Abstract
The process of cancer immunoediting generates a repertoire of cancer cells that can persist in immune competent hosts. In its most complex form, this process begins with the elimination of highly immunogenic unedited tumor cells followed by the escape of less immunogenic, edited cells. Although edited tumors can release immunosuppressive factors, it is unknown whether unedited tumors produce cytokines that enhance antitumor function. Utilizing gene microarray analysis, we found the cytokine interleukin 17D (IL-17D) was highly expressed in certain unedited tumors but not edited mouse tumor cell lines. Moreover, forced expression of IL-17D in edited tumor cells induced rejection by stimulating CCL2 production from tumor endothelial cells leading to the recruitment of natural killer (NK) cells. NK cells promoted M1 macrophage development leading to adaptive immune responses. IL-17D expression was also decreased in certain high-grade and metastatic human tumors, suggesting that it can be targeted for tumor immune therapy.
Keywords: tumor rejection, interleukin 17D, innate immunity, natural killer cells
Introduction
The cancer immunoediting process involves the initial elimination of highly immunogenic tumor cells from an “unedited” heterogeneous cell repertoire, followed by the eventual escape of poorly immunogenic, “edited” cells (Schreiber et al, 2011, Shankaran et al, 2001). Edited cell lines, which are derived from tumors that develop in wild-type (WT) mice, are termed “progressors” because they are poorly immunogenic and grow progressively when transplanted into syngeneic naïve WT mice. Unedited cell lines, which are derived from immune deficient mice, are often highly immunogenic and are termed “regressors” because they are rejected when transplanted into syngeneic naïve WT mice. Immune cells can infiltrate, recognize, become activated, and eliminate regressor but not progressor tumor cells (Bui et al, 2006, Flood et al, 1987, Shankaran et al, 2001).
Edited tumors possess antigens (Boon and van der Bruggen, 1996, DuPage et al, 2012) that can concomitantly immunize the host (Vaage, 1971), but the adaptive immune response to edited tumors ultimately fails, leading to cancer progression and death (Schreiber et al, 2011). The failure of the adaptive immune response to control antigenic tumors can involve multiple mechanisms that are intrinsic to the tumor cell, including antigen loss and acquisition of inhibitory ligands, or tumor-cell extrinsic effects, including immune suppressive cytokines, and antigen tolerance (Schreiber et al, 2011, Zitvogel et al, 2006, Zou and Chen, 2008). It is not known to what extent tumor extrinsic effects or intrinsic escape mechanisms contribute to cancer progression. Nevertheless, it is clear that progressor tumors express cytokines such as TGFβ that can inhibit antitumor immune responses (Bierie and Moses, 2010). In contrast, it has not been shown whether regressor cells can produce cytokines that serve to activate antitumor immunity. Importantly, cytokine-based immune therapy is a mainstay for treatment of human cancers such as melanoma and renal cell carcinoma (Nicholas and Lesinski, 2011, Rosenblatt and McDermott, 2011). In these diseases, treatment with IL-2 and IFNα is associated with severe toxic effects that limit therapeutic efficacy (Garbe et al, 2011, Hutson, 2011). Thus, discovering novel, safe, non-toxic cytokines that can mediate tumor rejection would have a high impact on tumor immune therapy.
The IL-17 family of cytokines is one of the most ancient cytokine families (Paul, 2013) and includes six members (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, IL-17F) identified by homology that possess a putative cysteine-knot structure (Iwakura et al, 2011, Kolls and Linden, 2004). IL-17A and IL-17F are defining members of the family and are produced by Th-17 cells to mediate immunity against extracellular bacteria and fungi. Recently, IL-17C was shown to have similar activity as IL-17A/IL-17F, although it is expressed by infected epithelial cells and not by T cells (Ramirez-Carrozzi et al, 2011, Song et al, 2011). IL-17D is a cytokine whose function is not well described, although similar to IL-17C, it is known to be expressed outside the immune system and can stimulate human umbilical vein endothelial cells to produce IL-6, IL-8, and GM-CSF (Starnes et al, 2002). It has also been found in rheumatoid nodules (Muller and Lamprecht, 2008) and is decreased in psoriatic skin (Johansen et al, 2009). Interestingly, IL-17D is considered to be the most ancient cytokine in the IL-17D family (Paul, 2013, Roberts et al, 2008) although there have been no studies addressing the function of IL-17D in cancer or any other disease model system.
In this study, we sought to identify tumor-secreted molecules that can mediate tumor rejection. We found that IL-17D is expressed in some regressor but not in progressor cell lines. Importantly, IL-17D is sufficient to induce rejection or growth delay when overexpressed in some progressor cells. We show that the mechanism of action of IL-17D is to stimulate production of monocyte chemotactic protein-1 (MCP-1, aka CCL2), which recruits natural killer (NK) cells to the tumor and leads to M1 macrophage development and productive antitumor adaptive immune responses. These observations identify IL-17D as a cytokine that can promote immune responses via recruitment of NK cells.
