Abstract
We created and produced a novel self-assembling nanoparticle platform for delivery of peptide epitopes that induces CD8+ and CD4+T cells that are protective against T. gondii infection. These self-assembling polypeptide nanoparticles (SAPNs) are composed of linear peptide (LP) monomers which contain two coiled-coil oligomerization domains, the dense granule 7 (GRA720-28 LPQFATAAT) peptide and a universal CD4+ T cell epitope (derived from PADRE). Purified LPs assemble into nanoparticles with icosahedral symmetry, similar to the capsids of small viruses. These particles were evaluated for their efficacy in eliciting IFN-γ by splenocytes of HLA-B*0702 transgenic mice and for their ability to protect against subsequent T. gondii challenge. This work demonstrates the feasibility of using this platform approach with a CD8+ epitope that binds HLA-B7 and tests the biological activity of potentially protective peptides restricted by human major histocompatibility complex (HLA) class I molecules in HLA transgenic mice.
Keywords: Toxoplasma gondii, HLA-B7, vaccine, nanoparticles
Introduction
Toxoplasmosis is a serious infectious disease for humans. It is caused by Toxoplasma gondii, an obligate, intracellular, apicomplexan parasite that infects all human cells and tissues but has special tropism for the eye and the brain. The disease is most severe in the fetus, newborn infants and in immunocompromised individuals [1]. Although antiparasitic drugs such as sulfadiazine and pyrimethamine are available, they do not eliminate the latent, cyst form of the parasite [2]. Thus, there is a great need for the development of a safe and potent vaccine.
A nanoparticle-based vaccine for malaria, a disease caused by the related apicomplexan plasmodial parasite, is being developed to deliver Plasmodium falciparum circumsporozoite protein (PfCSP) derived T and B- cell epitopes. They are self adjuvanting and have been successful in the induction of immune responses without additional adjuvants [3,4,5,6]. However, until now, an analogous nanoparticle-based vaccine has not yet been developed to prevent toxoplasmosis. Using self-adjuvanting nanoparticles in vaccines is promising because adjuvants have proven to be difficult to develop and manufacture, as they are limited by many factors such as toxicity, biodegradability, expenses, individual immunogenicity, and lack of interaction with the antigen itself [7]. Considerable effort has been made to identify promising vaccine candidate antigens for T. gondii. To date, these candidates include surface antigens (SAG), dense granule antigens (GRA), rhoptry proteins (ROP) and microneme proteins (MIC). Among them, GRA7 is a potent antigen expressed in all infectious stages of T. gondii [8]. It can trigger significant humoral and cellular immune responses against toxoplasmosis [9,10]. Our previous findings identified the T. gondii HLA-B*0702- restricted GRA720-28 (LPQFATAAT) peptide as one that confers protection against toxoplasmosis [10]. In conjunction with an universal CD4+ T cell epitope (PADRE) and adjuvant (a specially formulated TLR4 agonist called GLA-SE [11]), GRA7 peptide elicits IFN-γ from CD8+ T cells and controls parasite burden in HLA-B*0702 transgenic mice [12,13].
Herein, we constructed nanoparticles displaying the GRA720-28 in conjunction with PADRE and evaluated these vaccine components in HLA-B*0702 transgenic mice. Immunization of these mice activated CD8+ T cells to produce IFN-γ and protected against subsequent challenge with a high inoculum of type I and type II parasites. Our results highlight the potential for the use of these self-assembling nanoparticles as a platform for vaccine approach to protect against toxoplasmosis.
Materials and Methods
Peptides
The GRA720-28 (LPQFATAAT) peptide and PADRE-derived universal CD4 helper epitope (ERFVAAWTLRVRA) were used in the vaccine constructs [14].
