaction with PAK, activation of PAK, or JNK-mediated transcriptional responses. In contrast, the Y40C mutant abrogates PAK binding/activation and JNK/SAPK transcriptional responses but retains capacity for eliciting membrane ruffling [9–11]. Both the Rac1(Q61L, F37A) and Rac1(Q61L, Y40C) mutants show attenuated binding to p190B-MD in vitro (data not shown), suggesting that the p190B interaction may mediate multiple biological consequences of Rac1 activation. We next tested whether Rac1 and p190B interact in cells. We expressed N-terminal GST-tagged derivatives of Rac1 in HeLa cells along with Myc-tagged aa382–1007 of p190B. In pull-down assays using glutathione-sepharose beads we determined that while Rac1(Q61L) binds efficiently to aa382–1007 of p190B in cells, both effector domain mutants exhibit significantly reduced binding (Figure 2A). We obtained similar results in experiments with endogenous p190B (Figure 2B). Thus, contrary to previously established effector pathways of Rac1, the interaction with p190B-MD is attenuated equally by the F37A and Y40C mutations. Note that the GST-tagged derivatives used in this analysis retain the properties described in the literature (Figure S3).
Figure 2.
Characterization of the interaction between Rac1 and p190B. (A) An intact effector domain of Rac1 is required for the interaction with p190B aa382–1007 in cells. HeLa cells were transfected with mammalian expression constructs encoding GST alone or GST fusion proteins of Rac1(Q61L), Rac1(Q61L, F37A), or Rac1(Q61L, Y40C), as well as with Myc-tagged aa382–1007 fragment of p190B or empty vector control. 24 h after transfection the cells were lysed in Gold lysis buffer. Lysates were incubated with glutathione-sepharose beads for 4 h at 4°C, and bound proteins were processed for SDS-PAGE and western blotting to detect Myc- and GST-tagged proteins. (B) Activated Rac1 interacts with endogenous p190B. HeLa cells expressing GST alone, GST-Rac1(Q61L), or GST-Rac1 effector domain mutants were processed as described in (A) with the exception that western blots were probed with a mAb to p190B. (C) Activated Rac1 recruits aa382–1007 of p190B to membrane ruffles. HeLa cells were transfected as described in (A), and processed for immunofluorescence microscopy to detect GST, GST-tagged Rac1(Q61L), and Myc-tagged 382–1007 of p190B. (D) A Myc-tagged p190B fragment consisting of aa601–1007, which does not bind activated Rac1 (Figure S2A), exhibits reduced localization to membrane ruffles. Asterisks in (C) and (D) indicate untransfected cells that are only detectable upon prolonged exposure due to non-specific staining, and the hatched lines mark their perimeter.
We then examined the localizations of the Myc-tagged p190B fragments comprised by aa382–1007 and aa601–1007. In cells expressing GST alone, localization of the aa382–1007 fragment is diffuse, whereas the fragment localizes to membrane ruffles in GST-Rac1(Q61L) expressing cells (Figure 2C). This localization might result from entrapment of the aa382–1007 fragment within the cytoplasm of membrane ruffles induced by Rac1(Q61L). However, the aa601–1007 fragment, which fails to bind activated Rac1 (Figure S2A), exhibits substantially less localization to membrane ruffles in Rac1(Q61L) expressing cells (Figure 2D).
Activated Rac1 recruits p190B into a detergent-insoluble subcellular compartment
We next determined that overexpressed full-length p190B also colocalizes with Myc-tagged Rac1(Q61L) (Figure 3A), and that this localization is resistant to brief extraction with detergent (Figure 3B). Both p190A and p190B proteins can partition into detergent-insoluble compartments in response to various stimuli [12, 13]. To test whether activated Rac1 alters the solubility of p190B we subjected HeLa cells expressing Rac1(Q61L) to an established membrane fractionation procedure for p190B [13]. Virtually no endogenous p190B is detected in the cytosol. In control cells the majority of p190B is detergent-soluble. In contrast, expression of activated Rac1 elicits a significant shift in p190B localization to the detergent-insoluble fraction (Figure 3C and D). Attempts to examine the distribution of p190B mutants were unsuccessful because overexpression of p190B forms led to a "spill over" into the cytoplasmic and detergent-soluble pools.
Figure 3.
