Abstract
Infiltration of natural killer (NK) cells into solid organ allografts is observed in clinical and experimental transplantation. Studies suggest a role for NK cells in acute and chronic rejection of solid organ allografts; however, the effects of immunosuppressive agents on NK cells are not clearly established. Rat NK cell lines were analyzed for proliferation and cytotoxicity in the presence of cyclosporine, FK506, or rapamycin. Lewis recipients of DA liver allografts received immunosuppressive agents after transplantation. NK cells demonstrated robust function both in the absence and presence of cyclosporine and FK506. In contrast, rapamycin significantly inhibited proliferation and cytotoxicity of NK cells. NK cell numbers remained stable in graft recipients treated with cyclosporine and FK506, whereas there was a significant decrease in NK cells in rapamycin-treated recipients. These data indicate that immunosuppressive drugs have differential effects on NK cell function that may impact the immune response of transplant recipients.
Keywords: NK cells, Immunosuppression, Liver transplant
The relationship between the innate immune response and the subsequent alloimmune response in solid organ transplantation has not been fully established. Natural killer (NK) cells are effectors of the innate immune system that, unlike T cells, do not require prior sensitization to kill target cells (1). The role of NK cells in immune surveillance against virally infected cells or tumor cells is well established. According to the “missing self” hypothesis, NK cells can directly lyse target cells with deficient or aberrant self-major histocompatibility complex (MHC) class I molecules (2). Recognition of self-MHC molecules by inhibitory receptors on NK cells prevents lysis of autologous cells. Recently, receptors that activate NK cells independent of class I engagement have been described (3). This has lead to the conclusion that a balance of signals from both inhibitory and activating receptors regulates NK cell activation.
NK cells have been demonstrated to be potent effectors that mediate the rejection of both allogeneic bone marrow and xenogeneic solid organ grafts (4–8). Moreover, the infiltration of NK cells into solid organ allografts has been observed both in clinical transplantation and experimental animal models (9–15). In the first 24 hr posttransplant, more than 70% of the lymphocytes in rat liver allografts are NKR-P1+ NK cells (13). The importance of NK cells has been demonstrated in CD28−/− mice (11,15). In these studies, CD28−/− mice vigorously reject both skin and cardiac allografts. However, the depletion of NK cells and NKT cells with anti-NK 1.1 antibodies leads to markedly prolonged graft survival in CD28−/− recipients of allogeneic hearts. It should be noted that cardiac allograft survival was not similarly prolonged in CD28+/+ recipients depleted of NK and NKT cells (15). Thus, when costimulation is blocked, NK cells appear to play a role in mediating rejection. Little is known about the direct effects of immunosuppressive agents on NK cells and thus the purpose of this study was to determine the effects of cyclosporine (CsA), FK506 (FK), and rapamycin (Rapa) on NK cells.
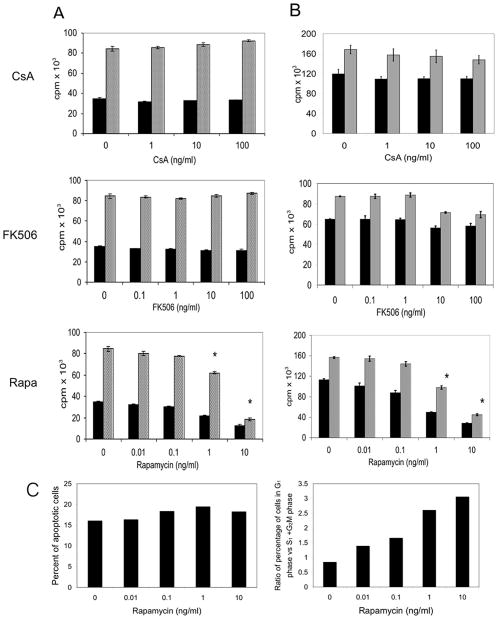
To determine if immunosuppressive agents alter the proliferation of NK cells, both RNK-16 cells, an established rat NK cell line, and primary rat NK cell lines were cultured with increasing doses of CsA (1–100 ng/ml), FK (0.1–100 ng/ml), or Rapa (0.1–10 ng/ml) for 24 or 72 hr (n=3). RNK-16 cells, which are cultured in the absence of interleukin (IL)-2, maintained robust proliferation in the presence of both CsA and FK (Fig. 1A). Similarly primary NK cells lines, which do require exogenous IL-2 to proliferate, were also resistant to the immunosuppressive drugs (Fig. 1B). In experiments conducted in parallel, we confirmed that the drugs inhibited proliferation in a mixed lymphocyte reaction (data not shown).
