Abstract
In the renal proximal tubule, the activities of the basolateral Na+/HCO3– cotransporter (NBC) and the apical Na+/H+ exchanger (NHE3) uniformly vary in parallel, suggesting that they are coordinately regulated. PKA-mediated inhibition of NHE3 is mediated by a PDZ motif–containing protein, the Na+/H+ exchanger regulatory factor (NHE-RF). Given the common inhibition of these transporters after protein kinase A (PKA) activation, we sought to determine whether NHE-RF also plays a role in PKA-regulated NBC activity. Renal cortex immunoblot analysis using anti-peptide antibodies directed against rabbit NHE-RF demonstrated the presence of this regulatory factor in both brush-border membranes (BBMs) and basolateral membranes (BLMs). Using a reconstitution assay, we found that limited trypsin digestion of detergent solubilized rabbit renal BLM preparations resulted in NBC activity that was unaffected by PKA activation. Co-reconstitution of these trypsinized preparations with a recombinant protein corresponding to wild-type rabbit NHE-RF restored the inhibitory effect of PKA on NBC activity in a concentration-dependent manner. NBC activity was inhibited 60% by 10–8M NHE-RF; this effect was not observed in the absence of PKA. Reconstitution with heat-denatured NHE-RF also failed to attenuate NBC activity. To establish further a physiologic role for NHE-RF in NBC regulation, the renal epithelial cell line B-SC-1, which lacks detectable endogenous NHE-RF expression, was engineered to express stably an NHE-RF transgene. NHE-RF–expressing B-SC-1 cells (B-SC-RF) exhibited markedly lower basal levels of NBC activity than did wild-type controls. Inhibition of NBC activity in B-SC-RF cells was enhanced after 10 μM of forskolin treatment, consistent with a postulated role for NHE-RF in mediating the inhibition of NBC activity by PKA. These findings not only suggest NHE-RF involvement in PKA-regulated NBC activity, but also provide a unique molecular mechanism whereby basolateral NBC and apical NHE3 activities may be coordinately regulated in renal proximal tubule cells.
J. Clin. Invest. 104:195–201 (1999).
Introduction
The renal proximal tubule reabsorbs more than 80% of the filtered bicarbonate load. The principal transport systems (1–3) responsible for transmembrane movement of hydrogen and bicarbonate ions in the proximal tubule cells are the apical Na+/H– exchanger type 3 isoform (NHE3) and the basolateral Na+/HCO3– cotransporter (NBC). It might be predicted that systemic and proximal tubule intracellular acid-base homeostasis requires that NHE3 and NBC function in a coordinate fashion. The uniformly parallel regulation of both transporters under a variety of physiologic conditions is consistent with such a hypothesis. NBC activity is enhanced by metabolic acidosis and inhibited by metabolic alkalosis (4). Similarly, chronic hypercapnia increases NBC activity, whereas chronic respiratory alkalosis has the opposite effect (5). NBC activity is also modulated by angiotensin II and parathyroid hormone (6, 7). Under identical conditions, NHE3 activity varies in parallel, suggesting that the activities of the 2 systems may be coordinately regulated (4). The parallel regulation of these transporters has also recently been extended to the level of specific regulatory protein kinases (8, 9). Phosphorylation of brush-border membrane (BBM) and basolateral membrane (BLM) proteins by either protein kinase A (PKA) or calcium calmodulin multifunction protein kinase II (Ca-CAMK II) is inhibitory for both NHE and NBC activities in the respective membranes, whereas protein kinase C (PKC) is stimulatory (8).
PKA inhibition of NHE3 activity requires the participation of a peptide cofactor, the recently cloned Na+/H– exchanger regulatory factor (NHE-RF) (10). The corresponding mechanism of PKA-mediated inhibition of basolateral NBC activity, however, is not known. An examination of the primary sequences of the recently cloned amphibian and human NBC cDNAs reveals conserved COOH-terminal sequences homologous with known PKA phosphorylation sites (2, 3), possibly suggesting that PKA regulation may involve direct phosphorylation of the transporter, as has been shown for NHE3 (11, 12). This has not yet been demonstrated for the NBC and thus requires further investigation. However, we have previously shown that limited trypsin digestion of solubilized renal BLMs is sufficient to dissociate NBC activity from its regulation by PKA (13). Co-reconstitution experiments have further suggested that a regulatory protein analogous to NHE-RF is necessary for NBC regulation by PKA (13). Taken together, these findings suggest a role for NHE-RF in PKA regulation of NBC activity. We therefore sought to examine this possibility, and demonstrate herein that NHE-RF mediates, in large part, the inhibition of NBC by PKA. Our findings suggest a common molecular mechanism for coordinate regulation of NBC and NHE3 activities in renal proximal tubule cells. They also provide the first evidence of a regulatory role for NHE-RF outside the context of NHE3.