Results
IL-17D is highly expressed by certain regressor but not progressor tumors
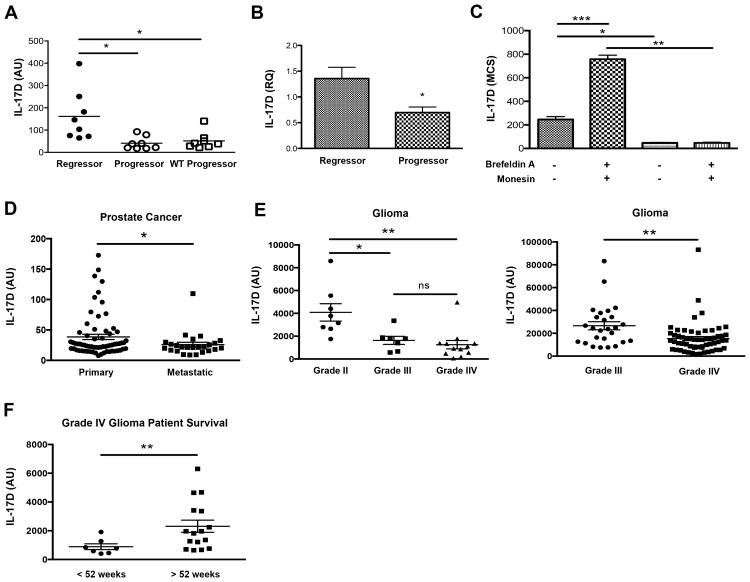
To identify genes that could induce tumor rejection we used a model system whereby edited progressors and unedited regressor methylcholanthrene (MCA)-induced sarcoma cell lines were derived from syngeneic WT and immune-deficient mice (Shankaran et al., 2001, O'Sullivan et al., 2012). We performed gene microarray studies on eight regressor and sixteen progressor cell lines (Fig. S1A). Among the many gene expression differences detected, we focused on the cytokine IL-17D due to its unknown function in tumor biology. We found that IL-17D was highly upregulated in some regressors but was not expressed in any progressor tumor cell line tested by microarray (Fig. 1A), qRT-PCR (Fig. 1B, S1B), and intracellular FACS and verified with an independent set of regressors/progressors from another strain (Fig. S1C, D) (O'Sullivan et al., 2012, Shankaran et al., 2001). Furthermore, treatment of regressor tumor cell lines with protein transport inhibitors doubled the amount of intracellular IL-17D signal, (Fig. 1C), confirming its secretion.
Figure 1. IL-17D is highly expressed in some regressor cell lines and is downregulated in progressor tumor cell lines and several human cancer samples.
(A) Plotted microarray data of IL-17D gene expression of regressor (n = 8) and progressor (n =16) tumor cell lines.
(B) qRT-PCR analysis of independent regressor (n = 4) and progressor (n =4) tumor cell lines.
(C) Quantitated IL-17D intracellular protein expression of 129/Sv RAG2-/- derived regressor (n =3) and progressor (n = 3) tumor cell lines incubated with or without brefeldin A and monensin. IL-17D MCS (mean channel shift) values are calculated by taking the mean florescence of IL-17D intracellular protein signal and subtracting the mean fluorescence signal of the isotype control stain for the same tumor cell line sample.
(D) IL17D gene expression was evaluated from publicly available NCBI GDS datasets from studies comparing indicated cancerous and metastatic tissue from human patients.
(E) Patient samples from the GDS1816 dataset who had been diagnosed as WHO Grade IV astrocytomas with necrosis were divided into low or high survival time categories, and IL17D gene expression was evaluated. Each point represents an individual patient sample.
Data from (B-C) are representative of two independent experiments. Samples were compared using an unpaired, two-tailed Student's t test with Welch's correction. Error bars are depicted as ±SEM. (*P < 0.05, **P < 0.01, *** P < 0.001; NS, not significant).
See also Figure S1.
To assess the expression of IL-17D in human cancers, we utilized publicly available NCBI GEO datasets to examine IL-17D expression in multiple malignant human tissues. Interestingly, IL-17D gene expression was decreased in metastatic prostate tumors compared to primary prostate tumors (Fig. 1D) and was also suppressed in more advanced, higher stage gliomas (WHO Grade III astrocytoma, Grade IV glioblastoma multiforme (GBM)) relative to less advanced, lower stage gliomas (WHO Grade II oligodendroglioma) (Fig 1E, left panel). Additional studies confirmed that IL-17D expression was suppressed in Grade IV GBM when compared to Grade III astrocytomas (Fig. 1E, right panel) and that high expression of IL-17D in tumor biopsies correlated with a greater survival time for a subset of patients with Grade IV GBM (Fig. 1F).
IL-17D promotes progressor tumor rejection, but is not required for regressor tumor rejection
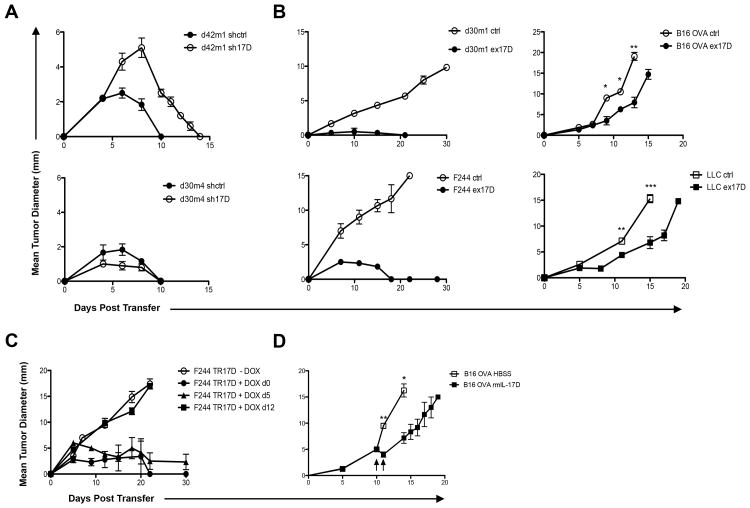
We then explored whether manipulating IL-17D expression could influence tumor growth IL-17D was silenced in regressor cell lines by 95-100% and overexpressed in progressors at approximately five-fold of control cells, a level similar to the expression in unmanipulated regressors (Fig. S2A-D). Silencing of IL-17D in regressor tumors led to a slight growth increase and delayed rejection in one regressor tumor (d42m1), while having no measurable effect in another regressor tumor (d30m4) (Fig. 2A). In four of the six progressor cell lines tested, the overexpression of IL-17D led to complete rejection (F244 and d30m1) or a significant delay in growth (B16.OVA and LLC) in WT mice (Fig. 2B). This effect of IL-17D was due to adaptive immune cells because in vitro and in vivo growth kinetics (in RAG2-/- mice) remained unchanged (Fig. S2F,G).
