Abstract
Using a case-crossover study design and conditional logistic regression, we compared the relative odds of transmural (full-wall) myocardial infarction (MI) calculated using exposure surrogates that account for human activity patterns and the indoor transport of ambient PM2.5 with those calculated using central-site PM2.5 concentrations to estimate exposure to PM2.5 of outdoor origin (exposure to ambient PM2.5). Because variability in human activity and indoor PM2.5 transport contributes exposure error in epidemiologic analyses when central-site concentrations are used as exposure surrogates, we refer to surrogates that account for this variability as “refined” surrogates. As an alternative analysis, we evaluated whether the relative odds of transmural MI associated with increases in ambient PM2.5 is modified by residential air exchange rate (AER), a variable that influences the fraction of ambient PM2.5 that penetrates and persists indoors. Use of refined exposure surrogates did not result in larger health effect estimates (ORs = 1.10 – 1.11 with each interquartile range increase.), narrower confidence intervals, or better model fits compared to the analysis that used central-site PM2.5. We did observe evidence for heterogeneity in the relative odds of transmural MI with residential AER (effect-modification), with residents of homes with higher AERs having larger ORs than homes in lower AER tertiles. For the level of exposure-estimate refinement considered here, our findings add support to the use of central-site PM2.5 concentrations for epidemiological studies that employ similar case-crossover study designs. In such designs, each subject serves as his or her own matched control. Thus, exposure error related to factors that vary spatially or across subjects should only minimally impact effect estimates. These findings also illustrate that variability in factors that influence the fraction of ambient PM2.5 in indoor air (e.g., AER) could possibly bias health effects estimates in study designs for which a spatio-temporal comparison of exposure effects across subjects is conducted.
INTRODUCTION
A recent meta-analysis, which reported a statistically significant 2.5% increase in the risk of myocardial infarction (MI) associated with each 10 μg/m3 increase in ambient (outdoor-generated) PM2.5 concentration lagged one day, concluded that acute increases in PM2.5 may trigger MI.1 In our previous work, which was included in this meta-analysis, we reported an increased risk of transmural (full wall) MI, but not non-transmural (subendocardial) MI, associated with increased PM2.5 concentration in the 24 hours before emergency department admission for that infarction.2 In all of these studies, PM2.5 measured at one or more nearby (within 10 km) central-site monitors was used as a proxy for a subject’s exposure to PM2.5 of outdoor origin (i.e., exposure to ambient PM2.5). This likely resulted in exposure error due, in part, to proximity to local sources, human activity patterns (e.g., time spent in various locations) and temporal and spatial variability in the efficiency with which ambient PM2.5 penetrates into and persists in the indoor environment. While other air pollution studies have explored exposure refinements that account for spatial variability in ambient PM2.5 due to local sources,e.g. 3–6 the variable effects of human activity patterns and ambient PM2.5 losses with outdoor-to-indoor transport are largely unexplored.
The fraction of ambient PM2.5 that penetrates and persists indoors (F) varies with multiple factors including particle size and chemical composition, housing characteristics (e.g., home age), meteorological conditions (e.g., wind speed and temperature),7,8 and human activities (e.g., opening windows or using air conditioning).9 Variability in the time spent in various locations (e.g., outdoors, indoors, or in a vehicle) also influences personal exposure to PM2.5 of outdoor origin due to spatial variability in both outdoor PM2.5 concentrations and the indoor transport of ambient PM2.5. This exposure error is likely a combination of Berkson and classical errors, which would bias effect estimates towards the null and/or inflate variances,10,11 hampering the detection of statistically significant associations between increased ambient PM2.5 exposures and the risk of MI. Therefore, ambient PM2.5 exposure surrogates that account for these factors could offer improvement over the direct use of central-site monitor PM2.5 concentrations in air pollution epidemiology studies.
Exposure errors associated with variability in F and human activity patterns may modify ambient-PM-mediated health effect estimates. Multiple studies have reported a lower risk of morbidity or mortality associated with increases in PM2.5 concentration in communities with a high prevalence of central air conditioning (AC), compared to risk estimates among communities with lower AC prevalence.12–16 Central AC use reduces F because indoor air is filtered as it is recirculated, thus increasing particle losses indoors.17–19 In a previous study, we reported that conditions resulting in lower calculated values of F (due to spatial variability in PM2.5 composition and/or residential AER) corresponded to circumstances under which lower effect estimates had been observed in previous epidemiological studies. We concluded that exposure misclassification due to variability in F could partially explain this observed geographic heterogeneity in ambient-PM-mediated health effect estimates.20
Using a case-crossover study design, herein we estimated the relative odds of transmural MI associated with increased ambient PM2.5 exposure in the previous 24 hours using three different PM2.5 exposure metrics that account for variability in human activity patterns and/or the indoor transport of ambient PM2.5: (a) a stochastic human exposure model that simulates the ambient PM2.5 concentration and time spent in each of several locations (i.e. outdoors, indoors, in a vehicle) to estimate population distributions of ambient PM2.5 exposure, (b) a deterministic mass-balance model that estimates residential, indoor concentrations of ambient (outdoor-generated) PM2.5 using a more refined treatment of residential air exchange rates (AERs) and PM2.5 penetration and losses with indoor transport, and (c) a hybrid of these two models (Baxter et al.21 in this issue of the journal). As noted above, variability in human activity patterns and the indoor transport of ambient PM2.5 can contribute to exposure error in epidemiologic analyses when central-site concentrations alone are used to estimate exposure to ambient PM2.5 and, thus, we refer to the exposure surrogates that account for this variability as “refined” exposure surrogates in the following text. We hypothesized that these refined ambient PM2.5 exposure surrogates would have less non-differential exposure error (which tends to bias effect estimates towards the null) and, thus, would result in larger health effect estimates, narrower confidence intervals, and better model fits compared to the analysis that used central-site PM2.5 concentrations alone as surrogates for ambient PM2.5 exposures. As an alternative analysis, we evaluated whether the association between ambient PM2.5 and transmural MI is modified by residential AER. For this analysis, we hypothesized that associations between transmural MI and ambient PM2.5 concentrations would be smaller for low AERs because a smaller fraction of ambient PM2.5 penetrates and persists indoors. Thus, at low AERs, the difference between central-site PM2.5 concentrations and actual ambient PM2.5 exposure is greater, resulting in proportionally more non-differential exposure misclassification and larger bias towards the null (i.e. greater underestimation of effect).