Methods
Preparation of membrane vesicles and proteoliposomes.
BLMs and BBMs were prepared from rabbit kidney cortex as described previously (8, 13, 14). BBM purity was assessed by enrichment of alkaline phosphatase activity, and specific activity was typically 10- to 14-fold greater than that of homogenates. BLM preparations were similarly 12- to 15-fold increased in Na+/K+/ATPase content, without a corresponding increase in the specific activities of luminal membranes markers (e.g., alkaline phosphatase and γ-glutamyl transferase). BLM and BBM proteins were solubilized and reconstituted into proteoliposomes as described (13, 15, 16). In brief, liposomal reconstitution of solubilized proteins (2.5 mg/mL) was accomplished by mixing 1.6 part protein (vol/vol) with 1.0 part of L-α phosphatidylcholine (35 mg/mL) and sonicating at maximal output and 4°C for 10 minutes. This mixture was then dialyzed for 18 hours against 50 mM HEPES plus 250 mM mannitol (pH 7.2) using a 6- to 8-kDa molecular exclusion cutoff. The resulting proteoliposomes were used for uptake studies, and reconstituted protein content was maintained constant at 5 μg per assay.
Limited membrane proteolysis.
Solubilized BLM protein preparations (2.5 mg/mL) were incubated with 100 μg/mL trypsin (Sigma Chemical Co., St. Louis, Missouri, USA) when appropriate, as described by Weinman et al. (16). After digestion for 15 minutes at 37°C, proteolysis of solubilized protein was stopped by the addition of a 10-fold molar excess of soybean trypsin inhibitor (Sigma Chemical Co.). Studies on the effect of PKA and trypsin were accomplished using trypsin cross-linked to agarose (Sigma Chemical Co.); digestion was stopped by 50-μM filtration to remove immobilized protease activity.
Assay of Na+/HCO3– cotransporter activity in proteoliposomes.
Initial rates of bicarbonate-dependent 22Na uptake were used to measure maximal NBC activity in proteoliposomes and were measured at 3 seconds by the rapid-filtration technique, as described previously and outlined later here (13, 15). After preincubation at room temperature for 1–2 hours in 200 mM sucrose, 1 mM gluconate, and 50 mM Tris-buffered HEPES (pH 7.5), proteoliposomes were pelleted at 30,000 g for 30 minutes at 4°C before resuspension in the same solution. Assays were then initiated at room temperature by the addition of 40 mM sodium gluconate, 60 mM potassium gluconate, 1 mM magnesium gluconate, and 50 mM HEPES (pH 7.5) containing 1 μCi 22Na in either the presence or absence of 25 mM NaHCO3 (HCO3–-free uptake medium was prepared by equimolar substitution of sodium gluconate). Uptakes were stopped after 3 seconds by the addition of 4 mL of ice-cold stop solution (200 mM sucrose and 50 mM Tris-buffered HEPES [pH 7.5]), followed by harvesting of a prewetted 0.45-μm Millipore filter (Millipore Corp., Bedford, Massachusetts, USA). Proteoliposomes were washed 3 additional times with ice-cold stop solution, and radioactivity was measured by liquid scintillation counting. NBC activity was taken as the initial rate of HCO3–-dependent 22Na uptake and was measured as the difference in 22Na uptake measured in both the presence and absence of an inwardly directed HCO3– gradient. To test the ability of recombinant NHE-RF to reconstitute PKA regulation of NBC activity in proteoliposomes prepared from trypsinized membrane preparations, artificial vesicles were incubated with 50 μM ATP and 100 μM MgCl2 (pH 7.4) in the presence of the catalytic subunit of PKA (40 mU/mL for 15 minutes at 30°C) before reconstitution into proteoliposomes (16). Dose dependency was assayed by co-reconstitution of 10–8 to 10–18 M recombinant NHE-RF with solubilized BLM proteins in the presence of PKA. Specificity was ensured by evaluating heat-denatured NHE-RF in parallel.
Measurement of NBC activity in cells.
NBC activity was monitored as specific changes in intracellular pH (pHi) using a fluorometric assay described previously (17). Briefly, pHi was continuously monitored using the pH-sensitive chemical fluorophore BCECF [1,2,7 biscarboxymethyl-5-(6)-carboxyfluorescein] as its caged acetoxymethyl ester, BCECF-AM. Confluent cell monolayers were loaded with 15 μM BCECF-AM at 37°C for 30 minutes. Cells grown on coverslips were analyzed at 37°C using a PTI RatioMaster dual excitation spectrofluorometer system (Photon Technology International, Monmoth Junction, New Jersey, USA) equipped with Felix version 1.1 software for fluorescence analysis (Photon Technology International, South Brunswick, New Jersey, USA). Changes in pHi were monitored as the ratio of fluorescence emission intensities resulting from dual excitation at pH-sensitive (500 nm) and pH-insensitive (430 nm) wavelengths. Calibration of BCECF fluorescence with pHi was routinely performed in the presence of the ionophore nigericin and 140 mM K+ after each experiment. The effect of forskolin stimulation was also evaluated in both B-SC-1 and B-SC-RF cells after incubation with either 10 μM of forskolin (18) or vehicle alone for 30 minutes at 37°C.