Figure 2. Expression of IL-17D mediates progressor tumor rejection.
(A) Tumor growth of indicated (ctrl, sh17D) regressor tumors transplanted into WT mice (n=5 for each tumor cell line).
(B) Tumor growth of indicated (ctrl, ex17D) progressor tumors transplanted into WT mice (n=5 for each tumor cell line).
(C) Tumor growth of inducible IL-17D progressor tumor cell line transplanted into WT mice receiving water or doxycycline continuously from d0 (n=5), d5 (n=5), or d12 (n=5).
(D) Tumor growth of B16.OVA melanoma tumor cell line transplanted into WT mice and either receiving intratumoral injections of IL-17D (2μg) or HBSS on d10 and d11. Data from (A-D) are representative of two independent experiments. Samples were compared using an unpaired, two-tailed Student's t test with Welch's correction. Error bars are depicted as ±SEM. (*P < 0.05, **P< 0.01, *** P < 0.001).
See also Figure S2.
To demonstrate the antitumor efficacy of IL-17D on pre-established tumors, we generated a progressor tumor cell line (F244TR17D) that expressed IL-17D upon administration of doxycycline (Fig. S2E). Induced expression of IL-17D caused the rejection of 25mm2 tumors, but not 100mm2 tumors (Fig. 2C), indicating IL-17D was most effective in inducing rejection of small tumors. We then tested whether intratumoral injections of recombinant IL-17D could mediate tumor regression of pre-established B16.OVA tumors transplanted into WT mice. Strikingly, intratumoral injections of recombinant IL-17D caused a significant growth delay compared to control treated tumors, demonstrating the antitumor efficacy of IL-17D (Fig. 2D).
IL-17D expression enhances recruitment of NK cells in progressor and regressor tumors
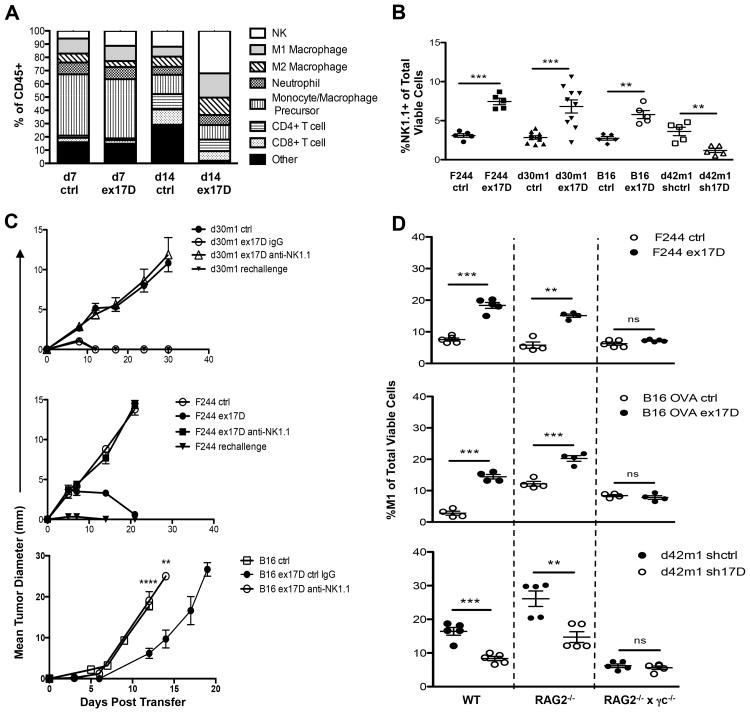
To define the mechanism of IL-17D-mediated tumor rejection, we characterized tumor infiltrating immune cells in tumors with high and low levels of IL-17D. We found an approximately two-fold increase in the amount of natural killer (NK) cells in tumors with high versus low IL-17D (Fig. 3A, B). These NK cells had similar phenotype to splenic NK cells and did not display markers found in immunoablative NK cells (Terme et al., 2012) or IKDCs (Bonmort et al., 2008) (Fig. S3). Notably, NK cells were required for tumor rejection, since mice treated with anti-NK1.1 but not control IgG failed to reject the IL-17D-overexpressing tumors (d30m1, F244) or showed increased growth (B16.OVA) (Fig. 3C). The recruitment of NK cells likely mediates IL-17D's antitumor activity, as we did not observe enhanced numbers of either neutrophils or monocytes in tumors expressing high versus low levels of IL-17D, and neutrophils were not required for IL-17D-mediated tumor rejection (data not shown).
Figure 3. Overexpression of IL-17D in progressor tumors recruits NK cells that are required for tumor rejection in WT mice and promote M1 macrophage infiltration.