METHODS
Study Population and Outcome Definition
The study population and definition of transmural infarction used in this study have been described previously.2 Briefly, all unscheduled hospital admissions with a primary diagnosis of acute myocardial infarction (International Classification of Diseases 9th Revision [ICD-9] code 410.01, 410.11, 410.21, 410.31, 410.41, 410.51, 410.61, 410.71, 410.31, 410.91) were extracted from the Myocardial Infarction Data Acquisition System (MIDAS), a New-Jersey-wide database of hospital discharges and death certificate registrations.22,23 We included only those patients who were admitted between January 2004 and December 2006, were ≥18 years of age, were residents of New Jersey at the time of their MI, and had no previous diagnosis of MI. These subjects (n = 1563) were primarily male (63%) and white (69%) and had a median age of 62. Only subjects who resided within 10 km of a central-site monitor at the time of their MI were included in this study. This study was approved by the University of Medicine and Dentistry of New Jersey Institutional Review Board and the University of Rochester Research Subjects Review Board. MIDAS was also approved by the New Jersey Department of Health and Senior Services Institutional Review Board.
Exposure Surrogates
We used four different exposure surrogates generated from central-site monitor concentrations to estimate personal exposure to PM2.5 of outdoor origin (i.e., exposure to ambient PM2.5). Because we only observed a significantly increased relative odds of transmural MI associated with average PM2.5 concentrations in the 24 hours preceding emergency department admission in the in initial analysis,2 here, hourly ambient PM2.5 exposures were computed and averaged over that 24 hour period for each exposure metric. Detailed descriptions of each exposure surrogate and comparisons between them are available elsewhere.21 In the following paragraphs, we provide a brief description of each exposure metric. The exposure surrogates are labeled based on their level of refinement and complexity, with higher-numbered Tiers corresponding to a greater degree of refinement.
Tier 1. Central-site PM2.5 Concentrations
For Tier 1, hourly ambient PM2.5 concentrations for the study period (January 2004 – December 2006) measured at 7 New Jersey Department of Environmental Protection monitors were retrieved from the United States Environmental Protection Agency website.24 The zip code of each patient’s residence at the time of MI was extracted from MIDAS and subjects were assigned 24 hour average PM2.5 concentrations, for all case and control periods, from the monitor closest to their residence.2 Tier 1 exposure estimates varied temporally within and across central-site-monitor regions with ambient PM2.5 concentrations. Because subjects residing within 10 km of the same monitor were assigned the same exposure value for a given 24-hour case or control period, there was no geographic variability in exposure estimates within that 10 km radius. Within a given case or control period, however, exposure estimates did vary across monitoring locations.
Tier 2a. SHEDS
In Tier 2a, the exposure-modifying effects of human activity patterns and the indoor transport of ambient PM2.5 were taken into account using the Stochastic Human Exposure and Dose Simulation (SHEDS) model.25 Distributions of ambient PM2.5 exposures were generated for a simulated population representative of the study population. For each census tract within 10 km of a central-site monitor, 10,000 representative individuals were simulated by sampling from census-tract level demographic data (gender, age, and employment status) from the 2000 U.S. Census. For each simulated individual, a time series of human activity patterns was simulated using diary data from the Consolidated Human Activity Database26 matched by age, gender, season, and day of week. Hourly central-site PM2.5 concentrations (Tier 1) were used as inputs, and personal exposure to PM2.5 of outdoor origin was calculated as a time-weighted average of the ambient PM2.5 concentrations in each microenvironment (e.g. home, office, outdoors). Note, indoor PM2.5 sources were set to zero to estimate the distribution of exposures to PM2.5 of outdoor origin only (i.e., ambient PM2.5 exposures) in each census tract. For residential microenvironments, SHEDS sampled from a representative distribution of housing types, AERs, particle penetration efficiencies, and indoor particle deposition rates. It should be emphasized that the AERs used in this version of SHEDS vary seasonally, but not spatially within the study domain. From the distribution of ambient PM2.5 exposures generated for each hour during the study period, we used the median to calculate 24 hour mean exposures for each case and control period. The 24 hour mean exposures calculated for each census tract were then averaged over the 10 km region surrounding each central-site monitor.
Tier 2b. The Aerosol Penetration and Persistence Model
Hourly PM2.5 concentrations measured at the central-site monitors (Tier 1) were modified to account for the effects of outdoor-to-indoor transport using the Aerosol Penetration and Persistence (APP) model8,27,28 and the Lawrence Berkeley National Laboratory (LBNL) Infiltration model.29,30 The APP model is a deterministic mass balance model that predicts the indoor concentration of ambient PM2.5 based on AER, outdoor PM2.5 concentrations, the efficiency of particle penetration into the home, the rate of depositional losses in indoor air, and, for ammonium nitrate, phase changes in the indoor environment.8,27,28 In addition to accounting for the semi-volatile nature of ammonium nitrate, daily variations in particle chemical composition were taken into account through the use of particle-size-resolved deposition loss rates specific to the size distributions of the major PM2.5 species (sulfate, nitrate, elemental carbon, and organic carbon). Central-site PM2.5 composition data from the EPA Speciation Trends Network (STN) is available for every third day and was downloaded from the US EPA website for this purpose.24 For days without measurements, PM2.5 species mass fractions were interpolated using a weighted average of the two nearest mass fraction measurements. Subjects were excluded if there was a period of more than nine days between STN measurements for the case period or all control periods. Because speciation measurements were not available for all central-site-monitor locations, values for the New Brunswick monitoring station, which were most highly correlated with data from other monitors across the state, were used. For details, see Hodas et al.20 and Baxter et al.21. With this approach, particle losses indoors varied daily with variations in PM2.5 composition. Note, however, that deposition loss rates did not vary spatially in this work.