NHE-RF immunoblot analysis.
Immunoblot analysis was performed according to the method of Towbin et al. (19) with the following modifications. In brief, solubilized proteins from purified BLM and BBM preparations were separated by SDS-PAGE before transfer to nitrocellulose membranes for immunoblotting. Membranes were blocked by incubating in Tris-buffered saline supplemented with 0.1% Tween-20 (TTBS) and 5% (wt/vol) dry milk for 6 hours at 4°C before incubation with affinity-purified polyclonal anti-peptide antibody directed against amino acid residues 2–10 at the NH2-terminus of rabbit NHE-RF (10). After thorough washing with PBS (pH 7.5), specific peptide bands were viewed using a commercially available enhanced chemiluminescence system (ECL; Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) according to the manufacturer’s instruction. Molecular weight determination was done using the Ambis system and software (Ambis Corp. San Diego, California, USA).
Generation of cultured B-SC-1 cells stably expressing the NHE-RF transgene.
A 1.9-kb HindIII-PstI DNA fragment containing the full-length rabbit NHE-RF cDNA was excised from the original pBluescript cloning vector (10) and was inserted into the HindIII-PstI multiple cloning site of pEGFP-N1 (CLONTECH Laboratories Inc., Palo Alto, California, USA). Subsequent excision of the PstI-NotI fragment containing the downstream EGFP reporter gene resulted in an NHE-RF mammalian expression plasmid (pCMV-NHE-RF) under the control of a human cytomegalovirus (CMV) immediate-early promoter and enhancer. Plasmids used for transfection were routinely double purified by sequential anion exchange chromatography (QIAGEN Inc., Valencia, California, USA) and isopycnic banding in a CsCl density gradient. Transient gene transfer was accomplished using Lipofectamine (GIBCO BRL, Gaithersburg, Maryland, USA) according to the manufacturer’s recommendations. Stable transfectants were then selected by G418 resistance (400 μg/mL) conferred by a coexpressed neoR gene in the parent vector. After clonal selection by limited dilution, stable transfectants were routinely maintained in normal growth medium supplemented with 200 μg/mL G418.
Northern blot analysis.
Isolation of poly(A)+ RNA was performed using a Poly(A) Pure Kit (Ambion Inc., Austin, Texas, USA) according to the manufacturer’s recommendations. Individual mRNA species were resolved by denaturing agarose gel electrophoresis of poly(A)+ RNA before Northern transfer to nitrocellulose. Membranes were then probed with random-primed digoxigenin-labeled NHE-RF cDNA probes using a DIG/Genius DNA labeling and detection system (Boehringer Mannheim Biochemicals Inc., Indianapolis, Indiana, USA).
Analysis of results.
Results of the experiments are presented as mean ± SEM. The Student’s t test for unpaired or paired data was used to analyze results whenever appropriate. The analysis of data was done using the Epistat software program (Math Archives, Round Rock, Texas, USA). The Western immunoblot and Northern blots shown were representative examples of at least 3 experiments. The blots were scanned, and molecular weight was determined using the Ambis system and software program.