(A) Percentage of (7AAD-, CD45+, CD3-, NK1.1+) NK cells, (7AAD-, CD45+, CD11b+, Ly6G+, MHCIIlo) neutrophils, (7AAD-, CD45+, CD11b+, Ly6Chi) monocytes/macrophage precursors, (7AAD-, CD45+, F4/80+, Ly6Clo, MHCIIhi, CD206lo ) M1 macrophages, (7AAD-, CD45+, F4/80+, Ly6Clo, MHCIIlo, CD206hi) M2 macrophages, (7AAD-, CD45+, CD3+, CD4+, CD8-) CD4+ T-cell, and (7AAD-, CD45+, CD3+, CD4-, CD8+) CD8+ T-cell infiltrating immune cells in F244 ctrl or ex17D tumors on d7 and d14 post transplantation in WT mice. (“Other” indicates infiltrating Ly6C-MHCII-NK1.1-CD3-immune cells).
(B) Percent infiltrating NK cells of total viable (7AAD-) cells from transduced regressor and progressor tumors on d7 post tumor transplant in WT mice.
(C) Tumor growth of IL-17D overexpressing (ex17D) progressor tumors transplanted into WT mice receiving either i.p. injections of anti-NK1.1/ctrl IgG, or pre-immunized with transplantation of IL-17D overexpressing (ex17D) tumor cell lines.
(D) Percentage of M1 macrophages of total viable cells on d14 post tumor transplant of progressor tumor cell lines into WT, RAG2-/-, or RAG2-/- × γc-/- hosts.
Data from (A-D) are representative of two independent experiments. Samples were compared using an unpaired, two-tailed Student's t test with Welch's correction. Error bars are depicted as ±SEM. (**P < 0.01, ***P < 0.001, ****P < 0.0001; NS, not significant). See also Figure S3.
Since it is known that NK-dependent tumor rejection can lead to priming of adaptive immune responses (Diefenbach et al, 2001, Kelly et al, 2002), we then tested whether mice that had rejected IL-17D-overexpressing tumors could reject a rechallenge with untransduced progressor tumors. Indeed, we found that parental cells were rejected in primed mice (Fig. 3C), confirming that edited tumors possess antigens and that initiating the “correct” innate cell response (via IL-17D) can result in productive antigen-specific antitumor responses.
Previously, we have found a requirement for NK cells and IFNγ in the accumulation of M1 macrophages in regressor tumors during cancer immunoediting (O'Sullivan et al, 2012). We also observed an approximately 1.5-fold enhancement in the accumulation of M1 macrophages in progressor tumors overexpressing IL-17D (Fig. 3D), whereas silencing of IL-17D in regressor tumors reduced M1 macrophages by approximately two-fold in both WT and RAG2-/-, but not RAG2-/- × γc-/- hosts, which are deficient in NK cells (Fig. 3D).
IL-17D recruits innate immune cells in an air pouch model of inflammation
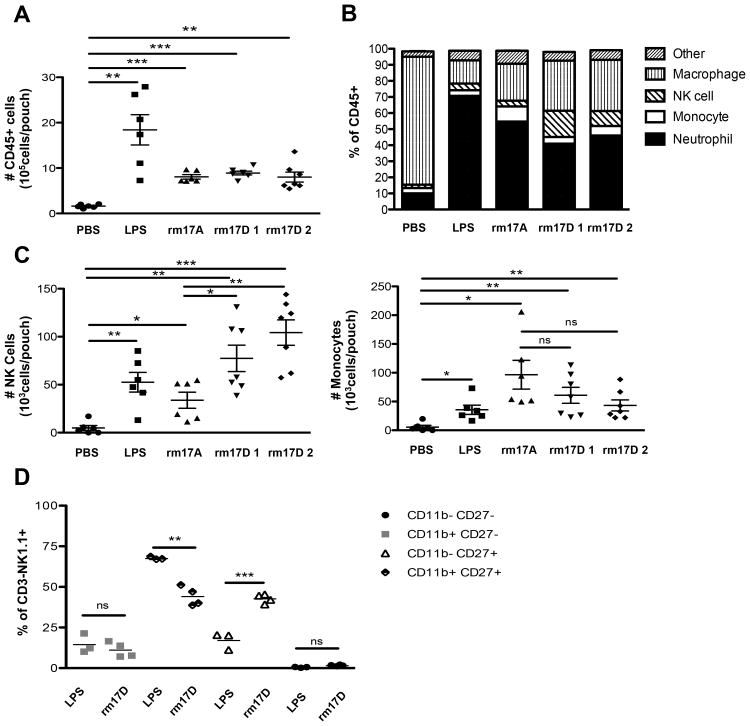
To show directly whether IL-17D can induce the recruitment of immune cells, we used an in vivo air pouch model of inflammation in WT mice. Sterile air pouches become well vascularized after a period of seven days (data not shown) and recruit immune cells rapidly after administration of lipopolysaccharide (LPS) (Pelletier et al, 2004). Indeed, we found that LPS, IL-17A, and IL-17D significantly recruited CD45+ immune cells into air pouches compared to phosphate buffered saline (PBS) control (Fig. 4A). When we examined the composition of the immune cells, we found that LPS and IL-17A recruited more neutrophils than any other cell type, whereas neutrophils constituted a smaller percentage of cells recruited by IL-17D (Fig. 4B). Interestingly, IL-17D recruited significantly more NK cells (Fig. 4C) but not monocytes (Fig. 4C), neutrophils or macrophages (Fig. S4A) compared to LPS and IL-17A. We found that the IL-17D-recruited NK cells were mostly CD27high (Fig. S4B) which could be a semi-mature population of NK cells that may participate in IFNγ-dependent T cell priming in lymph nodes (Martin-Fontecha et al, 2004, Watt et al, 2008). Interestingly, IL-17D recruited approximately twice the amount of CD27highCD11blow NK cells as LPS with no significant recruitment of mature CD27lo NK cells (Fig. 4D).