AERs calculated with the LBNL Infiltration model, which was modified to include air flow through open windows (details in Supplemental Information), were used as inputs to the APP model. The LBNL infiltration model predicts AER for single-family homes based on normalized leakage rates (which describe the effective area of openings in the building shell through which air can flow, normalized by home floor area and a parameter accounting for building height and validated against measurements in 70,000 closed homes) and meteorological conditions.29,30 Meteorological data were gathered from four airports in New Jersey (Newark, Caldwell, Somerset, and Trenton) and subjects were assigned the weather data from the monitor nearest their residence at the time of MI. The normalized leakage area was calculated using a model resulting from a statistical analysis relating leakage to housing characteristics (home age, floor area)31 using census-tract level housing data from the 2000 U.S. Census and the American Housing Survey. Notably, the model used to calculate normalized leakage rate differs for homes above and below the poverty line because home leakiness varies with resident poverty status, with low-income homes tending to be leakier.31 Thus, variations in calculated AERs arise from temporal and spatial variability in meteorological conditions and with spatial variability in housing stock. Unlike Tier 2a, detailed human activity patterns are not accounted for in this metric, but Tier 2b provides a more refined treatment of residential AER and PM2.5 penetration and losses with indoor transport. Census-tract-level ambient PM2.5 exposures were averaged over the 10 km area around each central-site monitor.
Tier 3. SHEDS and APP Hybrid
The final exposure metric combined the refined treatment of human activity patterns from Tier 2a, with the more temporally- and spatially-resolved estimates of residential AER from Tier 2b (but without variations in PM2.5 deposition rates with variations in PM2.5 composition). PM2.5 exposures were estimated with SHEDS as described above, but using residential AERs estimated with the LBNL Infiltration model.
Statistical Analyses
Study Design
For each ambient PM2.5 exposure surrogate (tier), we used the same time-stratified case-crossover design32,33 as in the initial analysis2 to estimate the relative odds of a transmural infarction associated with increased exposure in the previous 24 hours. In this design, each patient contributed information both as a case during the period immediately before the MI, and as a matched control during times when a MI did not occur. Since each subject serves as their own control, factors that differ only across subjects are controlled by design. Case periods were defined as the 24 hour period before emergency department admission for MI. Control periods (3–4 per case depending on the number of days in the calendar month), defined as 24 hour periods in which no MI occurred, were matched to the case period by day of the week, time of day, year, and calendar month. Central-site PM2.5 concentrations (Tier 1) and modeled ambient PM2.5 exposures (Tier 2a, 2b, 3) corresponding to these case and control periods were then contrasted in the statistical analyses.
Modeled Exposure Tier Analyses
We used the same conditional logistic regression model as in the initial analysis,2 stratified by study subject, to examine the multiplicative interaction between ambient PM2.5 exposure and transmural MI. We regressed case-control status (i.e., case period = 1, control period = 0) against the mean estimated ambient PM2.5 exposure in the 24 hour period before emergency department admission for the index infarction or the corresponding control period. We also included a natural spline (3 degrees of freedom) of the mean apparent temperature,34,35 from the same 24 hour period, to estimate each subjects’ perceived ambient air temperature. Hourly temperature and relative humidity data used to calculate apparent temperature were gathered from the same airports as the data used to calculate AER in Tiers 2b and 3. The relative odds of transmural MI was estimated using each exposure surrogate (Tier 1, 2a, 2b, or 3) scaled to the Tier-specific interquartile range (IQR) increase in the ambient PM2.5 exposure. For each Tier, we present the odds ratio (OR), its 95% confidence interval, and its Akaike’s Information Criterion (AIC) value, which was used to compare the fit of these non-nested models to Tier 1.
We also examined whether the refined exposure estimates (Tiers 2a, 2b, 3) added explanatory power over the Tier 1 estimate. In other words, we evaluated whether the refined exposure estimates provided supplementary exposure information beyond that accounted for in the Tier 1 estimates and whether including that information in effect-estimate calculations resulted in additional MI risk over that associated with the central-site PM2.5 concentrations (Tier 1 estimates) alone. For each case and control time period, the Tier 1 exposure estimate and each of the refined exposure estimates were converted to z-scores based on their respective means and standard deviations. The conditional logistic regression model described above was run again with the Tier 1 z-score and the z-score difference (e.g., the difference between the Tier 1 z-score and the Tier 2a z-score) as covariates. Z-scores were used in order to create scale- and location-invariant versions of the exposure metrics. Given that variables that differ only by scale and location may contribute equivalently to explaining a response in the context of linear modeling, entering the difference (between the refined and the original z-scores) into a linear model in addition to the original represents the additional contribution that the refined variable can make over the original in explaining the response in a linear model. The regression coefficient for the Tier 1 z-score, times the observed IQR, estimated the increase in log-odds of transmural infarction associated with each IQR increase in the Tier 1 PM2.5 concentration, while the regression coefficient for the “z-score difference” provided an estimate of the additional increase in log-odds of a transmural infarction associated with each IQR increase in the refined PM2.5 exposure estimate, independent of the Tier 1 PM2.5 concentration. A significance test of the “z-score difference” regression coefficient provides a test of whether the refined Tier adds any statistically significant relative odds beyond what is provided by Tier 1. We ran this same model separately for each refined metric (Tiers 2a, 2b, and 3).
AER Effect Modification Analyses
We also explored whether residential AER alone, without the other components contributing to the refined exposure surrogates, modified the association between the Tier 1 exposure surrogate and transmural infarction. We did this because AER estimates have smaller uncertainties than the more expansive exposure models and are important predictors of the fraction of ambient PM2.5 that penetrates and persists indoors. However, as explained below, this approach also differs from the main analysis in that it introduces a spatial comparison.
AERs from the Tier 2b exposure estimates were ranked into tertiles (high AER, middle AER, and low AER). See supplemental information, Table 2 for summary statistics of AERs in each tertile. We then re-ran the Tier 1 conditional logistic regression analysis adding two interaction terms to the model, as well as indicator variables for AER. The base model is
where Yij equals one if the jth period for the ith subject is a case and zero if control. Further, AERlow,i, AERmid,i and AERhigh,i are indicator variables equal to one if subject i has a low, middle or high AER and zero otherwise. The term f(Tempij; γ) represents the natural spline that is added to adjust for apparent temperature and αi represents the sum of a random intercept for subject i as well as any between-subject variables. Upon conditioning on subject, αi becomes a nuisance parameter which cancels out of the conditional logistic likelihood and is not estimated. From this model, we estimated the relative odds of a transmural infarction and its 95% confidence interval associated with a 10.3 μg/m3 (IQR) increase in Tier 1 PM2.5 concentration, within each tertile of AER. This was done for the cool (November to April) and warm (May to October) seasons separately because PM2.5 concentrations and composition are distinctly different over these two periods (Supplemental Information, Table 3).36
In this alternative analysis using interaction terms to estimate the relative odds of a transmural MI associated with increased PM2.5 concentration within the low, middle, and high AER groups, we essentially stratified the case-crossover analysis described above by modeled residential AER and compared estimates of the relative odds of transmural MI across AER tertiles. In contrast with the “Modeled Exposure Tier Analysis”, which was strictly a within-subject, temporal analysis, this “AER Effect Modification Analysis” is a spatio-temporal comparison of exposure effects across AER tertiles and, thus, across subjects. Here, we also focused on a single parameter that influences the indoor transport of ambient PM2.5 in order to reduce the number of assumptions and associated uncertainty in comparison to the more complicated refined exposure surrogates explored above.