Gene cloning of nanoparticle proteins
The GRA720-28 peptide sequence was cloned in between the NsiI/BamHI restriction sites of the modified pPEP-T vector [15] to yield the final LP amino acid sequence MGHHHHHHASERLPQFATAATGSWQTWNARWDQWSNDWNAWRSDWQAWR DDWARWRALWMGGRLLLRLEELERRLEELERRLEELERFVAAWTLRVRALERR LEELAGGSGDPPPNPNDPPPPNPNDK (GRA720-28 peptide is underlined). The sequence is composed of the his-tag sequence (1-12aa), the CD8+ epitope (13-21aa), the pentameric coiled coil (22-60aa), a glycine-glycine linker (61-62aa), the trimeric coiled coil (63-107aa) and a solubility tag (108-128). The trimeric coiled coil contains a PADRE derivative as a CD4+ epitope (86-98aa).
A control construct, P4c-RD, was made that contained a random peptide sequence, IPSTAFTDI AWVRLPNHY, at the N-terminal end in place of the GRA720-28 peptide.
Protein purification, refolding, and analysis of the nanoparticle polypeptide
LP monomers were expressed in the Escherichia coli BL21-CodonPlus strain (Stratagene). Expression clones were grown at 37°C in Luria broth medium containing 50 µg/µl kanamycin and 34 µg/µl chloramphenicol. A 1-liter culture of E. coli was grown to an A600 of 0.6, and protein expression was induced by addition of isopropyl-β-D- thiogalactopyranoside (1mM final concentration). Recombinant protein was extracted under native conditions by using the BugBuster protein extraction reagent (Novagen, 6 ml/g of cell pellet) containing a protease inhibitor mix (Roche Diagnostics) and 10 ug/ml lysozyme. All purification steps were done under 8M urea denaturing conditions. The His-tagged LPs were purified by using nickel-affinity chromatography and followed by Q-Sepharose. The eluate which contains 8M urea was dialyzed against a buffer containing 5 mM Hepes-KOH (pH 7.8) and 0.5 mM DTT. Upon removal of the denaturant the LPs self-assemble to form nanoparticles which were stored at 4°C. The purity of the recombinant LP was determined by SDS-PAGE, and the protein concentration was measured by the method of Bradford using BSA as a standard.
The shape and size of the nanoparticles were determined by using transmission electron microscopy (TEM) and dynamic light scattering (DLS) [16]. TEM analysis was performed as previously described [17] and photographed on a Zeiss EM910 transmission electron microscope (Carl Zeiss). The hydrodynamic diameter of the SAPN was measured using Wyatt DynaPro Nanostar (Wyatt technology) instrument in PBS at 25°C and pH 7.5.
Mice
Female HLA-B*0702 transgenic mice express a chimeric HLA-B07/H2-Db MHC Class I Molecule and are on a C57BL/6 x Balb/C background backcrossed through many generations. They were produced at Pharmexa-Epimmune (San Diego, CA) and bred at the University of Chicago as previously described (6). They were maintained in SPF conditions throughout. All studies were conducted with the approval of the Institutional Animal Care and Use Committee at the University of Chicago.
Immunizations of mice
To evaluate nanoparticle immunogenicity, HLA-B*0702 transgenic mice were inoculated subcutaneously (s.c.) at the base of the tail using a 30-gauge needle with 50 μg GRA720-28 SAPN or P4c-RD control SAPN three times at two weeks intervals.
ELISpot assay to determine immune responses with murine splenocytes
Mice (n=5 per group) were euthanized 14 days after immunization. Spleens were harvested, pressed through a 70 μm screen to form a single-cell suspension, and depleted of erythrocytes with AKC lysis buffer (160 mM NH4Cl, 10 mM KHCO3, 100 mM EDTA). Splenocytes were washed twice with Hank's Balanced Salt Solution (HBSS) and resuspended in complete RPMI medium (RPMI-1640 supplemented with 2 mM L-GlutaMax).