Results
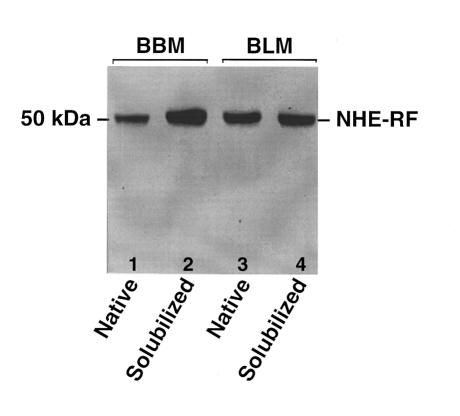
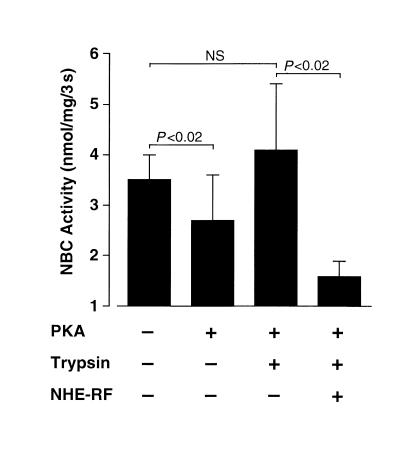
Immunoblot analysis confirmed the presence of endogenous NHE-RF in BLM preparations of normal rabbit kidney. As shown in Figure 1, polyclonal NHE-RF antibody reproducibly hybridized to a single polypeptide of appropriate size (∼50 kDa) in BLM and BBM preparations. To determine the specific role of NHE-RF in the regulation of basolateral NBC activity, the effect of recombinant NHE-RF on NBC activity was examined using solubilized rabbit renal BLM proteins reconstituted into artificial proteoliposomes. In control proteoliposomes, incubation with the catalytic subunit of PKA reduced NBC activity from 3.5 ± 0.51 nmol/mg/3 s to 2.7 ± 0.9 nmol/mg/3 s (P < 0.02). Trypsin pretreatment of solubilized protein abolished the inhibitory effect of PKA on cotransporter activity (3.5 ± 0.51 nmol/mg/3 s vs. 4.1 ± 1.3 nmol/mg/3 s; P = ns). Co-reconstitution of recombinant NHE-RF with proteoliposomes containing trypsin-treated proteins restored PKA inhibition of NBC activity (Figure 2), with activity decreasing from 4.1 ± 1.3 nmol/mg/3 s in the absence of NHE-RF to 1.6 ± 0.32 nmol/mg/3 s in the presence of NHE-RF (P < 0.02).
Figure 1.
Western blot analysis of the native and solubilized BBMs and BLMs probed with the specific anti-peptide antibody against the NHE-RF. Highly purified native and solubilized BBMs and BLMs were separated on SDS-PAGE, blotted onto nitrocellulose, and analyzed by Western blot with anti–NHE-RF antibody. Lanes 1 and 2 are from BBMs; lanes 3 and 4 represent the BLMs. Molecular mass standards in kilodaltons are shown on the left. NHE-RF protein recognition is indicated on the right.
Figure 2.
Effects of recombinant NHE-RF protein on NBC activity in proteoliposomes. NBC activity (nmol/mg/3 s) was measured by the rapid filtration techniques as the HCO3–-dependent 22Na uptake (see Methods). NBC activity was measured in different proteoliposome preparations under different conditions as HCO3–-dependent 22Na uptake in the presence or absence of inwardly directed HCO3– gradient (NaHCO3 was replaced by sodium gluconate). Solubilized BLM proteins with (+) or without (–) limited trypsin digestion were reconstituted into proteoliposomes in the absence (–) or presence (+) of PKA. Co-reconstitution with NHE-RF is indicated as shown. The presented values are the mean ± SEM of 6 different experiments.
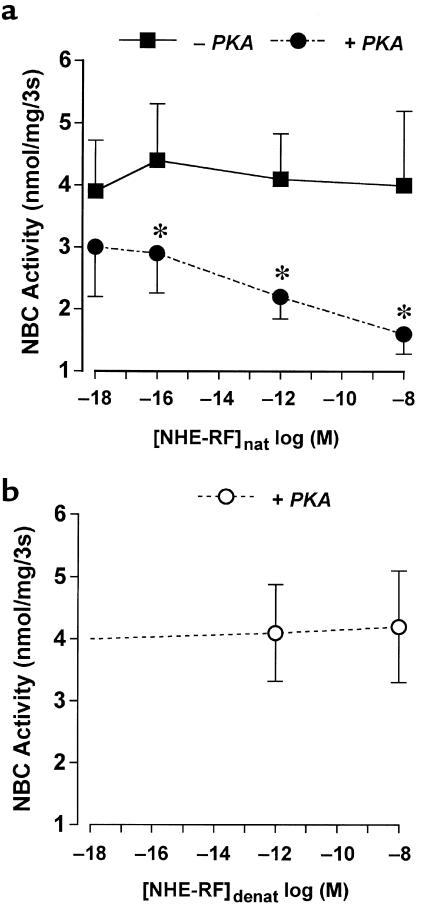
Figure 3 further demonstrates that NHE-RF restores PKA inhibition of NBC in a concentration-dependent manner. Increasing recombinant NHE-RF content in the reconstitution assay from 10–18 to 10–8 M resulted in corresponding increases in PKA-mediated inhibition of NBC activity. Heat denaturation of NHE-RF before co-reconstitution completely abrogated its effect on PKA-regulated NBC activity but had no effect on basal activity (Figure 3b). Additional studies were performed to demonstrate that the inhibition of NBC activity by NHE-RF required the presence of PKA. As shown in Figure 3a, in the absence of PKA, the inhibitory effect of NHE-RF on NBC activity was not observed.
Figure 3.