FIGURE 1.
Effects of immunosuppressive agents on NK cell proliferation. RNK-16 cells (A) or primary rat NK cells (B) were incubated with CsA (0–100 ng/ml), FK506 (0–100 ng/ml), or rapamycin (0–10 ng/ml) for 24 hr (black bars) or 72 hr (gray bars). 3H-Tdr incorporation during an additional 18-hr incubation was measured. Values are the means±SEM of 3H-TdR incorporation (cpm)×103. The data shown is representative of three separate experiments. (C) RNK-16 cells were incubated with Rapa (0–10 ng/ml) for 24 hr. Cell cycle analysis was carried out by incubating cells for 30 min with propidium iodide solution before flow cytometric analysis. The percentage of apoptotic cells was determined as the percentage of cells in sub-G1 phase (left panel). Growth arrest was determined as the ratio of cells in G1 phase vs. the S and G2/M phases (right panel). The data shown is representative of two separate experiments.
In marked contrast, Rapa significantly inhibited the proliferation of both RNK-16 cells and primary NK cell lines in a dose-dependent manner (Fig. 1A and B). In a representative experiment (n=3), the proliferation of primary NK cells is inhibited by 66% at 24 hr in the presence of Rapa at 1.0 ng/ml (Fig. 1B). To determine whether this decrease in proliferation was due to apoptosis or growth arrest, cell cycle analysis was performed on RNK-16 cells cultured in the absence or presence of increasing concentration of Rapa. The proportion of cells with sub-G1 DNA content was analyzed to assess the levels of apoptosis. The percentage of apoptotic cells was similar at all concentrations examined (Fig. 1C, left panel). In contrast, treatment with Rapa results in an increase in the proportion of cells in G1 phase compared with the proportion in S and G2/M indicating an arrest in the G1 to S transition (Fig. 1C, right panel). This effect was dose-dependent as it was seen at the lowest concentration of Rapa examined (0.01 ng/ml) and was more dramatic at the higher concentrations of Rapa.
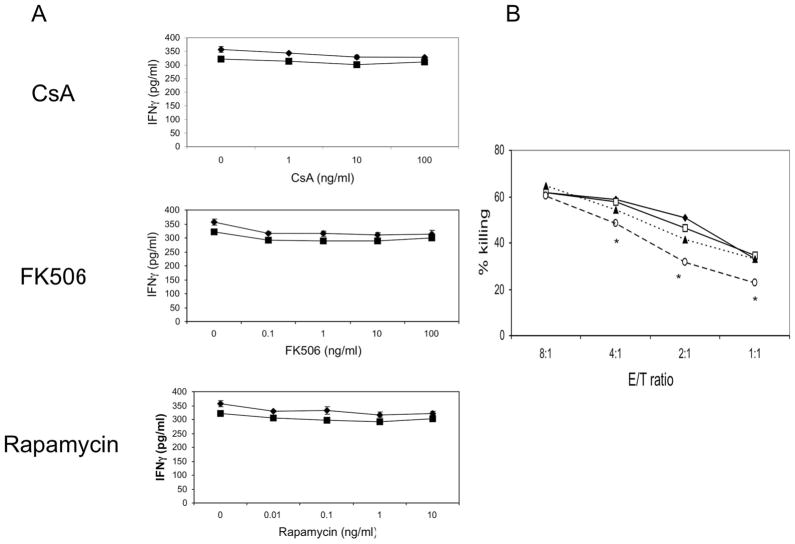
To examine the effects of immunosuppressant agents on NK cell function, we examined primary NK cell lines for interferon (IFN)-γ secretion and cytotoxicity in the absence and presence of CsA (1–100 ng/ml), FK (0.1–10 ng/ml), or Rapa (0.1–10 ng/ml). We have previously demonstrated that NK cells produce IFNγ and are a major source of IFNγ in allografts (13). Primary NK cells spontaneously secrete IFNγ in both the absence and presence of increasing concentrations of immunosuppressant drugs (Fig. 2A). Thus even though proliferation is diminished by Rapa, and there are less NK cells present, the levels of IFNγ in the supernatant remain comparable to levels in the control cultures. These findings are consistent with a report indicating that Rapa actually increases IFNγ production (16).