Parasites
The strains of parasite used in this study include type I RH-YFP that express fluorescence protein (YFP) gene (kindly provided by Boris Streipen, University of Georgia), and Type II Me49 strain that express the firefly luciferase (FLUC) gene constitutively by tachyzoites and bradyzoites. It was created, and kindly provided by Laura Knoll (Wisconsin).
Challenge with Type II parasites
For challenge studies, mice (n=6 for PBS and GRA720-28 SAPN, n=3 for ΔRPS13) were challenged intraperitoneally (i.p.) 14 days post-immunization using 2,000 Type II parasites.
In vivo bioluminescence imaging for determining outcomes of challenge with type II parasites
Mice infected with 2,000 Me49-FLUC tachyzoites were imaged 21 days post-challenge using the in vivo imaging system (IVIS; Xenogen, Alameda, CA). The type II parasites FLUC do not have a YFP cassette. Thus we used the luciferase cassette to track the cyst parasites in the brain.
Mice were injected retroorbitally with 200 μl of D-luciferin, anesthetized in an O2-rich induction chamber with 2% isoflurane, and imaged after 12 minutes. Photonic emissions were assessed using Living image® 2.20.1 software (Xenogen). Data are presented as pseudocolor representations of light intensity and mean photons/region of interest (ROI). All mouse experiments were repeated at least twice. There were 6 mice in each group.
Enumeration of cysts in mouse brains following type II parasite challenge
Mice were euthanized at 21 days after infection, and brains were collected, homogenized with 1 ml of saline (0.85% NaCl) and tissue cysts were counted microscopically in 50 μl of the homogenate, and the count was multiplied by 20 to obtain the number of tissue cysts per brain. This number was confirmed by staining brain cysts with fluorescein-labeled Dolichos biflorus agglutinin (Vector Laboratories) and quantitation using fluorescence microscopy.
Challenge of mice with Type I tachyzoites and determination of peritoneal parasite burden
Immunized HLA-B07 female mice (n=5 per group) were challenged i.p. with 2,000 RH T. gondii expressing stable YFP (YFP parasites). Peritoneal fluid was collected 120 hours post infection and parasite fluorescence and numbers were measured using a fluorometer and hemocytometer, respectively.
Statistical analyses
Data for each assay were compared by ANOVA and a Student t test using GraphPad Prism 5 software (GraphPad Software, San Diego, CA). Differences between the groups were identified by ANOVA and multiple comparison procedures, as we previously described [18]. Data are expressed as the means ± SD. Results were considered to be statistically significant at p < 0.05.
Results
Preparation and characterization of GRA720-28 SAPN
We expressed and purified from E. coli a protein composed of the five-fold coiled-coil domain from the so-called trp-zipper [19], a trimeric coiled-coil domain, and the CD8+T cell epitope restricted by HLA-B07 supertypes. After purification we changed the holding buffer to allow self-assembly of the LPs to form nanoparticles (Fig. 1A). The LP has a molecular mass of ∼12 kDa on SDS-PAGE (Fig. 1B). Transmission electron microscopy (Fig. 1C) and DLS (Fig. 1D) showed a relatively uniform distribution of non-aggregated nanoparticles of ∼38 nm in diameter.
Figure 1.