Figure 4. Recombinant mouse IL-17D recruits NK cells in an air pouch inflammation model.
(A) Total number of infiltrating immune cells per air pouch in WT mice receiving intra-pouch injections of PBS, LPS, IL-17A, IL-17D-1 (generated from E. coli.), or IL17D-2 (generated from C.reinhardtii).
(B) Percentages of NK cells, monocytes, neutrophils, and macrophages per air pouch receiving indicated intra-pouch injections. (Other indicates CD4+, CD8+ T cells or Ly6C-MHCII-NK1.1-CD3- recruited immune cells). Cell populations are defined as in Figure 3A
(C) Total number of NK cells and monocytes per air pouch receiving indicated intra-pouch injections.
(D) Immunophenotypic analysis of infiltrating NK1.1+CD3- NK cells in mouse air pouches receiving intra-pouch injections of LPS or rmIL-17D.
Data from (A-D) are representative of two independent experiments. Each point represents an individual mouse. Samples were compared using an unpaired, two-tailed Student's t test with Welch's correction. Error bars are depicted as ±SEM. (*P < 0.05, **P < 0.01, ***P < 0.001). See also Figure S4.
IL-17D indirectly recruits NK cells in vivo by stimulating the production of MCP-1
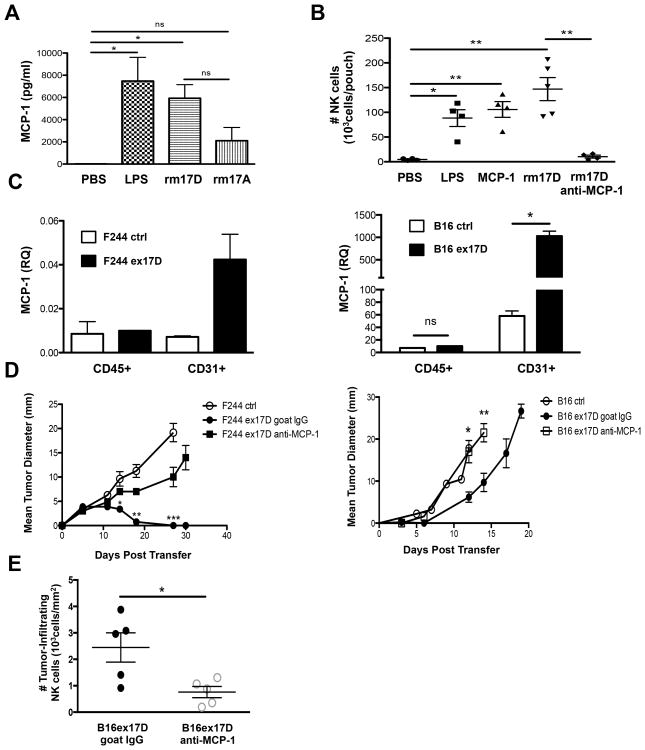
Since IL-17A is known to induce IL-8 from endothelial cells to recruit neutrophils (Roussel et al, 2010), we examined whether IL-17D utilized a similar mechanism. Indeed, we found that IL-17D induced the expression of MCP-1 in mouse air pouch lavage fluid (Fig. 5A). We then repeated air pouch experiments in the presence of blocking antibodies specific for MCP-1 and found that anti-MCP1, but not control IgG, completely inhibited IL-17D mediated recruitment of NK cells (Fig. 5B) monocytes, and neutrophils (Fig. S5A). Furthermore, qRT-PCR analysis of purified tumor endothelial cells from two IL-17D-overexpressing tumors (Fig. S5B) showed a 4-17X increase in MCP-1 transcript compared to control tumors respectively (Fig. 5C), while maintaining similar levels of VEGFR1 (Fig. S5C). Notably, depletion of MCP-1 led to increased growth of two IL-17D-overexpressing tumors (Fig. 5D). These results were likely due to reduced overall numbers of infiltrating NK cells, as MCP-1 depletion reduced the density of tumor infiltrating NK cells compared to control depletion in tumors overexpressing IL-17D (Fig. 5E).
Figure 5. IL-17D indirectly recruits NK cells through tumor endothelial cell production of MCP-1.
(A) Air pouch lavage fluid chemokine levels of MCP-1
(B) Total number of NK cells per air pouch for WT mice receiving intra-pouch injections of PBS, LPS, IL-17A, IL-17D, MCP-1, or IL-17D and anti-MCP-1 monoclonal antibodies.
(C) qRT-PCR analysis of MCP-1 expression from purified tumor leukocytes and endothelial cells harvested from d7 F244 or B16.OVA ctrl or ex17D tumors.
(D) Tumor growth of F244 or B16 OVA ctrl and ex17D tumors transplanted into WTmice receiving either i.p. injections of goat polyclonal anti-MCP-1 or ctrl goat IgG.
(E) Number of tumor-infiltrating NK cells per square mm of tumor from d7 B16 OVAex17D tumors transplanted into WT mice receiving either i.p. injections of goat polyclonal anti-MCP-1 or ctrl goat IgG.
Data are representative of two independent experiments. Each point represents a single mouse. Samples were compared using an unpaired, two-tailed Student's t test with Welch's correction. Error bars are depicted as ±SEM. (*P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant). See also Figure S5.