Activated Rac1 recruits p190B into a detergent-insoluble plasma membrane compartment. (A) and (B) Overexpressed p190B colocalizes with Rac1(Q61L) in membrane ruffles of untreated (A), as well as detergent extracted HeLa cells (B). HeLa cells were transfected with Myc-tagged Rac1(Q61L) in combination with full-length p190B, and either fixed in 2% PFA directly (A), or extracted with 0.5% Triton X-100 in MES buffer for 4 min on ice prior to fixation to remove soluble proteins from the cytoplasm (B). The cells were next processed for immunofluorescence microscopy. The p190B mAb used here is not sufficiently sensitive to detect endogenous p190B in HeLa cells by conventional fluorescence microscopy. (C) Endogenous p190B partitions into a detergent-insoluble membrane fraction from cells expressing activated Rac1. The cell fractionation procedure is detailed in Supplementary Experimental Procedures. Note that p190B shifts to the detergent-insoluble fraction in cells expressing activated Rac1(Q61L). (D) Quantification of the p190B in detergent-soluble and Triton-insoluble fractions was performed by densitometry. Data are expressed as mean ± SD from four independent experiments. The shift in p190B distribution between vector control and Rac1(Q61L) transfected cells is statistically significant (*, p<0.01, unpaired two-tailed Student t test), irrespectively of whether the detergent-soluble or detergent-insoluble pools are compared. (E) RBD pull-down assay to determine the levels of membrane-associated GTP-bound RhoA in the triton-soluble, post 100,000× g membrane fraction from cells expressing activated Rac1. For comparison total RhoA in 5% of the cell extract is shown. (F) Quantification of GTP-bound RhoA from four independent RBD pull-down assays. Data are expressed as mean ± SD from four independent experiments. In 4/4 experiments RhoA-GTP levels were reduced in membranes isolated from cells expressing Rac1(Q61L).
In parallel studies, we determined the effects of activated Rac1 on membrane associated, GTP-bound RhoA using the Rhotekin (RBD) pulldown assay. Strikingly, expression of Rac1(Q61L) depletes GTP-bound RhoA from membranes (Figures 3E and 3F). This is consistent with a scenario in which recruitment of p190B by activated Rac1 promotes localized hydrolysis of GTP-bound Rho, thus implicating p190B as a local antagonist of Rho signaling.
Role of the Rac1 binding sequence in p190B in regulating cell shape
Intriguingly, whereas Rnd proteins elicit cell rounding by enhancing the GAP activity of p190 molecules [5], expression of activated Rac1 promotes membrane ruffling and cell spreading (Figure 2C) [14]. Overexpression of p190B elicits cytoskeletal collapse that is counteracted by expression of Rac1(Q61L) (Figures 3A and S4). Consistent with the biochemical data presented in Figure 1B, activated forms of other Ras-like G proteins, which do not bind p190B-MD, fail to prevent cytoskeletal collapse elicited by overexpression of p190B (Figure S4). These findings raise the possibility that activated Rac1 restricts p190B-mediated RhoGAP activity within cells. We therefore engineered a p190B mutant (ΔRBS) lacking aa381–607, which is required for the interaction with GTP-bound Rac1 (Figure S2A), as well as a mutant lacking the GAP domain (ΔGAP; Figure 4A). Both mutants were expressed at levels comparable to exogenous wild-type p190B in HeLa cells (Fig. 4B). Moreover, the ΔRBS mutant retained GAP function, as it effectively elicits cytoskeletal collapse in HeLa cells (Figure 4C). Accordingly, the ΔGAP mutant is defective in causing cytoskeletal collapse (Figure 4C). We further verified that the cytoskeletal collapse elicited by the ΔRBS mutant was due to effects on Rho signaling, as it was abrogated by co-expression of ROCK1Δ3 (Figure S5), which is a constitutively active form of the Rho effector molecule ROCK1 [15].