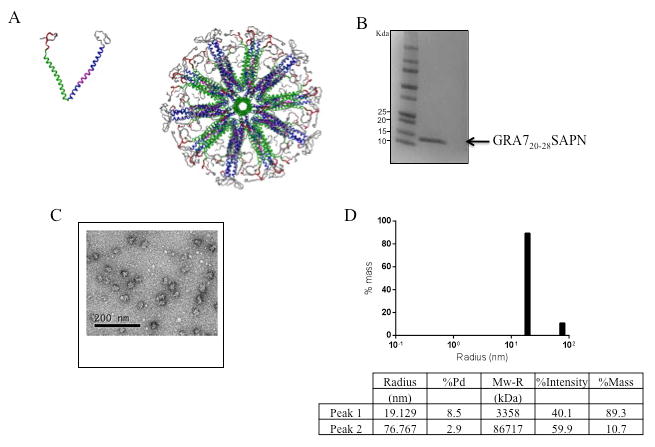
Assembly of GRA720-28 SAPN. A, Three-dimensional model of the nanoparticle. Left: single protein chain of the nanoparticle. Right: assembled nanoparticle with icosahedral symmetry viewing down the five-fold symmetry axis. Green: pentameric coiled coil; Blue: trimeric coiled coil; Magenta: CD4 epitope as an integral portion of the trimeric coiled coil; red: CD8 epitope; Gray: His-tag; Light gray: solubilization tag. B, SDS-PAGE 4-20% of the purified LP. C, Transmission electron microscopy of a nanoparticle preparation containing one CD8+ T cell epitope from dense granule protein GRA7 (GRA720–28) and the universal CD4+ TH epitopte (PADRE). D, DLS plot of GRA720-28 nanoparticles after final purification and assembly. For this product the diameter of 89.3% of the protein is ∼38nm.
PADRE-GRA720-28 SAPN elicit HLA-B07-restricted CD8+ T cells responses in immunized mice
Splenocytes were isolated from immunized HLA-B07 transgenic mice 2 weeks after final immunization. Their ability to generate IFN-γ and lymphocyte proliferation in response to the protein was assessed. The data in Fig. 2A indicate IFN-γ secretion was significantly enhanced by immunization with GRA720-28 nanoparticles and not with control nanoparticles or PBS when cells are stimulated with GRA720-28 peptide. Significant responses were also observed when splenocytes were tested for IFN-γ in an ELISpot assay. Fig. 2B shows the representative data of the IFN-γ spot formation from immunized mice stimulated with PADRE and GRA720-28 peptide. These results demonstrate that the CD4+ T cell epitope PADRE in this nanoparticle vaccine delivers help for IFN-γ production. Thus, the association of GRA720-28 peptide and PADRE contributes to an enhancement of IFN-γ production in HLA-B07 transgenic mice.
Figure 2.
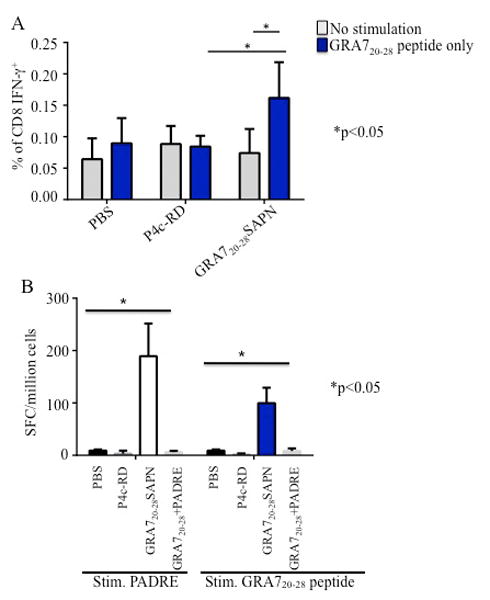
GRA720-28 nanoparticles elicit GRA7 peptide-specific immune response. A, T. gondii CD8+ T cell responses from immunized mice. B, PADRE and GRA720-28 specific IFN-γ spot formation and spleen cell proliferation were tested using splenocytes from GRA720-28 nanoparticles immunized HLA-B07 transgenic mice. GRA720-28 peptide and PADRE dissolved in PBS were used as a control for comparison.