To evaluate whether spatially varying factors in addition to AER (e.g. PM2.5 chemical composition, study population characteristics) could contribute to variability in relative risk of MI across AER tertiles, we also conducted a case-crossover analysis stratified by monitoring-site community and compared study population characteristics across AER tertiles. All data sets were constructed using SAS software (version 9.1.3; SAS Institute Inc., Cary, NC), and all analyses were conducted using R (version 2.6.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Modeled Exposure Tier Analyses
The summary statistics for Tiers 1, 2a, 2b, and 3 ambient PM2.5 exposure estimates have been described previously.2,21 While the refined ambient PM2.5 exposure concentrations (Tiers 2a, 2b, 3) for each case and control period were approximately half of Tier 1 (central-site) values on average, they were all highly correlated with the Tier 1 concentrations (r = 0.98, 0.98, and 0.98 for Tiers 2a, 2b, and 3, respectively). All relative odds estimates reported below were scaled to the IQR increase of each Tier: Tier 1 (10.3 μg/m3), Tier 2a (5.4 μg/m3), Tier 2b (5.4 μg/m3), and Tier 3 (5.4 μg/m3).
Each 10.3 μg/m3 increase in the Tier 1 PM2.5 concentration was associated with a significant increase in the odds of transmural MI (OR = 1.10, 95% CI = 1.01, 1.19). Each IQR increase (5.4 μg/m3) in the Tier 2a, Tier 2b, and Tier 3 PM2.5 concentrations was associated with the same size increase in the relative odds of a transmural MI with similar 95% confidence intervals across exposure tiers (Tier 2a: OR = 1.10, 95% CI = 1.01, 1.20; Tier 2B: 1.10, 95% CI = 1.01, 1.20; Tier 3: 1.11, 95% CI = 1.02, 1.20; Table 1). Similarly, model fits, as measured by the AIC value, were not substantially different across exposure tiers (Table 1). Further, using the z-score method, we found no additional significant relative increase in odds of transmural MI associated with the refined exposure estimates in addition to that associated with Tier 1 PM2.5 concentrations (Table 2). For example, each IQR (1.22 μg/m3) increase in the z-score for Tier 1 PM2.5 concentration was associated with a significant increase in the relative odds of a transmural infarction (OR = 1.11, 95% CI = 1.00, 1.23), but an IQR (0.21 μg/m3) increase in the Tier 2a z-score difference was associated with only a small, non-significant increase in the relative odds (OR = 1.03, 95% CI = 0.90, 1.18). Similarly, increases in the relative odds of MI associated with IQR increases in Tier 2b and Tier 3 z-score differences (0.21 and 0.28 μg/m3, respectively) were small and not statistically significant (Table 2) and, thus, added no explanatory power over the Tier 1 estimate.
Table 1.
Relative increase in odds of a transmural infarction associated with an IQR increase in PM2.5 concentration, by exposure Tier
| Tier | IQR | N | AIC | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Tier 1 | 10.3 | 1561 | 4397.4 | 1.10 | 1.01, 1.19 | 0.03 |
| Tier 2A SHEDS | 5.4 | 4397.2 | 1.10 | 1.01, 1.20 | 0.03 | |
|
| ||||||
| Tier 1 | 10.3 | 1552* | 4367.7 | 1.09 | 1.01, 1.19 | 0.04 |
| Tier 2B APP | 5.4 | 4366.8 | 1.10 | 1.01, 1.20 | 0.02 | |
|
| ||||||
| Tier 1 | 10.3 | 1561 | 4397.4 | 1.10 | 1.01, 1.19 | 0.03 |
| Tier 3 HYBRID | 5.4 | 4396.1 | 1.11 | 1.02, 1.20 | 0.01 | |
Subjects were excluded if there was a period of more than nine days between STN PM2.5 species concentration measurements for the case period or all control periods
Table 2.
Relative increase in odds of a transmural infarction associated with each IQR increase in PM2.5 concentration, by exposure Tier. Z-score method. For Tier 1, IQR refers to the interquartile range of z-scores, while for the refined exposure models (Tiers 2a, 2b, and 3), it refers to the interquartile range of the z-score difference (e.g., the difference between the Tier 1 and Tier 2a z-scores).
| Tier | IQR | N | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Tier 1 | 1.22 | 1561 | 1.11 | 1.00, 1.23 | 0.04 |
| Tier 2a SHEDS | 0.25 | 1.03 | 0.90, 1.18 | 0.65 | |
|
| |||||
| Tier 1 | 1.22 | 1552* | 1.12 | 1.02, 1.23 | 0.02 |
| Tier 2b APP | 0.21 | 1.05 | 0.97, 1.14 | 0.21 | |
|
| |||||
| Tier 1 | 1.22 | 1561 | 1.12 | 1.03, 1.22 | 0.01 |
| Tier 3 HYBRID | 0.18 | 1.05 | 0.99, 1.11 | 0.12 | |
Subjects were excluded if there was a period of more than nine days between STN PM2.5 species concentration measurements for the case period or all control periods
AER Effect Modification Analyses
As an alternative analysis, we evaluated whether modeled residential AERs in the 24 hour period immediately before emergency department arrival modified our estimate of the relative odds of a transmural MI associated with each 10.3 μg/m3 (IQR) increase in the Tier 1 PM2.5 concentration. MIs were evenly distributed between the warm (May to October) and cool (November to April) seasons. Summary statistics of the AER distributions for the warm and cool seasons are shown in Supplemental Information, Table 2.