FIGURE 2.
Effects of immunosuppressive agents on NK cell function. (A) Primary NK cells were incubated with CsA, FK506, or rapamycin for 24 hr (diamonds) or 72 hr (squares) and supernatants analyzed for IFNγ levels. The data shown is representative of three separate experiments. (B) Primary NK cells were incubated alone (filled diamonds) or with CsA (open squares), FK506 (filled triangles) or Rapamycin (open circles) for 24 hr and then used as effectors in a killing assay against YAC-1 targets at E:T ratios of 8:1 to 1:1. P≤0.05 (NK cells alone vs. NK cells incubated with rapamycin). The data shown are representative of three separate experiments.
To further examine whether immunosuppressive agents alter NK cell function, we cultured primary NK cells in the absence and presence of CsA (100 ng/ml), FK (10 ng/ml), or Rapa (10 ng/ml), washed the NK cells, and used them as effectors in a killing assay against NK cell-sensitive labeled YAC-1 tumor targets (104 targets cells/well). CsA and FK did not affect NK cell-mediated killing against YAC-1 targets while cytotoxicity was modestly, but reproducibly and significantly (P<0.05), inhibited in the presence of Rapa (Fig. 2B).
To determine the in vivo effects of these immunosuppressive agents on NK cells posttransplantation, groups of Lewis recipients (n=3), received livers from DA donors. Immunosuppressive agents (1.0 mg/kg/day) were initiated after transplantation and continued for 6 days. Blood was obtained on days 1, 3, 5, 7, 10, and 14 for flow cytometric analysis of NK cells, NKT cells were excluded from the analysis. Peripheral NK cell levels in graft recipients treated with FK remained relatively stable whereas there was a slight decrease in the levels of NK cells in recipients treated with CsA (Fig. 3A). In contrast, there was a significant decrease in NK cells, starting on day 5 posttransplant in graft recipients that received Rapa (Fig. 3A). In the DA→Lewis transplant model used in this study the survival time is 10–12 days with only a fraction of the graft recipients surviving for 14 days. Indeed only two rats, in each group, were available for analysis on days 10 and 14. In the two rats that survived for 14 days the numbers of peripheral NK cells (26.4±8.1) was more than three times the number observed in rats treated with Rapa (6.8±2.8; Fig. 3B).
FIGURE 3.
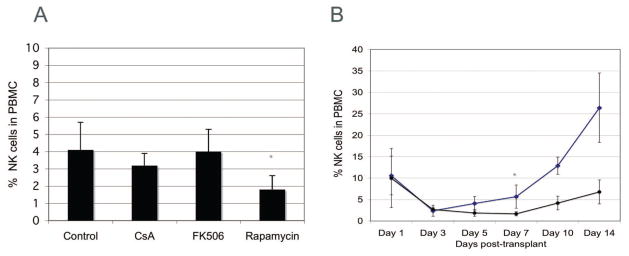
Rapamycin treatment decreases NK cell numbers posttransplantation. Lewis recipients of DA livers were treated with vehicle or CsA, FK506 and rapamycin for 7 days. (A) The proportion of NK cell levels in the peripheral blood on day 5 posttransplant. Data shown are the means ± SEM of three rats for each treatment. *P≤0.05 (vehicle treated graft recipients vs. graft recipients treated with Rapamycin as determined by Mann-Whitney U test). (B) The proportion of NK cell levels in the peripheral blood in control (diamonds) and rapamycin (circle) graft recipients. Data shown are the means ± SEM of three rats at each timepoint. *P<0.05 (vehicle treated graft recipients vs. graft recipients treated with Rapamycin as determined by Mann-Whitney U test).