Protective immune responses of GRA720-28 SAPN against T. gondii type II strain in mice
Mice were challenged 2 weeks after the last immunization. Brains from these mice were imaged 21 days after they had been challenged with 2,000 Me49 (Fluc) using a Xenogen in vivo imaging system. Initially, we performed pilot studies and found no difference on cyst burden between control PBS mice and control SAPN (data not shown). Thus, for subsequent studies we used PBS (as negative control), ΔRPS13 (as positive control) and nanoparticles for characterization. As shown in Fig. 3A-B, the numbers of luciferase expressing parasites in HLA-B07 mice immunized with GRA720-28 SAPN were significantly reduced compared to the mice immunized with control SAPN or PBS. This correlates with the reduction of the number of cysts per brain in GRA720-28 SAPN immunized mice (Fig. 3C). The attenuated parasites by knockout of the ribosomal protein 13 gene (rps13Δ) were used as positive control.
Figure 3.
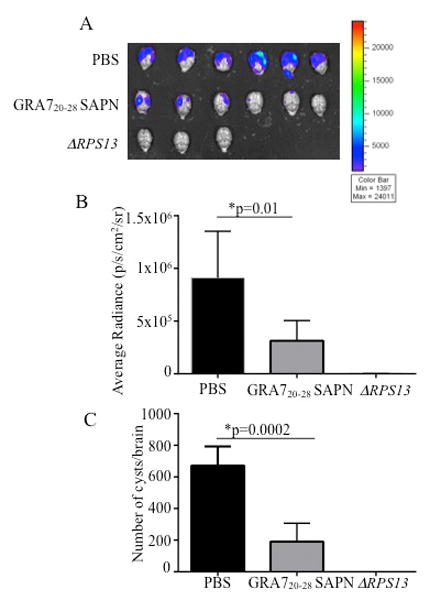
T. gondii brain cysts luciferase expression was significantly reduction in HLA-B07 mice immunized with GRA720-28 SAPN assayed at 21 days after challenge with 2000 Me49 (Fluc) T. gondii expressing luciferase B, Xenogen imaging of brain ex vivo following the injection of luciferin into the retroorbital plexus and then exposure of the brain to luciferin solution. C, Enumeration of cyst was performed with brains of mice challenged 21 days after final immunization.
Vaccination with GRA720-28 SAPN provides protection to mice against T. gondii RH strain
We immunized mice with GRA720-28 SAPN or a control SAPN and then challenged with type I strain of T. gondii (2,000 RH tachyzoites). Peritoneal fluid was collected 120 hours post-infection and parasite fluorescence and numbers were measured using a fluorometer and hemocytometer, respectively. Compared to control SAPN immunized mice, fluorescence from GRA720-28 SAPN immunized mice was significantly lower (Fig. 4A). This reduction was also observed in the measurements of the total parasite burden (Fig. 4B). In separate experiments GRA720-28 and PADRE peptides delivered in saline were used but conferred no protection in mice, nor did they lead to the production of IFN-γ (data not shown). Together these data indicate that GRA720-28 SAPN can protect mice against T. gondii infection.
Figure 4.
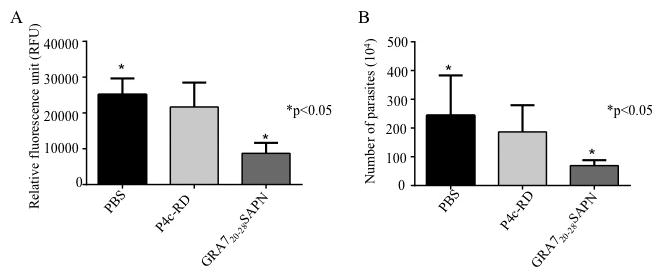
GRA720-28 SAPN reduce T. gondii type I parasite burden in vivo. YFP-parasites quantified using a (A) fluorimeter and (B) hemocytometer. These experiments were performed at least three times, and each value is the mean ± SD of 5 mice per group.
Discussion
Herein, we present a novel way to present immunogenic peptide epitopes to a host's immune system based on a monomeric linear protein that self assembles into a nanoparticle. This platform technology has been considered an effective approach to stimulate an antigen specific immune response that can help a host control an infectious agent [20,21,22].