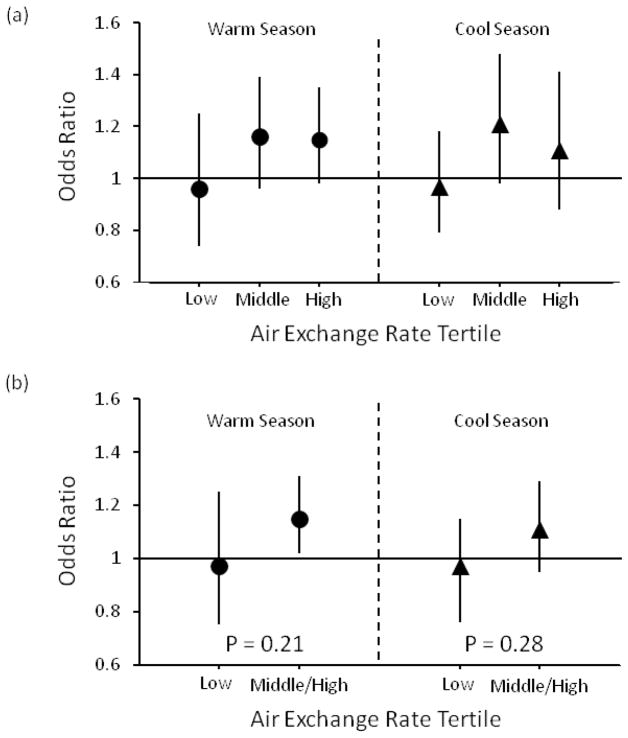
We observed heterogeneity in the relative odds of transmural MI across AER tertiles, with homes in higher AER tertiles having larger ORs than homes in the low AER tertile. In the warm season, each 10.3 μg/m3 increase in the Tier 1 PM2.5 concentration was associated with increased relative odds of a transmural MI in the middle AER tertile (OR = 1.16, 95% CI = 0.96, 1.39) and high AER tertile (OR = 1.15, 95% CI = 0.98, 1.35), but not the low AER tertile (OR = 0.96, 95% CI = 0.74, 1.25) (Figure 1). When we combined the middle and high AER tertiles and re-ran the model, each 10.3 μg/m3 increase in Tier 1 PM2.5 concentration was associated with a significant increase in the relative odds of a transmural MI for the middle and high AER tertiles, combined (OR = 1.15, 95% CI = 1.02, 1.31). Similarly, in the cool season, we observed an increase in the relative odds of a transmural MI associated with each 10.3 μg/m3 increase in Tier 1 concentrations for the middle and high AER tertiles (both individually and combined), but not for the low AER tertile (Figure 1).
Figure 1.
Relative odds of transmural infarction associated with each interquartile range increase in Tier 1 (central-site) PM2.5 concentration, stratified by air exchange rate tertile for (a) low, middle, and high AER tertiles and (b) for low and middle/high AER tertiles combined
To further explore the apparent effect-modification by AER, we assessed whether AER was actually a surrogate for another spatially-varying factor that might explain the observed variability in relative odds of transmural MI across AER tertiles. First, we evaluated the distribution of monitoring sites to which MI patients were assigned within each AER tertile and conducted a case-crossover analysis stratified by monitoring-site community. In the low AER tertile, the majority of study subjects were residents of the New Brunswick monitoring-site community. In the high AER tertile, the majority of subjects were residents of the Elizabeth monitoring-site community (Supplemental Information, Table 4). Each monitor-specific IQR increase in ambient PM2.5 concentration was associated with a non-statistically-significant increase in the relative odds of transmural MI in both New Brunswick (OR = 1.15, 95% CI = 0.95, 1.39) and Elizabeth (OR = 1.11, 95% CI = 0.97, 1.27; Table 3). For the other 5 monitors, ORs ranged from 0.78 in Millville to 1.23 in Rahway. However, given the sample sizes, ORs, and 95% confidence intervals within each monitoring location (Table 3), there is no clear difference in the relative odds of transmural MI associated with each IQR increase in PM2.5 concentration across monitors.
Table 3.
Relative odds of transmural infarction associated with each interquartile range increase in PM2.5 concentration, stratified by monitoring-site, in order of increasing median air exchange rate
| Monitor location | Median Air Exchange Rate (h−1) | IQR (μg/m3) | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Flemington | 0.32 | 8.9 | 0.98 | 0.40, 2.39 | 0.96 |
| New Brunswick | 0.41 | 8.4 | 1.15 | 0.95, 1.39 | 0.15 |
| Camden | 0.50 | 10.3 | 1.04 | 0.86, 1.25 | 0.68 |
| Millville | 0.50 | 9.5 | 0.78 | 0.47, 1.30 | 0.34 |
| Rahway | 0.52 | 9.3 | 1.23 | 0.87, 1.74 | 0.24 |
| Elizabeth | 0.60 | 11.7 | 1.11 | 0.97, 1.27 | 0.13 |
| Jersey City | 0.66 | 12.2 | 1.17 | 0.86, 1.59 | 0.32 |
DISCUSSION
In this case-crossover study of transmural myocardial infarction, use of refined surrogates of personal exposure to PM2.5 of outdoor origin that account for the exposure-modifying effects of human activity patterns and/or the indoor transport of ambient PM2.5, did not result in larger estimates of the relative odds of a transmural infarction associated with each IQR increase in PM2.5 concentration in the previous 24 hours, smaller confidence intervals, nor better model fits compared to analyses that used PM2.5 concentrations measured at central-site monitors. However, we did find effect modification of this relative odds estimate by estimated residential AER. This may be attributable to a greater degree of exposure error and resulting bias towards the null in the low AER tertile (less penetration of ambient PM indoors, and therefore more error in estimating one’s personal exposure to PM2.5 of outdoor origin) compared to the higher AER tertiles (more penetration of ambient PM indoors and therefore less exposure error), or residual confounding by some unmeasured factor.