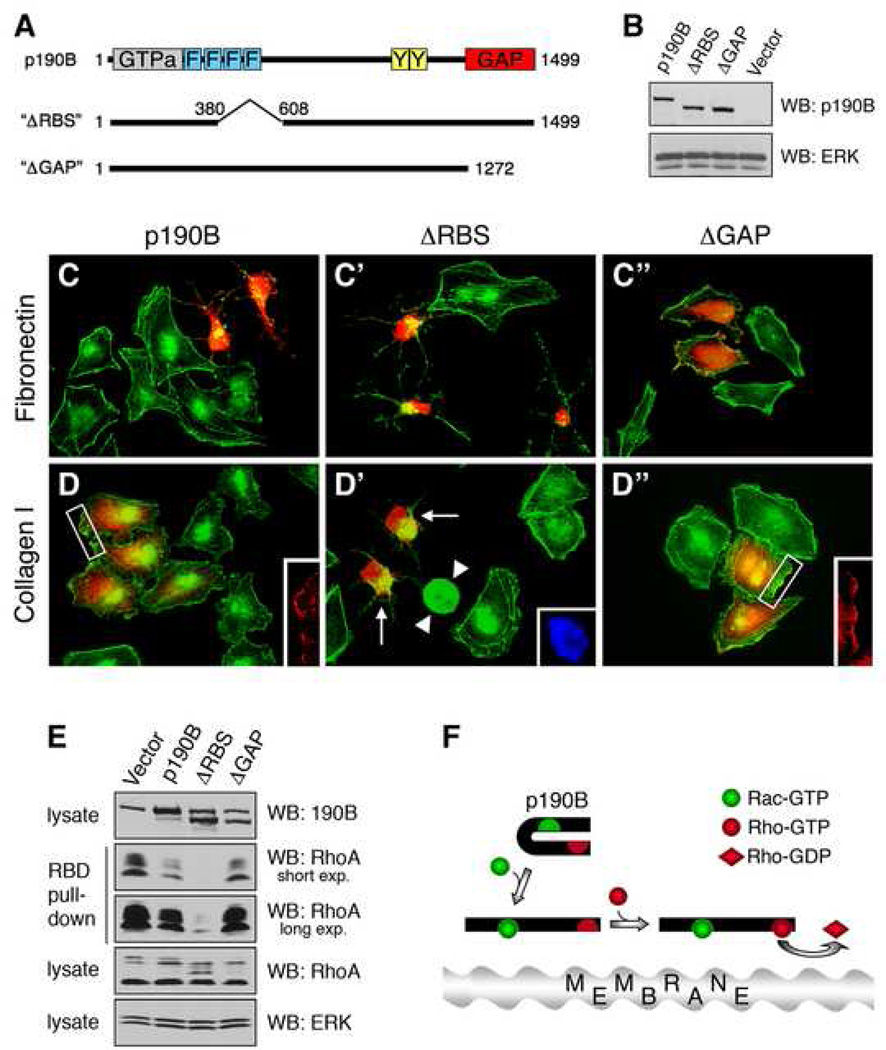
Figure 4.
The minimal Rac1-binding sequence (RBS) is required for modulation of p190B RhoGAP function. (A) Cartoon of p190B(ΔRBS) and p190B(ΔGAP) mutants. (B) Western blot analysis of wild-type p190B, ΔRBS, or ΔGAP protein levels in HeLa cells transfected with expression constructs. ERK is shown as a loading control. (C) and (D) The ΔRBS mutant is defective in regulating GAP function in response to integrin engagement. HeLa cells were transfected with expression constructs encoding wild-type p190B, ΔRBS, or ΔGAP and plated for 15 h on fibronectin (C), or collagen type-I (D). The cells were then processed for detection of transfected p190B forms (red), polymerized actin (green) and nuclei (blue). Inserts in panels (D) and (D") showing wild-type p190B or ΔGAP transfected cells, respectively, demonstrate the localization of these proteins to peripheral membrane ruffles. Arrows in panel (D') point to ΔRBS expressing cells exhibiting cytoskeletal collapse, which are easily distinguished from rounded mitotic cells (arrowheads) identified by DAPI staining (D', insert). (E) RBD pulldown assay conducted on 293 cells transfected with vector control, p190B, ΔRBS, or ΔGAP expression constructs. Note that the ΔRBS mutant is substantially more potent at hydrolysing RhoA-GTP than wild-type p190B. (F) Model of Rac1-mediated modulation of p190B RhoGAP function. Activated Rac1 interacts directly with the p190B-MD and recruits p190B to peripheral membrane ruffles, where p190B upon undergoing a conformational change exhibits enhanced RhoGAP activity leading to inactivation of RhoA.
Integrins are receptors for extracellular matrix constituents and signal upstream of Rac1[16], and p190B is recruited to the plasma membrane upon integrin ligation [4]. To test the functional significance of the Rac1-p190B interaction, we examined the effects of integrin ligands on cell shape. HeLa cells spread significantly faster on collagen type-I than on fibronectin, a response that is abrogated by dominant-negative Rac1 (data not shown). In short-term transfection experiments (15 h) both wild-type p190B and the ΔRBS mutant elicited cytoskeletal collapse on fibronectin (Figure 4C). In contrast, wild-type p190B overexpressing cells maintain a spread cell shape on collagen type-I, whereas ΔRBS expressing cells undergo cytoskeletal collapse (Figure 4D). To quantify the effects of wild-type p190B and the ΔRBS mutant on cell shape in an unbiased manner, we used phase-contrast microscopy (Figure S6A). In a double-blind quantification assay, we observed significant increases in the fraction of ΔRBS transfected cells exhibiting cytoskeletal collapse relative to cells transfected with wild-type p190B (Figure S6B). Collectively, our results suggest that activated Rac1, through direct interaction with p190B, locally modulates p190B RhoGAP function to regulate cell shape in response to integrin signaling.