A number of studies have demonstrated that the GRA proteins of T gondii are involved in parasite survival and virulence. GRA7 is secreted and has been found to be a promising candidate for the development of a vaccine against T. gondii in sheep. It was more effective in eliciting significant immunity against T. gondii when administrated with GRA1 or GRA4 [13,23,24].
Our previous studies have shown that the T gondii–specific HLA-B*07–restricted CD8+ T cell epitope LPQFATAAT derived from GRA720–28 elicited IFN-γ from human PBMCs [10]. In mice, LPQFATAAT elicited CD8+ T cell specific IFN-γ with the help of a universal CD4+ epitope and adjuvants, GLA-SE (TLR4 agonist) and Pam2Cys and confers protection of HLA-B07 transgenic mice from type II parasite challenge.
The inclusion of PADRE, a synthetic peptide that binds promiscuously to variants of the human MHC class II molecule DR and is effective in mice, also augments CD8+ T cell effector functions by producing IL2 which augments induction of CD4+ T helper cells [24,25]. Both CD4+ and CD8+ epitopes are important components in the formulation of successful vaccines by driving a protective response [26]. Adjuvants also contribute to the success of vaccination. Our recent studies show GLA-SE, a specially formulated Toll like receptor 4 (TLR4) agonist, is very effective as an adjuvant providing CD8+ T cells producing IFN-γ when used for immunizations against T. gondii in mice [10,27].
This current study is intended to develop new tools in a rational design for developing a vaccine against toxoplasmosis. To further evaluate the protective efficacy of GRA720–28, we designed and produced a prototypic Toxoplasma vaccine based on a highly versatile self-assembling protein nanoparticle platform that can repetitively display PADRE-GRA720–28 antigenic epitopes and tested whether it could be a potent vaccine against the challenge with different type of T. gondii strains. The nanoparticles are small ∼ 38nm in diameter, and contain strong hydrophobic oligomerization domains that drive self-assembly of the monomer proteins. Recently, these particles have shown to be taken up by mouse macrophages and bone marrow-derived DC [28]. In other studies, they have been used to induce in vivo immune neutralizing antibodies against a SARS virus epitope [29].
In the present study, we evaluated the immunogenic and protective potential of these nanoparticles with PADRE and GRA720–28 in human transgenic HLA-B*07 mice. Our data showed that HLA-B*07 mice immunized with the nanoparticles elicit protective immune responses against T. gondii infection. Consistent with former studies, mice immunized with the linked peptides in particles (PADRE and GRA7) elicit higher IFN-γ production than mice immunized with single PADRE and GRA7 peptides in saline . We have also found that the particles without these peptides (control SAPN) are not immunogenic by themselves and did not contribute to significant immune responses. In the GRA720–28 SAPN, the contribution of GRA720–28 induction of CD8+ to produce IFN-γ is significantly increased but still not high (Fig 2A, B). PADRE contributes to production of more IFN-γ response on the IFN-γ ELISpot assay (Fig 2B). This work indicates that PADRE is an effective CD4+ Th epitope that contributes to the peptide construct.
This is in correlation with our previous studies that showed GRA7 peptide in combination with PADRE and the adjuvant GLA-SE elicits IFN-γ in splenocytes of HLA-B*0702 [10]. Challenges with the type II parasites in HLA-B*0702 mice demonstrated a 54% reduction from a mean of 335 to 155 cysts per brain [10] . In this current study, our data using GRA720-28 nanoparticles showed a 72% reduction from a mean of 685 to 192 cysts. Thus, GRA720-28 SAPNs are capable of more protection against type II T. gondii strains.
However, none of these vaccine regimens provided complete protection against T. gondii type I and II strains since brain cysts and parasites in peritoneal fluid are still detected. By varying the dose amount or interval, use of an adjuvant, or inclusion of other T helper epitopes we may be able to improve upon this initial response.