Spatial variability, time activity, and losses with outdoor-to-indoor transport are all sources of exposure error in epidemiologic analyses that use central site monitor concentrations as surrogates for exposure to ambient (outdoor-generated) PM2.5. Several recent studies have reported larger effect estimates and/or smaller confidence intervals when exposures were estimated using models that account for spatial variability in outdoor air pollutant concentrations on local scales (e.g. interpolation methods, land use regression) in place of concentrations measured at a single monitor or averaged over all monitors in a region.e.g. 3–6 However, none have directly compared central-site PM2.5 with models accounting for human activity patterns and the indoor transport of ambient PM2.5 in a large epidemiologic study. Ebelt et al.37 estimated individual-level ambient PM2.5 exposure in a panel study of 16 subjects using individual-level time-activity diaries (to estimate time spent indoors) and indoor PM2.5 concentrations estimated using a mass balance model. Associations between cardiopulmonary outcomes (e.g., heart rate variability, forced expiration volume) and ambient PM2.5 exposure were calculated with this exposure metric, as well as ambient PM2.5 concentrations measured at central-site monitors. Contrary to our findings, the Ebelt et al.37 analyses that used individual-level information to model ambient PM2.5 exposures resulted in larger health effect estimates and smaller confidence intervals compared to the analyses that used central-site ambient PM2.5 concentrations.
Multiple factors could have contributed to the differences between our findings and those of Ebelt et al.37 One possibility is that many of the factors that are accounted for in the refined exposure estimates would not be expected to contribute to exposure error (or bias) in the case-crossover design. Because cases serve as their own controls in this design, factors that differ across subjects, but are largely constant within subjects (e.g., proximity to local PM2.5 sources, and differences in AERs or particle losses that stem from differences in housing stock, air conditioning prevalence or human activity patterns) would be expected to have a minimal impact on effect estimates. Similarly, with the case-control period confined to one calendar month, any factors that vary on time-scales longer than a month (e.g., seasonal variability in AER driven by indoor-outdoor temperature differences, natural ventilation, or air conditioning use) would be expected to have little or no effect on the relative odds estimates. Control periods are also matched to case periods by weekday, calendar month, and hour of the day, likely reducing the influence of much of the within-subject variability in human activity patterns occurring on these time-scales. Although not directly evaluated in this study, time-series analyses, which are also temporal contrasts of daily pollutant concentrations and daily counts of health outcomes, may also be only minimally impacted by these factors.
In addition, the refined exposure estimates used in Ebelt et al.37 were based on subject- level time-activity diaries and home-specific penetration and persistence of ambient PM2.5, while here, human activities and the indoor transport of ambient PM2.5 were modeled using census-tract level data and were then averaged over the area within a 10 km radius of each central-site monitor. For example, human activity patterns simulated with SHEDS for Tier 2a exposure estimates were estimated based on census-tract level demographic data. Similarly, modeled AER distributions for each census tract were used in the calculation of Tier 2b APP and Tier 3 exposure surrogates, rather than individual-level AERs. Further, species mass fractions were not available in every monitoring area and were estimated as the mass fractions measured at the New Brunswick monitor, which was most highly correlated with the other monitors across the state. These were used with local mass concentrations. The spatial resolution of data used to calculate the refined exposure estimates is a limitation of this study. Uncertainty resulting from these limitations could have contributed to exposure error in the refined exposure surrogates and, thus, the potential benefits of the refined exposure surrogates may not have been fully realized.38 However, when we estimated exposure at the zip-code level, rather than averaging over 10 km (Supplemental Information, Table 5), we observed no increase in ORs, reduction in 95% CIs, nor improved model fits. The potential for uncertainty due to averaging and the associated exposure error and bias was likely reduced in the “AER Effect Modification” analyses because we focused on a single parameter, requiring fewer assumptions and, thus, reduced possibility of compounding of exposure prediction errors. It is possible that simpler methods to account for variability in exposure to PM2.5 of outdoor origin resulting mostly from variability in the indoor transport of ambient PM2.5 (e.g., including AER as an interaction term in the conditional logistic regression model) may more accurately capture variability in effect than these more complicated exposure models, which could be subject to greater uncertainty.
The differences in the results of our “Modeled Tiered Exposure” and “AER Effect Modification” analyses may also be explained, in part, by differences in study design. In the tiered exposure analysis, we essentially compared the relative odds of transmural MI within different time periods during each subject’s person-time. Therefore, non-time varying confounders such as subject characteristics (age, health history, etc.), residential location (and any potential differences in the pollutant mixture due to different pollution sources, source proximity), and housing characteristics (leakage) were controlled by design. In the effect modification analysis, each relative odds estimate within each AER tertile also has this feature. However, when we then contrast these AER-tertile-specific relative odds estimates, we are comparing different subjects with their inherent differences in these characteristics. Thus, these characteristics may now act as confounders in this analysis. As a result, differences in these AER-tertile-specific relative odds estimates could be due, in part, to differences in AER, as well as differences in subject characteristics (e.g. age, co-morbidity, proximity to sources, housing stock, access to healthcare, smoking status, etc.) if those characteristics are covariant with AER. For example, low socio-economic status (SES) has been identified as a predictor of susceptibility to negative health outcomes associated with PM exposure.39 Further, low income residents tend to live in homes with higher AERs and, therefore, are exposed to a larger fraction of ambient PM2.5 (and smaller fraction of indoor emissions) than residents with higher SES (see also Sarnat JA et al.40 and Sarnat SE et al.41 in this issue of the Journal). In fact, because SES is a predictor of AER, poverty status is included in the residential AER model (see Supplemental Information).31 Thus, it is conceivable that our results showing effect modification of the PM2.5- MI association by AER could actually reflect effect modification by SES or a combination of AER and SES. It is also possible that higher AERs, in addition to access to health care and other factors, help to explain the associations between low SES and adverse health outcomes observed in previous studies. Notably, we did not observe differences in age, gender, race/ethnicity, and co-morbidities by AER tertile (Supplemental Information, Tables 6 and 7). Further, if location- specific factors other than AER were contributing to our findings, we would expect an increased relative odds of transmural MI in the monitoring-site community in which the majority of subjects were assigned to the high-AER tertile (i.e., Elizabeth) and a smaller effect estimate in communities in which the majority subjects were assigned to the low-AER tertile (i.e. New Brunswick). Instead, we observed larger relative odds of transmural MI in New Brunswick compared to Elizabeth (Table 3), which suggests that the observed effect modification is related to variability in AER.