The difference in cytoskeletal collapse between wild-type p190B and ΔRBS expressing cells cannot solely be explained by a mechanism whereby activated Rac1 "sequesters" p190B away from GTP-bound Rho. Although a fraction of wild-type p190B and the ΔGAP mutant localizes to peripheral actin-enriched membrane ruffles (Figures 4D and 4D", inserts), this fraction is relatively minor. The bulk of wild-type p190B localizes to the cytoplasm, as does the ΔRBS mutant. We therefore compared the GAP activity of the ΔRBS mutant to that of wild-type p190B. Strikingly, the ΔRBS mutant exhibits dramatically enhanced GAP activity (Figure 4E). This result demonstrates that the aa382–607 sequence in p190B, which is required for the interaction with activated Rac1, represses the GAP activity of p190B. Taken together, our data establish that activated Rac1 binds p190B directly and recruits it to membrane microdomains. Our results are also consistent with a model whereby binding of activated Rac1 enhances p190B RhoGAP activity by relieving autoinhibition, possibly through an allosteric mechanism (Figure 4F).
Discussion
A substantial body of work has linked the activities of Rho and Rac GTPases. It was initially demonstrated that activated Rac1 elicits membrane ruffling and subsequent actin stress fiber formation in fibroblasts, suggesting that Rac positively regulates Rho function [14]. This finding has been validated in cells derived from Rac1-deficient mice [17]. Subsequent studies have established that Rac also can function antagonistically to Rho through various mechanisms, all of which involve several intermediates [18–21]. Here, we describe a novel mechanism of cross-talk between Rac and Rho GTPases in which activated Rac1, through direct binding to the middle domain, recruits p190B to the plasma membrane and restricts p190B function within the cells. We further demonstrate that activated Rac1 depletes RhoA-GTP from membranes of HeLa cells. Moreover, we establish that the region comprised by aa382–607 of p190B, which is required for the interaction with Rac1 and Rnd3, potently represses the GAP activity of p190B. Collectively, these observations are consistent with a model in which Rac1-mediated recruitment of p190B locally antagonizes RhoA function by relieving autoinhibition of p190B RhoGAP activity.
In contrast to Rnd proteins, which we previously demonstrated enhance the GAP activity of p190 proteins in vitro [5], we have not yet been able to address directly whether Rac1 exerts a similar effect, perhaps because p190 molecules can also function as GAPs for Rac proteins [22]. Thus, in GAP activity assays in vitroRac1(Q61L) may "clog up" the GAP domain of p190B thereby preventing access to GTP-bound RhoA. It will thus be of great interest to determine whether Rnd and Rac proteins bind to the same motif within p190 molecules, akin to what has been observed for Plexin-B1 [23]. This would be consistent with a non-CRIB motif-mediated interaction, as suggested by the nondiscriminating effects of the F37A and Y40C effector domain mutants on the Rac1-p190B interaction. However, sequence gazing failed to detect a region in p190B with similarity to the motif in the Plexin-B1 tail that binds Rac1 and Rnd1. Thus, further analysis of the Rac1 and Rnd interactions with p190B will be required to identify the motif(s) in p190 proteins that mediate these interactions.
Given the similarities between the Rac1-p190B and the Rnd3-p190B interactions, it is intriguing that while Rac1 activation generally promotes membrane ruffling and cell spreading, expression of Rnd proteins elicits cytoskeletal collapse in various cell types [2, 24]. Obviously, Rac1 and Rnd proteins engage additional effector molecules beyond p190 RhoGAPs, which undoubtedly contributes to the distinct cellular effects of activated Rac and Rnd protein expression in cells. However, another potentially important difference between Rac and Rnd proteins in terms of p190 RhoGAP function relates to kinetics. While Rac proteins continuously cycle between an inactive, GDP-bound, and an active, GTP-bound state, Rnd proteins appear to be constitutively GTP-bound and prevailing evidence indicates that activity of Rnd proteins is regulated through their level of expression [24]. Thus, while it is probable that effects of Rnd proteins on p190 signaling may be sustained and possibly global, the effects of Rac1 are likely transient and local.