Lastly, the identification of an immunogenic peptide, which is capable of generating parasite specific CD4+ T cells and antibodies to protect mice, might help develop more robust vaccines against T. gondii. In this regard, it is possible that the following will improve our nanoparticle platform by adding protective peptides with HLA class I, II and B-cell epitopes. It has already been shown that the P. falciparum circumsporozoite protein (PfCSP) derived B-cell epitopes SAPN elicited higher amounts of antibodies with greater avidity than antibodies produced against a near full length recombinant PbCSP delivered with ISA-720 adjuvant [4]. Antibodies have not been considered to be the primary protective mechanism for T. gondii but rather cell mediated immunity with the induction of cytolytic T cells and interferon gamma production is [25,30,31,32]. Nonetheless antibody may contribute to protection as well.
It also will be of interest to transfer the GRA720-28 (LPQFATAAT) epitope to the GRA6 C-terminus. Previous studies have shown that optimal processing and immunodominance is determined by the location of the peptide epitope at the C-terminus of the GRA6 antigenic precursor. Thereby this determines immunogenicity and protection against the Toxoplasma gondii parasite [33].
Our future approach also will include the identification of additional peptides using bioinformatics, binding affinity assays, and study of responses of other supertypes of mice, e.g., HLA-A02 supertype family, which is present in 47% of the Europeans and 25% of the world population [18] and HLA-A*1101 [27].
In summary, our study shows that GRA720-28 SAPNs are capable of eliciting immune responses and protection against type I and II T. gondii strains. These SAPN proteins are inexpensive, can be produced in high quantities, and do not require, at least in mice, a separate adjuvant. They hold promise as vaccines. Potential improvements to this vaccine could be made with the addition of epitopes from various T. gondii proteins encompassing several of the parasite life stages in a single platform that could elicit a protective immune response for supermotifs present for all the human population, which could elicit protective immunity.
Acknowledgments
We gratefully thank Kevin Muite and Ani Solanki for technical help, Ernest Mui for helpful suggestions, Laura Knoll (Wisconsin) for the luciferase expressing parasites, and Boris Streipen, (University of Georgia) for the RH-YFP parasites. We gratefully acknowledge support of this work from the Mann Cornwell, Engel and Rooney-Alden, Langel, Rosenthal and Jensen families. This work also was supported by DMID-NIAID U01 AI77887, R01 27530 (to RM), Knights Templar Eye Foundation (to KE), and The Research to Prevent Blindness Foundation (to RM).
The funding sources had no influence in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
PB has an interest in the company Alpha-O Peptides that has patents or patents pending on the technology.
All authors contributed to concept, design, experiments, analysis, writing, and final approval of this manuscript.
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–344. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAuley J, Boyer KM, Patel D, Mets M, Swisher C, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 3.McCoy ME, Golden HE, Doll TA, Yang Y, Kaba SA, et al. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine. Malar J. 12:357. doi: 10.1186/1475-2875-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, et al. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183:7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaba SA, McCoy ME, Doll TA, Brando C, Guo Q, et al. Protective antibody and CD8+ T-cell responses to the Plasmodium falciparum circumsporozoite protein induced by a nanoparticle vaccine. PLoS One. 7:e48304. doi: 10.1371/journal.pone.0048304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Q, Dasgupta D, Doll TA, Burkhard P. Lanar DE Expression, purification and refolding of a self-assembling protein nanoparticle (SAPN) malaria vaccine. Methods. 60:242–247. doi: 10.1016/j.ymeth.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivakumar SM, Safhi MM, Kannadasan M, Sukumaran N. Vaccine adjuvants - Current status and prospects on controlled release adjuvancity. Saudi Pharm J. 19:197–206. doi: 10.1016/j.jsps.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson DJ, Jacobs D, Saman E, Dubremetz JF, Wright SE. In vivo expression and distribution of dense granule protein 7 (GRA7) in the exoenteric (tachyzoite, bradyzoite) and enteric (coccidian) forms of Toxoplasma gondii. Parasitology. 1999;119(Pt 3):259–265. doi: 10.1017/s0031182099004692. [DOI] [PubMed] [Google Scholar]