Discussion
The IL-17 family of cytokines promotes immune responses by inducing the expression of pro-inflammatory cytokines and chemokines, leading to recruitment of neutrophils, and other innate immune cells (Pappu et al., 2011). IL-17A/IL-17F are produced by Th-17 cells and are involved in autoimmune disease and host responses to tissue infection. IL-17C may have similar inflammatory functions to IL-17A/IL-17F, although IL-17C is expressed in epithelial cells and is induced by microbial ligands. Our discovery that IL-17D is expressed outside the immune system and functions to recruit NK cells suggests that the IL-17 family may have evolved to evoke distinct arms of the immune response, presumably to deal with specific pathogen insults. We speculate that similar to IL-17C, the expression of IL-17D in non-immune tissues may represent an early evolutionary adaptation to mediate local anti-viral immunity through the recruitment of NK cells. Notably, our preliminary studies indeed have found increased IL-17D transcripts in virus-infected skin (RSK and JDB, preliminary observations). Future studies on the endogenous role of IL-17D in the context of infection, autoimmunity, and cancer and its regulation are certainly warranted.
Our studies have shown that IL-17D is poorly expressed in cancer cells that grow progressively (mouse MCA-induced sarcomas and certain human cancers) but, comparatively, can be more highly expressed in certain immunogenic MCA-induced sarcoma cells and in low-stage tumors. It is not clear what regulates the constitutive expression of IL-17D in certain cells, but it is clear that high expression of IL-17D may not be compatible with tumor progression, since advanced stage human and edited mouse cancer cells have lower levels of IL-17D, and the ectopic expression of IL-17D in progressor cells led to NK dependent tumor rejection. Overexpression of immune cell-derived chemokines and cytokines such as GM-CSF (Dranoff, 2004, Dranoff et al, 1993) and IL-15 (Liu et al, 2012) have already been demonstrated to have potent antitumor efficacy. Our findings are unique in that IL-17D is tumor-expressed (rather than immune cell-derived) and thus likely represents an endogenous tumor surveillance activity.
It should be noted that not all regressors have high levels of IL-17D (Fig. 1) and that there are multiple genes that are differentially expressed in regressor tumors (Fig. S1), thus indicating that IL-17D is one of many genes that could participate in tumor surveillance. This is likely due to the heterogeneity and redundancy that is inherent in our system (and possibly in normal tumor surveillance mechanisms). For example, we have found that some regressors are well recognized by NK cells whereas others are not, and this is not always correlated with NKG2D ligand expression (O'Sullivan et al, 2011) even though NK cells and NKG2D are important for tumor surveillance (Guerra et al, 2008, Smyth et al, 2005). Furthermore, some regressors require type I IFN for their rejection whereas others do not (Dunn et al, 2005), even though IFNAR-/- mice lacking IFNα/β responsiveness are more susceptible to cancer (Diamond et al, 2011, Dunn et al, 2005, Fuertes et al, 2011), and IFNα/β is used in the treatment of melanoma (Garbe et al, 2011). We therefore conclude that IL-17D is one of many genes that regressor cells produce that can stimulate antitumor immunity. The identification of other genes that can differentiate regressor from progressor cell lines will involve future studies likely combining proteomic, gene expression, and exome sequencing approaches (Matsushita et al, 2012).
NK cells are known to be integral mediators of tumor surveillance (Bui and Schreiber, 2007, Smyth et al, 2001), but little is known about how they are recruited to sites of stress or transformation. Interestingly, in a model of liver carcinoma, the process of senescence induced MCP-1 and increased NK cell infiltration, leading to tumor suppression (Xue et al, 2007, Iannello et al, 2013), but IL-17D was not measured in this study. A recent study showed that the novel chemokine chemerin can recruit NK cells to mediate tumor surveillance (Pachynski et al, 2012), but it remains unclear what induces chemerin during inflammation. The chemokine receptor CXCR3 is expressed on NK cells (Uppaluri et al, 2008) and it ligands ITAC, MIG, and IP-10 can be induced by interferons during tumor development, but this receptor-ligand axis is not involved in the surveillance of MCA-induced sarcomas (Winkler et al, 2011). On the other hand, CXCR3 is thought to be the receptor that mediates the recruitment of cytokine-secreting CD27high NK cells into lymph nodes (Martin-Fontecha et al, 2004, Watt et al, 2008), and it may be possible that IL-17D can also induce CXCR3 ligands, either directly or indirectly via NK-cell production of IFNγ.
Other studies of IL-17 family members in tumor progression have focused on IL-17A and Th-17 cells. These studies have shown both tumor promoting and tumor inhibiting roles for IL-17A/Th-17 cells. For instance, transfection of IL-17A can augment the progression of human tumor cell lines transplanted in nude mice by increasing neovascularization (Numasaki et al., 2003, Numasaki et al., 2005, Tartour et al., 1999), whereas in a mouse syngeneic system, IL-17A promotes tumor rejection by boosting T cell responses (Benchetrit et al., 2002, Hirahara et al., 2001). Th17 cells have also been associated with tumor rejection and good prognosis in some studies (Kryczek et al., 2007, Muranski et al., 2008), whereas other studies indicate that Th17 cells promote tumor growth (Xiao et al., 2009, Zhang et al., 2008). One potential explanation for these conflicting results is that IL-17A can activate and recruit neutrophils, which recently have been shown to have both tumor-promoting and tumor-inhibiting activities (Fridlender et al., 2009). In contrast, it is well-established that NK cells have antitumor activities (Bui and Schreiber, 2007, Smyth et al., 2001), and in fact, can promote antitumor T cell (Diefenbach et al., 2001, Kelly et al., 2002) (Figure 2C) and macrophage responses (O'Sullivan et al., 2012) (Figure 3D). Therefore, unlike IL-17A, IL-17D may induce more consistent antitumor responses through NK cell recruitment that could be more effectively translated to cancer immune therapy. On the other hand, since enforced IL-17D expression induced rejection of some but not all progressor cell lines, it is likely that IL-17D-based therapy, acting through NK cells, will need to be used in combination with checkpoint blockade or inhibitors of T-regulatory cells, which can prevent NK cell activation (Ghiringhelli et al., 2005, Smyth et al., 2006). It is not clear what the total effect enforced IL-17D expression would have on adaptive immunity, but nevertheless, adaptive immunity is required (Supplementary Figure 2) and induced (Figure 3C) in IL-17D-mediated rejection. Finally, we speculate that the selective expression of IL-17D in neoplastic cells as opposed to immune cells would translate to a more benign side effect profile since this ancient cytokine may have evolved to mediate early, and clinically silent, innate tissue surveillance of stress, transformation, and/or pathogen infection.