The modification of MI risk by community-average AER is consistent with the results of previous studies that found that percent increases in short-term mortality associated with given increases in outdoor ozone and PM10 concentrations were larger for cities with higher annual average AERs compared to those with smaller AERs.42,43 Previous studies have also shown that home-ventilation conditions (e.g., infiltration through cracks in the building shell, air flow through open windows) and activities that affect particle losses indoors (e.g., AC use) impact ambient PM2.5 exposures.e.g.9,44 Sarnat et al.44 concluded that ambient monitors were good surrogates for exposure in well-ventilated homes, but were poor exposure surrogates in homes with windows and doors closed. Our results are also consistent with studies that have demonstrated a reduced risk of morbidity and mortality with increased prevalence of central AC.12–16 As noted above, F tends to be lower for homes with central AC in use due to increased particle losses in AC filters.17–19 Further, AERs tend to be lower for homes with AC in use compared to those with open windows,45 which also contributes to lower F values.
CONCLUSIONS
Use of refined exposure surrogates that account for human activity patterns and/or the indoor transport of ambient PM2.5 in this case-crossover study did not result in larger health effect estimates, narrower confidence intervals, or better model fits compared to the analyses that used central-site PM2.5 concentrations to estimate PM2.5 exposure. For the level of exposure- estimate-refinement considered here, our findings add support to the use of central-site PM2.5 concentrations for epidemiological studies that employ similar case-crossover study designs and other similar temporal analytic methods. These findings also illustrate that variability in factors that influence the fraction of ambient PM2.5 in indoor air (e.g., AER) can bias health effects estimates in study designs for which a spatio-temporal comparison of exposure effects across subjects is conducted.
Supplementary Material
Acknowledgments
This research was funded, in part, by the U.S. Environmental Protection Agency (Cooperative Agreement CR-83407201-0), NIEHS-sponsored UMDNJ Center for Environmental Exposures and Disease (NIEHS P30ES005022), and the New Jersey Agricultural Experiment Station. Natasha Hodas was supported by a Graduate Assistance in Areas of National Need Fellowship and an EPA STAR Fellowship. Although this work was reviewed by EPA and approved for publication, it may not necessarily reflect official Agency policy.
References
- 1.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 2.Rich DQ, Kipen HM, Zhang J, Kamat L, Wilson AC, Kostis JB. Triggering of transmural infarctions, but not non-transmural infarctions, by ambient fine particles. Environ Health Perspect. 2010;118:1229–1235. doi: 10.1289/ehp.0901624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouliou T, Kanaroglou PS, Elliott SJ, Penegelly D. Assessing the health impacts of air pollution: a re-analysis of the Hamilton children’s cohort data using a spatial analytic approach. Int J Environ Heal R. 2008;18:17–35. doi: 10.1080/09603120701844290. [DOI] [PubMed] [Google Scholar]
- 4.Kim SY, Sheppard L, Kim H. Health effects of long-term air pollution: influence of exposure prediction methods. Epidemol. 2009;20:442–450. doi: 10.1097/EDE.0b013e31819e4331. [DOI] [PubMed] [Google Scholar]
- 5.Sahsuvaroglu T, Jerrett M, Sears MR, McConnell R, Finkelstein N, Arain A, et al. Spatial analysis of air pollution and childhood asthma in Hamilton, Canada: comparing exposure methods in sensitive subgroups. Environ Health. 2009 doi: 10.1186/1476-069X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son JY, Bell ML, Lee JT. Individual exposure to air pollution and lung function in Korea: spatial analysis using multiple exposure approaches. Environ Res. 2010;110:739–749. doi: 10.1016/j.envres.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Sarnat SE, Coull BA, Ruiz PA, Koutrakis P, Suh HH. The influences of ambient particle composition and size on particle infiltration in Los Angeles, CA, Residences. J Air Waste Manag Assoc. 2006;56:186–196. doi: 10.1080/10473289.2006.10464449. [DOI] [PubMed] [Google Scholar]
- 8.Hering SV, Lunden MM, Thatcher TL, Kirchstetter TW, Brown NJ. Using regional data and building leakage to assess indoor concentrations of particles of outdoor origin. Aerosol Sci Tech. 2007;41:639–654. [Google Scholar]
- 9.Meng QY, Spector D, Colome S, Turpin BJ. Determinants of indoor exposure to PM2.5 of indoor and outdoor origin during the RIOPA study. Atmospheric Environ. 2009;43:5750–5758. doi: 10.1016/j.atmosenv.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateson TF, Coull BA, Hubbell B, Kazuhiko I, Jerrett M, Lumley T, et al. Panel discussion review: session 3 – issue involved in interpretation of epidemiological analyses – statistical modeling. J Expo Anal Environ Epidemiol. 2007;17:S90–S96. doi: 10.1038/sj.jes.7500631. [DOI] [PubMed] [Google Scholar]
- 12.Janssen NA, Schwartz HJ, Zanobetti A, Suh HH. Air conditioning and source-specific particles as modifiers of the effect of PM10 on hospital admissions for heart and lung disease. Environ Health Perspect. 2002;110:43–49. doi: 10.1289/ehp.0211043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeka A, Zanobetti A, Schwartz J. Short-term effects of particulate matter on cause specific mortality: effects of lags and modification by city-specific characteristics. Occup Environ Med. 2005;62:718–725. doi: 10.1136/oem.2004.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17:279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 15.Bell ML, Ebisu K, Peng RD, Dominici F. Adverse health effects of particulate air pollution: modification by air conditioning. Epidemiol. 2009;19:680–689. doi: 10.1097/EDE.0b013e3181aba749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter LK, Özkaynak H, Franklin M, Schultz BD, Neas LM. The Use of Improved Exposure Factors in the Interpretation of Fine Particulate Matter Epidemiological Results. Air Qual Atmos Health. 2013;6:195–204. [Google Scholar]
- 17.Thornburg JW, Ensor DS, Rodes CE, Lawless PA, Sparks LE, Mosely RB. Penetration of particles into buildings and associated physical factors, Part I: model development and computer simulations. Aerosol Sci Tech. 2001;34:284–296. [Google Scholar]
- 18.Waring MS, Siegel JA. Particle loading rates for HVAC filters, heat exchangers, and ducts. Indoor Air. 2008;18:209–224. doi: 10.1111/j.1600-0668.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 19.Stephens B, Siegel JA. Comparison of test methods for determining the particle removal efficiency of filters in residential and light-commercial central HVAC systems. Aerosol Sci Tech. 2012;46:504–513. [Google Scholar]
- 20.Hodas N, Lunden M, Meng QY, Baxter L, Özkaynak H, Burke J, et al. Variability in the fraction of ambient fine particulate matter found indoors and heterogeneity in health effect estimates. J Expo Sci Environ Epidemiol. 2012;22:448– 454. doi: 10.1038/jes.2012.34. [DOI] [PubMed] [Google Scholar]
- 21.Baxter L, Burke J, Lunden M, Turpin BJ, Rich DQ, Thevenet-Morrison K, et al. Influence of human activity patterns and residential air exchange rates on modeled distributions of PM2.5 exposure compared to central-site monitoring data. J Expo Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2012.118. e-pub ahead of print 16 January 2013. [DOI] [PubMed] [Google Scholar]
- 22.Kostis JB, Wilson AC, O’Dowd K, Gregory P, Chelton S, Cosgrove NM, et al. Sex differences in the management and long-term outcome of acute myocardial infarction. A statewide study. MIDAS Study Group. Myocardial Infarction Data Acquisition System. Circulation. 1994;90:1715–1730. doi: 10.1161/01.cir.90.4.1715. [DOI] [PubMed] [Google Scholar]
- 23.Kostis JB, Wilson AC, Lacy CR, Cosgrove NM, Ranjan R, Lawrence-Nelson J. Time trends in the occurrence and outcome of acute myocardial infarction and coronary heart disease death between 1986 and 1996 (a New Jersey statewide study) Am J Cardiol. 2001;88:837–841. doi: 10.1016/s0002-9149(01)01888-4. [DOI] [PubMed] [Google Scholar]
- 24.US EPA. [accessed 19 July 2010];Technology Transfer Network (TTN)—AQS Datamart. 2008 Available: http://www.epa.gov/ttn/airs/aqsdatamart/index.htm.