- 9.Kong JT, Grigg ME, Uyetake L, Parmley S, Boothroyd JC. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis. 2003;187:1484–1495. doi: 10.1086/374647. [DOI] [PubMed] [Google Scholar]
- 10.Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, et al. Toxoplasma gondii HLA- B*0702-restricted GRA7(20-28) peptide with adjuvants and a universal helper T cell epitope elicits CD8(+) T cells producing interferon-gamma and reduces parasite burden in HLA-B*0702 mice. Hum Immunol. 73:1–10. doi: 10.1016/j.humimm.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, et al. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min J, Qu D, Li C, Song X, Zhao Q, et al. Enhancement of protective immune responses induced by Toxoplasma gondii dense granule antigen 7 (GRA7) against toxoplasmosis in mice using a prime-boost vaccination strategy. Vaccine. 30:5631–5636. doi: 10.1016/j.vaccine.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Jongert E, de Craeye S, Dewit J, Huygen K. GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunol. 2007;29:445–453. doi: 10.1111/j.1365-3024.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 14.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 15.Kammerer RA, Schulthess T, Landwehr R, Lustig A, Engel J, et al. An autonomous folding unit mediates the assembly of two-stranded coiled coils. Proc Natl Acad Sci U S A. 1998;95:13419–13424. doi: 10.1073/pnas.95.23.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman S, Machaidze G, Lustig A, Aebi U, Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomedicine. 2006;2:95–102. doi: 10.1016/j.nano.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science. 1996;274:761–765. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- 18.Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, et al. Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLA-A*0201. Vaccine. 29:754–762. doi: 10.1016/j.vaccine.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Yong W, Deng Y, Kallenbach NR, Lu M. Atomic structure of a tryptophan-zipper pentamer. Proc Natl Acad Sci U S A. 2004;101:16156–16161. doi: 10.1073/pnas.0405319101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials. 2007;28:3427–3436. doi: 10.1016/j.biomaterials.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 22.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Hiszczynska-Sawicka E, Oledzka G, Holec-Gasior L, Li H, Xu JB, et al. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii. Vet Parasitol. 177:281–289. doi: 10.1016/j.vetpar.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 24.Brown CR, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 25.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 26.Alexander J, Oseroff C, Dahlberg C, Qin M, Ishioka G, et al. A decaepitope polypeptide primes for multiple CD8+ IFN-gamma and Th lymphocyte responses: evaluation of multiepitope polypeptides as a mode for vaccine delivery. J Immunol. 2002;168:6189–6198. doi: 10.4049/jimmunol.168.12.6189. [DOI] [PubMed] [Google Scholar]
- 27.Cong H, Mui EJ, Witola WH, Sidney J, Alexander J, et al. Human immunome, bioinformatic analyses using HLA supermotifs and the parasite genome, binding assays, studies of human T cell responses, and immunization of HLA-A*1101 transgenic mice including novel adjuvants provide a foundation for HLA-A03 restricted CD8+T cell epitope based, adjuvanted vaccine protective against Toxoplasma gondii. Immunome Res. 6:12. doi: 10.1186/1745-7580-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCoy ME, Golden HE, Doll TA, Yang Y, Kaba SA, et al. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine. Malar J. 12:136. doi: 10.1186/1475-2875-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pimentel TA, Yan Z, Jeffers SA, Holmes KV, Hodges RS, et al. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Des. 2009;73:53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, et al. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 32.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 33.Feliu V, Vasseur V, Grover HS, Chu HH, Brown MJ, et al. Location of the CD8 T cell epitope within the antigenic precursor determines immunogenicity and protection against the Toxoplasma gondii parasite. PLoS Pathog. 9:e1003449. doi: 10.1371/journal.ppat.1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]