The effects of different concentrations of recombinant NHE-RF protein on NBC activity. (a) Trypsin-treated solubilized proteins reconstituted into proteoliposomes were incubated with (filled circles) or without (filled squares) catalytic subunit of PKA (PKA) in the presence of varying concentrations of recombinant nondenatured NHE-RF ([NHE-RF]nat). NBC activity (HCO3–-dependent 22Na uptake) was measured as the difference in 22Na uptake in the presence or absence of inwardly directed HCO3– gradient (NaHCO3 was replaced by sodium gluconate). The data represent the mean ± SEM of 6 independent experiments in different proteoliposome preparations. *P < 0.05 by t test. (b) The effects of different concentrations of denatured recombinant NHE-RF protein ([NHE-RF]denat) on NBC activity in the presence of catalytic subunit of PKA (PKA). Recombinant NHE-RF protein that was heat denatured was incubated with trypsin-treated solubilized BLMs in the presence of catalytic subunit of PKA (open circles). NBC activity (HCO3–-dependent 22Na uptake) was measured as the difference in 22Na uptake in the presence or absence of inwardly directed HCO3- gradient (NaHCO3 was replaced by sodium gluconate). The presented values are the mean ± SEM of 6 separate experiments in different proteoliposome preparations.
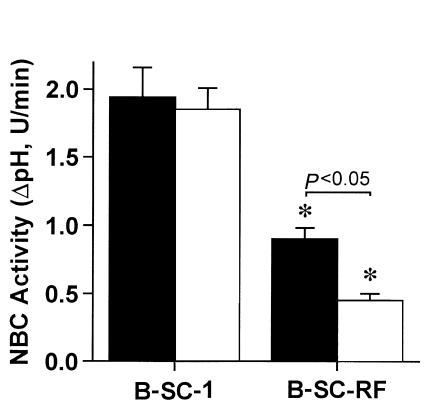
To study further the role of NHE-RF in regulation of NBC activity, the effects of NHE-RF were studied using B-SC-1 (African Green Monkey Kidney) cells stably expressing a rabbit NHE-RF transgene (B-SC-RF cells). Wild-type B-SC-1 cells exhibit endogenous NBC activity (20). However, Northern blot analysis of B-SC-1 poly(A)+ RNA failed to detect NHE-RF mRNA. In contrast, B-SC-RF cells expressed 1.8- to 1.9-kb transcripts that hybridized with NHE-RF cDNA probes at high stringency (Figure 4). Compared with wild-type cells, NBC activity in B-SC-RF cells was markedly decreased (B-SC-1 wild-type, 1.94 ± 0.22 ΔpH, U/min vs. B-SC-RF, 0.90 ± 0.08 ΔpH, U/min; P < 0.001) (Figure 5). To control for transfection, NBC activity was also determined in B-SC-1 cells transfected with β-galactosidase expression vector and in cells treated with Lipofectamine alone. NBC activity did not differ between untransfected controls (1.94 ± 0.22 ΔpH, U/min), β-galactosidase–expressing controls (1.89 ± 0.10 ΔpH, U/min), or mock-transfected cells (1.80 ± 0.14 ΔpH, U/min), suggesting that our findings are specific for heterologous NHE-RF expression and not the gene transfer procedure per se. In wild-type B-SC-1 cells, NBC activity was not altered (Figure 5) in the absence (1.94 ± 0.22 ΔpH, U/min) or presence (1.85 ± 0.16 ΔpH, U/min) of 10 μM of forskolin. Treatment of B-SC-RF cells with forskolin to stimulate PKA activity resulted in a significant inhibition of NBC activity from 0.90 ± 0.08 ΔpH, U/min in the absence of forskolin to 0.45 ± 0.05 ΔpH, U/min (P < 0.01) in the presence of the drug.
Figure 4.
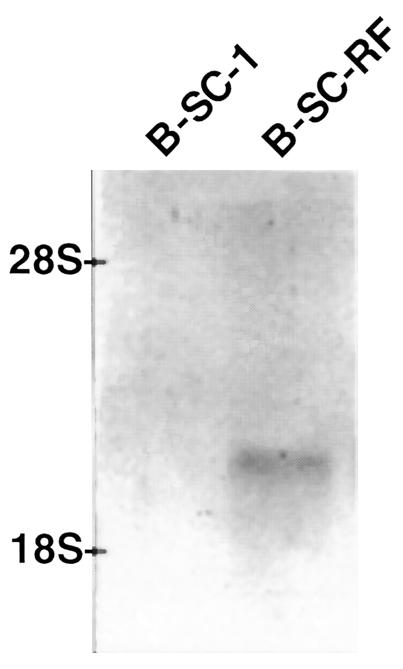
Representative Northern blot analysis of NHE-RF in B-SC-1 cells. NHE-RF expression was examined in B-SC-1 cells stably transfected with the NHE-RF cDNA (B-SC-RF) and the wild-type B-SC-1 (B-SC-1). Two micrograms of poly(A)-enriched RNA from B-SC-1 cells was electrophoresed per lane. Hybridization was performed under high-stringency conditions.