- 25.Burke JM, Zufall MJ, Özkaynak H. A population exposure model for particulate matter: Case study results for PM2.5 in Philadelphia. PA J Expo Anal Environ Epidemiol. 2001;11:470–489. doi: 10.1038/sj.jea.7500188. [DOI] [PubMed] [Google Scholar]
- 26.McCurdy T, Glen G, Smith L, Lakkadi Y. The National Exposure Research Laboratory’s Consolidated Human Activity Database. J Expo Anal Environ Epidemiol. 2000;10:1–13. doi: 10.1038/sj.jea.7500114. [DOI] [PubMed] [Google Scholar]
- 27.Lunden MM, Thatcher TL, Hering SV, Brown NJ. The use of time- and chemically-resolved particulate data to characterize the infiltration of outdoor PM2. 5 into a residence in the San Joaquin Valley. Environ Sci Technol. 2003;37:4724–4732. doi: 10.1021/es026387i. [DOI] [PubMed] [Google Scholar]
- 28.Lunden MM, Revzan KL, Fischer ML, Thatcher TL, Littlejohn D, Hering SC, et al. The transformation of outdoor ammonium nitrate aerosols in the indoor environment. Atmospheric Environ. 2003;37:5633–5644. [Google Scholar]
- 29.Sherman MH, Grimsrud DT. Lawrence Berkeley National Laboratory Report. LBNL- 10852. Berkeley, CA: 1980. Measurement of infiltration using fan pressurization and weather data. [Google Scholar]
- 30.Sherman MH, Dickerhoff DJ. Airtightness of US dwellings. ASHRAE Transactions. 1998;104:1359–1367. [Google Scholar]
- 31.Chan WR, Nazaroff WW, Price PN, Sohn MD, Gadgil AJ. Analyzing a database of residential air leakage in the United States. Atmospheric Environ. 2005;39:3445–3455. [Google Scholar]
- 32.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiol. 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Steadman RG. The assessment of sultriness. Part II: effects of wind, extra radiation and barometric pressure on apparent temperature. J Appl Meteorol. 1979;18:874–885. [Google Scholar]
- 35.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuersuwan N, Turpin BJ. Evaluation of time resolved PM2. 5 data in urban/suburban areas of New Jersey. J Air Waste Manag Assoc. 2000;50:1780–1789. doi: 10.1080/10473289.2000.10464214. [DOI] [PubMed] [Google Scholar]
- 37.Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: a comparison of health effects. Epidemiol. 2005;16:296–405. doi: 10.1097/01.ede.0000158918.57071.3e. [DOI] [PubMed] [Google Scholar]
- 38.Szpiro AA, Paciorek CJ, Sheppard L. Does more accurate exposure prediction necessarily improve health effect estimates? Epidemiol. 2011;22:680–685. doi: 10.1097/EDE.0b013e3182254cc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119:446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarnat JA, Sarnat SE, Chang H, Mulholland J, Özkaynak H, Isakov V. Spatiotemporally-resolved air exchange rate as a modifier of acute air pollution related morbidity. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2013.32. (in press) [DOI] [PubMed] [Google Scholar]
- 41.Sarnat SE, Sarnat JA, Mulholland J, Isakov V, Özkaynak H, Chang H, et al. Application of alternative spatiotemporal metrics of ambient air pollution exposure in a time-series epidemiological study in Atlanta. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2013.41. (submitted) [DOI] [PubMed] [Google Scholar]
- 42.Chen C, Zhao B, Weschler CJ. Assessing the influence of indoor exposure to “outdoor ozone” on the relationship between ozone and short-term mortality in U.S. communities. Environ Health Perspect. 2012;120:235–240. doi: 10.1289/ehp.1103970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Zhao B, Weschler CJ. Indoor exposure to “outdoor PM10”: assessing its influence on the relationship between PM10 and short-term mortality in U.S. cities. Epidemiol. 2012;23:870–878. doi: 10.1097/EDE.0b013e31826b800e. [DOI] [PubMed] [Google Scholar]
- 44.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J Air Waste Manag Assoc. 2000;50:1184–1198. doi: 10.1080/10473289.2000.10464165. [DOI] [PubMed] [Google Scholar]
- 45.Breen MS, Breen M, Williams RW, Schultz BD. Predicting residential air exchange rates from questionnaires and meteorology: model evaluation in central North Carolina. Environ Sci Technol. 2010;44:9349–9356. doi: 10.1021/es101800k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.