Our data clearly indicate that NK cells proliferate and retain cytotoxic capabilities in the presence of CsA and FK. Earlier studies demonstrated that rats that received FK retained normal NK cell function however this study did not address NK cell numbers and function posttransplant (12). Similarly, most studies indicate that CsA has little effect on NK cell proliferation and cytotoxicity (17–20). However, it has been recently reported that a subpopulation of human NK cells demonstrates reduced proliferation in the presence of CsA (21) but retained cytotoxicity against tumor targets. Indeed, we did see a trend towards decreased proliferation of NK cells exposed to CsA suggesting that a subpopulation may indeed be sensitive to this immunosuppressant. Further studies are necessary to clarify if certain subsets of NK cells are more sensitive to CsA and FK.
Interestingly, NK cells exposed to Rapa, in vitro or in vivo, demonstrate decreased proliferation. Similar to what has been reported for T and B cells, our data indicate that Rapa blocks NK cell progression from the G1 to S phase of the cell cycle. Moreover, NK cell killing against tumor targets is significantly diminished in the presence of 10 ng/ml Rapa in agreement with earlier studies suggesting that Rapa inhibits cytotoxicity and antibody-dependent cell-mediated cytotoxicity (22). Additionally, Rapa can inhibit growth of malignant B cells such as chronic lymphocytic B-cell leukemia and post-transplant lymphoproliferative disease–related B-cell lymphomas (23–25).
We demonstrate that CsA and FK spare NK cells, suggesting that NK cells would still function to eliminate virally infected or transformed cells in transplant patients. In contrast, NK cell proliferation and function are diminished by Rapa. Although NK cells were long thought to be innocent bystanders in the rejection of organ allografts, recent studies indicate that, under some conditions, NK cells can actively contribute to both acute and chronic allograft rejection (9–15). Understanding the differential effects of immunosuppressive drugs on NK cell effector function is important in clinical transplantation. Further research is necessary to specifically determine how NK cells contribute to the rejection response and to develop immunosuppressive agents that can target the deleterious effects of NK cells in graft rejection while preserving the beneficial functions of NK cells against viruses and tumors.
Acknowledgments
This project was supported by the National Institutes of Health (RO1AI44095), a fellowship from the National Science Scholarship (Singapore Agency for Science, Technology and Research to L.W.), and a fellowship funded by the Transplant and Tissue Engineering Center of Excellence at Lucile Packard Children’s Hospital (to M.F.).