Figure 5.
Effects of NHE-RF overexpression on NBC activity in B-SC-1 cells. Wild-type B-SC-1 cells and B-SC-cells stably expressing the NHE-RF (B-SC-RF) were assayed for NBC activity (ΔpH, U/min) using a fluorometric assay with BCECF as the pH change indicator, as described in Methods. Forskolin (10 μM) was added as indicated. (filled bars = without Forskolin, open bars = with Forskolin). Data showed represent mean ± SEM of 7 different experiments.
Discussion
We have previously shown that brush-border NHE3 and basolateral NBC activities vary in parallel in a remarkably coordinate fashion under a variety of physiologic conditions. Acidosis or PKC activation uniformly stimulate both transporters, whereas PKA and Ca-CaMKII are both inhibitory. We therefore hypothesized that coordinate regulation of these important ion transporters, particularly in response to PKA activation, could be mediated by common mechanisms.
Previous studies of PKA-inhibited brush-border NHE3 activity have shown that limited trypsin digestion of solubilized BBM preparations can dissociate NHE3 activity from its regulation by PKA (9, 21). This observation ultimately led to the isolation and cloning of an obligatory cofactor for PKA inhibition of Na+/H+ exchange activity (10). This factor, NHE-RF, was the first of a number of polyvalent PDZ motif–containing regulatory proteins identified in the kidney (22, 23). PDZ domains are known to function as protein-recognition modules, similar to the Src homology domains, and have been shown to mediate specific protein-protein interactions involved in a number of cell functions including signal transduction at the plasma membranes (24, 25).
In parallel and independent studies of PKA-regulated NBC activity, it was found that limited trypsin proteolysis of renal BLM proteins also dissociated NBC activity from its regulation by PKA and that the PKA regulatory response could be restored by a dissociable peptide regulatory factor (13). These similar effects of trypsin were observed on the PKA regulatory responses of both NHE3 and NBC. Even more striking was the ability of specific membrane fractions to restore the PKA responsiveness of both transporters. Given the demonstrated role of NHE-RF in the regulation of the NHE3, it was attractive to consider the possibility that NHE-RF could function similarly in the regulation of NBC activity by PKA. Immunoblotting with specific antisera directed against the rabbit NHE-RF detected a single polypeptide of the appropriate size in both native and detergent-solubilized rabbit BLM and BBM preparations. It would therefore appear that NHE-RF is associated with both BLM and BBM in rabbit kidney cells, and the demonstration of its association with BLM provided a compelling rationale for more detailed studies of its possible role in PKA regulation of NBC.
Functional studies suggested a physiologic role for NHE-RF in NBC regulation. It is of great interest that a peptide regulatory factor known to mediate PKA inhibition of NHE3 activity at the apical membrane can also mediate similar functions at the BLM of the same cell type. We assayed NBC activity in proteoliposomes reconstituted with rabbit renal BLM proteins in both the presence and the absence of recombinant NHE-RF. In the absence of NHE-RF, proteoliposomes containing trypsinized proteins did not exhibit PKA-inhibitable NBC activity. However, co-reconstitution of these preparations with recombinant NHE-RF restored the inhibitory effect of PKA. NHE-RF affected the response to PKA in a concentration-dependent manner. NHE-RF was required for full PKA inhibition, and this effect was completely abrogated by heat-denaturing NHE-RF prior to reconstitution into artificial vesicles.
To extend our observations further, we also examined the functional consequences of NHE-RF transgene expression in cultured B-SC-1 cells. This epithelial-like renal cell line is an appropriate model for study, as it exhibits NBC activity but does not possess detectable levels of endogenous NHE-RF expression. B-SC-1 cells stably expressing an NHE-RF transgene (B-SC-RF) exhibit markedly decreased basal NBC activity compared with the wild-type. This effect was specific for NHE-RF expression, because NBC activity in control cells overexpressing a β-galactosidase transgene or treated with Lipofectamine alone was similar to that observed in wild-type cells. PKA stimulation of B-SC-RF cells by forskolin caused a further reduction on NBC activity, whereas control cells expressing a β-galactosidase transgene were not similarly affected. Additionally NBC activity in wild-type B-SC-1 cells was not inhibited by PKA. Taken together, these observations provide clear evidence for a regulatory role of NHE-RF in modulating the effect of PKA on NBC activity.