Experimental Procedures
All experiments involving mice were conducted under animal protocols approved by the Washington University Animal Studies Committee and the University of California, San Diego Institutional Animal Care and Use Committee (IACUC protocol #S06201) and were in accordance with their ethical guidelines.
Cell lines and mice
MCA sarcoma cell lines are a kind gift from Dr. Robert Schreiber and were generated as described (Shankaran et al, 2001). All experiments were done with cells passaged between 4 and 12 cycles. 129/Sv, C57BL/6 × 129/Sv F1, 129/Sv RAG2-/-, C57BL/6 RAG2-/-, and RAG2-/-× γc-/- mice used were used for tumor transplantation experiments. Cell lines were maintained in RPMI 1640 supplemented with 10% FCS, L-glutamine, NEAA, sodium pyruvate, sodium bicarbonate, pen/strep, and β-mercaptoethanol.
Microarray and clustering analysis
Murine Genome U74v2 Set GeneChip Array (Affymetrix) was used for analysis of cDNA generated from regressor and progressor tumor cell lines. Details of RNA preparation, cDNA preparation, microarray setup and clustering analysis are described in the Supplemental Experimental Procedures.
Human cancer microarray data analysis
For human clinical samples, IL-17D gene expression was evaluated from NCBI Gene Expression Omnibus (GEO) datasets from studies comparing primary and metastatic tumors (GDS2546), or low-grade versus high-grade glioma patient samples (GDS4467, GDS1976, GDS1816) as described previously (Pachynski et al, 2012).
Generation of IL-17D-deficient and overexpressing tumor cell lines
Cell lines were generated as decribed in the Supplemental Experimental Procedures.
Antibodies and FACS analysis of tumor cells
For intracellular staining, cells were either incubated with or without 2μM monensin (Sigma) and 1μg/ml Brefeldin A (BD biosciences) and then harvested by trypsinization, washed once with PBS, stained, and analyzed for intracellular IL-17D signal as described in the Supplemental Experimental Procedures.
Tumor transplantation and TIL analysis
Subconfluent tumor cell lines were harvested and injected subcutaneously into syngeneic recipient WT, RAG2-/-, or RAG2-/-× γc-/- mice at either 1 × 106 cells/mouse (for all growth exps) or 5-10 × 106 cells/mouse (for TIL analysis), as previously described (Bui et al, 2006). Tumor rechallenge was performed 3 months after mice had rejected transplanted tumors by injecting 1 × 106 cells/mouse subcutaneously with parental tumor cell lines. In vivo depletion of various immune subsets, doxycycline administration, and intratumoral injection of IL-17D are described in the Supplemental Experimental Procedures. Tumor growth and immune infiltration were analyzed as described in the Supplemental Experimental Procedures.
Mouse air pouch experiments
C57BL/6 × 129/Sv F1 mice were injected s.c with 3ml of sterilized air filtered through a 0.2μm Millipore filter (Bellerica) to form air pouches on day 0 and re-inflated again on day 3. On day 7, either 1ml of LPS (1μg/ml), IL-17A (5μg/ml) (R&D Systems), IL-17D (5μg/ml) (R&D Systems), IL-17D (5μg/ml) (Mayfield Lab), MCP-1 (5μg/ml) (Peprotech), or IL-17D (5μg/ml) + anti-MCP-1 polyclonal antibodies (25μg/ml) (R&D Systems) was injected into mouse air pouches 8h before air pouch harvest. Air pouches were lavaged with 2ml PBS and centrifuged at 1250 rpm for 5 min at room temperature. Supernatant was harvested and analyzed for chemokine protein levels using the mouse chemokine flowcytomix kit from eBioscience. Infiltrating air pouch cells were resuspended in FACS stain buffer, counted on a hemocytometer, and analyzed by cell surface markers as described in the Supplemental Experimental Procedures.
Chemokine secretion assay
On days 7 and 14 post-transplantation, tumors were harvested and single cell suspensions were prepared as described for the TIL analysis. Filtered tumor/immune cell suspensions were plated in triplicate wells at 40,000 cells/well in 100 μL for 24 hrs at 37°C. Supernatant was analyzed for chemokines using the mouse chemokine flowcytomix kit from eBioscience.