We provide evidence that cross-talk between Rac and Rho GTPases through p190B is required for control of cell shape by integrin signaling. Additional novelty of the mechanism defined here resides in the more nuanced manner in which Rac1 may regulate Rho function, rather than through direct antagonism. For instance, at physiological levels of p190B expression, one can now envision that activated Rac, during cell spreading and migration, may not only utilize p190B to locally inactivate RhoA to permit membrane ruffling and focal complex formation, but that GTP-bound Rac in addition sequesters p190B to permit Rho activation and stress fiber formation in adjacent regions of the cell. Another possible context for such regulation is in chemotaxing neutrophils, where activated Rac promotes membrane ruffling at the front and Rho elicits contraction at the rear of the cell [25]. Rac1-mediated recruitment of p190B to the front of a chemotaxing neutrophil could account for the striking polarity of this cell type. Thirdly, direct interaction between Rac1 and p190B might serve to establish the mutually excluding zones of active Rac and Rho along expanding adherens junctions in polarizing epithelial cells [26]. Thus, the findings of the present study open multiple new avenues for further studies that will provide a better understanding of the coordination of Rho and Rac GTPase signaling.
Supplementary Material
Acknowledgements
We are grateful to Tobias Meyer, Martin A. Schwartz, and Arthur S. Alberts for providing reagents, to Ashley D. Pollock and Jessica Wagner for technical assistance, and to Lynne Coluccio and Scott R. Frank for helpful discussions. This work was funded by R01 NIH CA62142 to JS, R01 NIH CA092354 to SHH, and funds from The Roy and Lynne Frank Foundation and Children's Hospital to SHH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 3.Malliri A, Collard JG. Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol. 2003;15:583–589. doi: 10.1016/s0955-0674(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 4.Burbelo PD, Miyamoto S, Utani A, Brill S, Yamada KM, Hall A, Yamada Y. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J Biol Chem. 1995;270:30919–30926. doi: 10.1074/jbc.270.52.30919. [DOI] [PubMed] [Google Scholar]
- 5.Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol. 2003;13:1106–1115. doi: 10.1016/s0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heo WD, Meyer T. Switch-of-function mutants based on morphology classification of Ras superfamily small GTPases. Cell. 2003;113:315–328. doi: 10.1016/s0092-8674(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 7.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 8.Bedford MT, Leder P. The FF domain: a novel motif that often accompanies WW domains. Trends Biochem Sci. 1999;24:264–265. doi: 10.1016/s0968-0004(99)01417-6. [DOI] [PubMed] [Google Scholar]
- 9.Diekmann D, Nobes CD, Burbelo PD, Abo A, Hall A. Rac GTPase interacts with GAPs and target proteins through multiple effector sites. Embo Journal. 1995;14:5297–5305. doi: 10.1002/j.1460-2075.1995.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase [published erratum appears in Science 1997 Apr 11;276(5310):185] Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 11.Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen JC, Zhuang S, Nguyen TH, Boss GR, Pilz RB. Oncogenic Ras leads to Rho activation by activating the mitogen-activated protein kinase pathway and decreasing Rho-GTPase-activating protein activity. J Biol Chem. 2003;278:2807–2818. doi: 10.1074/jbc.M207943200. [DOI] [PubMed] [Google Scholar]
- 13.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 14.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. Febs Letters. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 16.Grande-Garcia A, Echarri A, Del Pozo MA. Integrin regulation of membrane domain trafficking and Rac targeting. Biochem Soc Trans. 2005;33:609–613. doi: 10.1042/BST0330609. [DOI] [PubMed] [Google Scholar]
- 17.Guo F, Debidda M, Yang L, Williams DA, Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J Biol Chem. 2006;281:18652–18659. doi: 10.1074/jbc.M603508200. [DOI] [PubMed] [Google Scholar]
- 18.van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nature Cell Biology. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 19.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 20.Meng W, Numazaki M, Takeuchi K, Uchibori Y, Ando-Akatsuka Y, Tominaga M, Tominaga T. DIP (mDia interacting protein) is a key molecule regulating Rho and Rac in a Src-dependent manner. Embo J. 2004;23:760–771. doi: 10.1038/sj.emboj.7600095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenfeldt H, Castellone MD, Randazzo PA, Gutkind JS. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. Journal of molecular signaling. 2006;1:8. doi: 10.1186/1750-2187-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligeti E, Dagher MC, Hernandez SE, Koleske AJ, Settleman J. Phospholipids can switch the GTPase substrate preference of a GTPase-activating protein. J Biol Chem. 2004;279:5055–5058. doi: 10.1074/jbc.C300547200. [DOI] [PubMed] [Google Scholar]
- 23.Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J Biol Chem. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 26.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.