The precise mechanism by which NHE-RF facilitates PKA-mediated inhibition of NBC is not known at this time. Recent studies have proposed a model for PKA regulation of NHE3 involving a signal-complex of proteins including PKA, ezrin, and NHE-RF (26, 27). Studies by Moe and colleagues (11) have provided evidence that PKA phosphorylated specific residues in the COOH-terminal tail of NHE3 and that PKA phosphorylation of the transporter by PKA is required for inhibition of activity. Recent studies have indicated that NHE-RF is required for PKA-mediated regulation of NHE3 (22). It is of interest that NBC contains potential PKA phosphorylation sites in its COOH-terminus (2, 3), and it might be suggested that a signal complex similar to that regulating NHE3 operates to inhibit NBC. It is not proved, however, that the NHE3/NHE-RF model is applicable to regulation of other NHE-RF–interacting proteins. For example, NHE-RF coimmunoprecipitates NHE3 in unstimulated cells, suggesting a physical interaction between these proteins in the absence of PKA activation (28). On the other hand, NHE-RF binds to the β2-adrenergic receptor only when the receptor is occupied with its agonist (29). Accordingly, the relation between NBC and NHE-RF will require additional study. The role of phosphorylation of NHE-RF in its interaction with NBC is also unknown at this time. Although in vitro studies have indicated that NHE-RF is phosphorylated by PKA, in vivo studies using HEK293 cells (30), OK cells (28), and PS120 fibroblast cells (22) all indicate that NHE-RF is constitutively phosphorylated in vivo and that its phosphorylation is not increased by cAMP. Moreover, a nonphosphorylated mutant of NHE-RF functions in vivo as a regulatory cofactor in PKA-mediated inhibition of NHE3 (E.J. Weinman et al., manuscript submitted for publication). The relation between phosphorylation of NHE-RF in its interaction with NBC will require additional study.
In summary, we have provided compelling evidence for a role of NHE-RF in the PKA regulation of NBC activity in renal cells. Although it has been suggested that NHE-RF may participate in the regulation of cell functions other than Na+/H+ exchange, this has heretofore not been demonstrated. To our knowledge, the present report is the first to document the involvement of NHE-RF in a process other than PKA regulation of NHE3 activity in renal epithelial cells. Given the uniformly parallel regulation of NHE3 and NBC in these cells under a variety of experimental conditions, the demonstration of a common regulatory factor for both transporters is thus of great interest. The regulation of both NBC and NHE3 by PKA via NHE-RF also suggests a common molecular mechanism whereby these transporters exhibit coordinate regulation after PKA activation in renal proximal tubule cells. Additional studies will be necessary to define the exact mechanism(s) whereby NHE-RF functions as a physiologic regulator of both NBC and NHE3 activities. However, our findings provide a model system to study further the mechanism(s) of the coordinate NBC and NHE regulation in renal cells.
Acknowledgments
The authors acknowledge the excellent secretarial assistance of Elisa Leaños in the preparation of the manuscript. We are grateful to Jose A.L. Arruda for his comments on the manuscript. The work was supported by grants from the American Heart Association of Metropolitan Chicago and the National Kidney Foundation of Illinois Inc. (to A.A. Bernardo), and by the National Institutes of Health (grant DK-37319) and the Research Service of the Department of Veterans Affairs (to E.J. Weinman).
References
- 1.Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev. 1990;70:79–114. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression, cloning and characterization of a renal electrogenic Na+/HCO3– cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- 3.Burnham GE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3– cotransporter. J Biol Chem. 1997;272:19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- 4.Akiba T, Rocco VK, Warnock DG. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987;80:308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz OS, Arruda JAL, Talor Z. Na-HCO3 cotransporter and Na-H antiporter in chronic respiratory acidosis and alkalosis. Am J Physiol. 1989;256:F414–F420. doi: 10.1152/ajprenal.1989.256.3.F414. [DOI] [PubMed] [Google Scholar]
- 6.Gerber J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchanger and Na+/HCO3 cotransporter in the rabbit proximal tubule. Proc Natl Acad Sci USA. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastoriza-Munoz E, Harrington RM, Graber M. Parathyroid hormone decrease HCO3– reabsorption in the rat proximal tubule by stimulating phosphatidyl inositol metabolism and inhibiting base exit. J Clin Invest. 1992;89:1485–1489. doi: 10.1172/JCI115739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz OS, Arruda JAL. Regulation of the renal Na-HCO3 cotransporter by cyclic AMP and calcium dependent protein kinase. Am J Physiol. 1992;256:F560–F565. doi: 10.1152/ajprenal.1992.262.4.F560. [DOI] [PubMed] [Google Scholar]