Generation of cDNA and quantitative PCR
Tumor cell lines were plated in triplicate at 6 × 104 cells/well in a 6 well plate and incubated for 48 hours at 37°. Supernatant was aspirated and cells were washed twice with PBS before addition of 1ml Trizol reagent (Invitrogen). CD31+ and CD45+ tumor-derived cell populations were washed twice with PBS before addition of 1ml Trizol reagent (Invitrogen). Details describing RNA extraction, cDNA preparation, qPCR reactions and analysis are described in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
IL-17D is highly expressed in some unedited, but not edited tumor cell lines
IL-17D expression is decreased in certain high-grade and metastatic human Tumors
IL-17D overexpression in some poorly immunogenic tumors mediates rejection
IL-17D recruits NK cells through MCP-1 production from tumor endothelial cells
Acknowledgments
We would like to thank C. Peinado for tumor mixing assistance, P. Lee for cloning assistance, and R. Schreiber for critical review of the manuscript. J.D.B is supported by grants from the Hartwell Foundation, NIH (CA128893, CA157885), the American Cancer Society (ACS-IRG #70-002), the Cancer Research Coordinating Committee (6-444951-34384), and the Concern Foundation. Funds for IL-17D production in the Mayfield lab came from the California Energy Commission award 500-10-039.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmort M, Dalod M, Mignot G, Ullrich E, Chaput N, Zitvogel L. Killer dendritic cells: IKDC and the others. Curr Opin Immunol. 2008;20:558–65. doi: 10.1016/j.coi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Boon T, Vander Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–8. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res. 2006;66:7301–9. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- Dupage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–9. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood PM, Schreiber H, Ron Y. Protective immunity to progressive tumors can be induced by antigen presented on regressor tumors. J Immunol. 1987;138:3573–9. [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16(Suppl 2):14–22. doi: 10.1634/theoncologist.2011-S2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–69. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–24. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- Liu RB, Engels B, Arina A, Schreiber K, Hyjek E, Schietinger A, Binder DC, Butz E, Krausz T, Rowley DA, Jabri B, Schreiber H. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res. 2012;72:1964–74. doi: 10.1158/0008-5472.CAN-11-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–4. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Lamprecht P. Interleukin-17 in chronic inflammatory and autoimmune diseases: rheumatoid arthritis, Crohn's disease and Wegener's granulomatosis. Z Rheumatol. 2008;67:72–4. doi: 10.1007/s00393-007-0236-7. [DOI] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas C, Lesinski GB. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunotherapy. 2011;3:673–90. doi: 10.2217/imt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, Mcallister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–89. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- O'Sullivan T, Dunn GP, Lacoursiere DY, Schreiber RD, Bui JD. Cancer immunoediting of the NK group 2D ligand H60a. J Immunol. 2011;187:3538–45. doi: 10.4049/jimmunol.1100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, Smyth MJ, Schreiber RD, Bui JD. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209:1869–82. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachynski RK, Zabel BA, Kohrt HE, Tejeda NM, Monnier J, Swanson CD, Holzer AK, Gentles AJ, Sperinde GV, Edalati A, Hadeiba HA, Alizadeh AA, Butcher EC. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med. 2012;209:1427–35. doi: 10.1084/jem.20112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. Fundamental Immunology. Lippincott Williams & Wilkins, a Wolters Kluwer business; 2013. [Google Scholar]
- Pelletier M, Bouchard A, Girard D. In vivo and in vitro roles of IL-21 in inflammation. J Immunol. 2004;173:7521–30. doi: 10.4049/jimmunol.173.12.7521. [DOI] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, De Voss J, Balazs M, Gonzalez L, Jr, Singh H, Ouyang W, Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–66. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- Roberts S, Gueguen Y, De Lorgeril J, Goetz F. Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev Comp Immunol. 2008;32:1099–104. doi: 10.1016/j.dci.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Mcdermott DF. Immunotherapy for renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:793–812. doi: 10.1016/j.hoc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–7. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–9. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–8. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12:1151–8. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–6. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, Wijdenes J, Lebecque S, Sautes-Fridman C. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–704. [PubMed] [Google Scholar]
- Terme M, Ullrich E, Aymeric L, Meinhardt K, Coudert JD, Desbois M, Ghiringhelli F, Viaud S, Ryffel B, Yagita H, Chen L, Mecheri S, Kaplanski G, Prevost-Blondel A, Kato M, Schultze JL, Tartour E, Kroemer G, Degli-Esposti M, Chaput N, Zitvogel L. Cancer-induced immunosuppression: IL-18-elicited immunoablative NK cells. Cancer Res. 2012;72:2757–67. doi: 10.1158/0008-5472.CAN-11-3379. [DOI] [PubMed] [Google Scholar]
- Uppaluri R, Sheehan KC, Wang L, Bui JD, Brotman JJ, LU B, Gerard C, Hancock WW, Schreiber RD. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86:137–47. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaage J. Concomitant immunity and specific depression of immunity by residual or reinjected syngeneic tumor tissue. Cancer Res. 1971;31:1655–62. [PubMed] [Google Scholar]
- Watt SV, Andrews DM, Takeda K, Smyth MJ, Hayakawa Y. IFN-gamma-dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J Immunol. 2008;181:5323–30. doi: 10.4049/jimmunol.181.8.5323. [DOI] [PubMed] [Google Scholar]
- Winkler AE, Brotman JJ, Pittman ME, Judd NP, Lewis JS, Jr, Schreiber RD, Uppaluri R. CXCR3 enhances a T-cell-dependent epidermal proliferative response and promotes skin tumorigenesis. Cancer Res. 2011;71:5707–16. doi: 10.1158/0008-5472.CAN-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Wang C, Zhang J, LI Z, Zhao X, Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69:2010–7. doi: 10.1158/0008-5472.CAN-08-3479. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–7. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.