References
- 1.Lanier LL. Turning on natural killer cells. J Exp Med. 2000;191:1259. doi: 10.1084/jem.191.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 3.Bottino C, Biassoni R, Millo R, et al. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum Immunol. 2000;61:1. doi: 10.1016/s0198-8859(99)00162-7. [DOI] [PubMed] [Google Scholar]
- 4.Auchincloss H, Jr, Sachs DH. Xenogeneic transplantation. Annu Rev Immunol. 1998;16:433. doi: 10.1146/annurev.immunol.16.1.433. [DOI] [PubMed] [Google Scholar]
- 5.Blakely ML, Van der Werf WJ, Berndt MC, et al. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation. 1994;58:1059. [PubMed] [Google Scholar]
- 6.Gourlay WA, Chambers WH, Monaco AP, Maki T. Importance of natural killer cells in the rejection of hamster skin xenografts. Transplantation. 1998;65:727. doi: 10.1097/00007890-199803150-00021. [DOI] [PubMed] [Google Scholar]
- 7.Manilay JO, Sykes M. Natural killer cells and their role in graft rejection. Curr Opin Immunol. 1998;10:532. doi: 10.1016/s0952-7915(98)80219-7. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin WM, 3rd, Larsen CP, Fairchild RL. Innate immune responses to transplants: a significant variable with cadaver donors. Immunity. 2001;14:369. doi: 10.1016/s1074-7613(01)00117-0. [DOI] [PubMed] [Google Scholar]
- 10.Kitchens WH, Uehara S, Chase CM, et al. The changing role of natural killer cells in solid organ rejection and tolerance. Transplantation. 2006;81:811. doi: 10.1097/01.tp.0000202844.33794.0e. [DOI] [PubMed] [Google Scholar]
- 11.Maier S, Tertilt C, Chambron N, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 12.Markus PM, Van den Brink MR, Luchs BA, et al. Effects of in vivo treatment with FK506 on natural killer cells in rats. Transplantation. 1991;51:913. doi: 10.1097/00007890-199104000-00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obara H, Nagasaki K, Hsieh CL, et al. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am J Transplant. 2005;5:2094. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersson E, Ostraat O, Ekberg H, et al. Allogeneic heart transplantation activates alloreactive NK cells. Cell Immunol. 1997;175:25. doi: 10.1006/cimm.1996.1031. [DOI] [PubMed] [Google Scholar]
- 15.McNerney ME, Lee KM, Zhou P, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 16.Wieder KJ, Hancock WW, Schmidbauer G, et al. Rapamycin treatment depresses intragraft expression of KC/MIP-2, granzyme B, and IFN-gamma in rat recipients of cardiac allografts. J Immunol. 1993;151:1158. [PubMed] [Google Scholar]
- 17.Kosugi A, Shearer GM. Effect of cyclosporin A on lymphopoiesis. III. Augmentation of the generation of natural killer cells in bone marrow transplanted mice treated with cyclosporin A. J Immunol. 1991;146:1416. [PubMed] [Google Scholar]
- 18.Lefkowitz M, Kornbluth J, Tomaszewski JE, Jorkasky DK. Natural killer-cell activity in cyclosporine-treated renal allograft recipients. J Clin Immunol. 1988;8:121. doi: 10.1007/BF00917900. [DOI] [PubMed] [Google Scholar]
- 19.Petersson E, Qi Z, Ekberg H, et al. Activation of alloreactive natural killer cells is resistant to cyclosporine. Transplantation. 1997;63:1138. doi: 10.1097/00007890-199704270-00014. [DOI] [PubMed] [Google Scholar]
- 20.Vampa ML, Norman PJ, Burnapp L, et al. Natural killer-cell activity after human renal transplantation in relation to killer immunoglobulin-like receptors and human leukocyte antigen mismatch. Transplantation. 2003;76:1220. doi: 10.1097/01.TP.0000083896.91215.C7. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Grzywacz B, Sukovich D, et al. The unexpected effect of cy-closporin A on CD56+CD16− and CD56−CD16− natural killer cell subpopulations. Blood. 2007;110:1530. doi: 10.1182/blood-2006-10-048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H, Chen H, Daloze P, Wu J. Effects of rapamycin on human HLA-unrestricted cell killing. Clin Immunol Immunopathol. 1992;65:60. doi: 10.1016/0090-1229(92)90248-m. [DOI] [PubMed] [Google Scholar]
- 23.Decker T, Hipp S, Ringshausen I, et al. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003;101:278. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 24.Nepomuceno RR, Balatoni CE, Natkunam Y, et al. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Ep-stein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472. [PubMed] [Google Scholar]
- 25.Vaysberg M, Balatoni CE, Nepomuceno RR, et al. Rapamycin inhibits proliferation of Epstein-Barr virus-positive B-cell lymphomas through modulation of cell-cycle protein expression. Transplantation. 2007;83:1114. doi: 10.1097/01.tp.0000260142.38619.9c. [DOI] [PubMed] [Google Scholar]