- 9.Weinman EJ, Dubinsky WP, Dinh Q, Steplock D, Shenolikar S. Effects of limited trypsin digestion on the renal Na+-H+ exchanger and its regulation by cAMP-dependent protein kinases. J Membr Biol. 1989;109:233–241. doi: 10.1007/BF01870280. [DOI] [PubMed] [Google Scholar]
- 10.Weinman EJ, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na+-H+ exchanger. J Clin Invest. 1995;95:2143–2149. doi: 10.1172/JCI117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe OW, Amemiya M, Yamaji Y. Activation of protein kinase A acutely inhibits and phosphorylates Na/H exchanger NHE-3. J Clin Invest. 1996;96:2187–2194. doi: 10.1172/JCI118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, et al. Acute inhibition of Na/H exchanger NHE-3 by cAMP-role of protein kinase A and NHE-3 and NHE-3 phosphoserine 552 and 605. J Biol Chem. 1999;274:3978–3987. doi: 10.1074/jbc.274.7.3978. [DOI] [PubMed] [Google Scholar]
- 13.Bernardo AA, et al. Renal cortical basolateral Na+-H+ exchanger III: evidence for a regulatory protein in the inhibitory effect of protein kinase A. J Membr Biol. 1995;145:67–74. doi: 10.1007/BF00233307. [DOI] [PubMed] [Google Scholar]
- 14.Talor Z, Richison G, Arruda JAL. High affinity binding sites in luminal and basolateral renal membranes. Am J Physiol. 1985;248:F472–F481. doi: 10.1152/ajprenal.1985.248.4.F472. [DOI] [PubMed] [Google Scholar]
- 15.Bernardo AA, Kear FT, Ruiz OS, Arruda JAL. Renal cortical basolateral Na+/HCO3- cotransporter I: partial purification and reconstitution. J Membr Biol. 1994;140:31–37. doi: 10.1007/BF00234483. [DOI] [PubMed] [Google Scholar]
- 16.Weinman EJ, Dubinsky WP, Shenolikar S. Reconstitution of cAMP-dependent protein kinase regulated renal Na+-H+ exchanger. J Membr Biol. 1988;101:11–18. doi: 10.1007/BF01872815. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz OS, Wang LJ, Pahlavan P, Arruda JAL. Regulation of the renal Na+/HCO3- cotransporter III: presence and modulation by glucocorticoids in primary cultures of the proximal tubule. Kidney Int. 1995;47:1669–1676. doi: 10.1038/ki.1995.231. [DOI] [PubMed] [Google Scholar]
- 18.Green JA, Kleeman CR. The role of calcium and cAMP messenger systems in intracellular pH regulation of osteoblastic cells. Am J Physiol. 1992;262:C111–C121. doi: 10.1152/ajpcell.1992.262.1.C111. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jentsch TJ, Janicke I, Sorgonfrei D, Keller S, Wiederholdt M. The regulation of intracellular pH in monkey kidney epithelial cells (B-SC-1) J Biol Chem. 1986;261:12120–12127. [PubMed] [Google Scholar]
- 21.Weinman EJ, Steplock D, Shenolikar S. cAMP mediated inhibition of the renal brush border membrane Na+/H+ exchanger requires a dissociable phosphoprotein cofactor. J Clin Invest. 1993;92:1781–1786. doi: 10.1172/JCI116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun CH, et al. cAMP-mediated inhibition of the epithelial brush border Na/H exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA. 1997;94:3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Custer MB, Spindler B, Verrey F, Murer H, Biber J. Identificaton of a new gene product (Diphor-1) regulated by dietary phosphate. Am J Physiol. 1997;273:F801–F806. doi: 10.1152/ajprenal.1997.273.5.F801. [DOI] [PubMed] [Google Scholar]
- 24.Morais Cabral JH, et al. Crystal structure of PDZ domain. Nature. 1996;382:649–652. doi: 10.1038/382649a0. [DOI] [PubMed] [Google Scholar]
- 25.Harrison SC. Peptide surface association: the case of PDZ and PTB domains. Cell. 1996;86:341–343. doi: 10.1016/s0092-8674(00)80105-1. [DOI] [PubMed] [Google Scholar]
- 26.Murthy A, et al. NHE-RF a regulatory cofactor for Na+-H+ exchanger is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem. 1998;273:1273–1276. doi: 10.1074/jbc.273.3.1273. [DOI] [PubMed] [Google Scholar]
- 27.Yun CH, Lamprecht G, Forster DV, Sidor A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+-H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem. 1998;273:25856–25863. doi: 10.1074/jbc.273.40.25856. [DOI] [PubMed] [Google Scholar]
- 28.Lamprecht G, Weinman EJ, Yun CH. The role of NHE-RF and E3KARP in the cAMP-mediated inhibition of NHE-3. J Biol Chem. 1998;273:29972–29978. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- 29.Hall RA, et al. The beta–2-adrenergic receptor interacts with the Na+/H+ exchanger regulatory factor to control Na+/H+ exchanger. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 30.Weinman EJ, et al. Structure-function of recombinant Na/H exchanger regulatory factor (NHE-RF) J Clin Invest. 1998;101:2199–2206. doi: 10.1172/JCI204. [DOI] [PMC free article] [PubMed] [Google Scholar]