Abstract
Background
Clinical consequences of active surveillance compared with immediate treatment have been evaluated in patients with low-risk prostate cancer; yet, its financial benefits have not been adequately studied in Canada or elsewhere. Our study objective was to evaluate the direct costs associated with active surveillance and immediate treatment in the Canadian context.
Methods
We developed a Markov model with Monte Carlo microsimulations to estimate the Canadian cost of prostate cancer associated with immediate treatment and active surveillance strategies. The patients receiving active surveillance were assumed to receive delayed treatment at a rate of 8.35%, 4.17% and 2.1% per year for the first 2 years, years 3 to 5, and years 6 to 10 of follow-up, respectively. All costs were assigned in Canadian dollars and reflect Quebec’s health system.
Results
With active surveillance, the mean cost of prostate cancer management over the first year and 5 years of follow-up was estimated at $6200 (95% confidence interval [CI] $6083–$6317) per patient. The mean cost corresponding to immediate treatment was estimated at $13 735 (95% CI $13 615–$13 855) per patient. We estimated that patients receiving active surveillance who received delayed treatment incurred higher costs of $16 257 per patient.
Interpretation
Active surveillance could offer important economic benefits to the Canadian health system, estimated at $96.1 million for each annual cohort of incident prostate cancer. These results add to the economic rationale advocating active surveillance for eligible men with low-risk prostate cancer.
Prostate cancer is the most common cancer and the third leading cause of cancer-related mortality in Canadian men. In Canadian Cancer Statistics 2011, an incidence of 122 per 100 000 person-years was reported, which is twice the incidence of lung or colorectal cancer, respectively the second and third leading causes of cancer.1 Whereas the incidence of colorectal cancer has been stable over the last 30 years, the incidence of prostate cancer has increased by 50%. Correspondingly, the economic burden of prostate cancer is also very high in Canada and elsewhere.2–6
Studies have shown that most cancers at diagnosis are low to intermediate risk.7,8 The natural history of prostate cancer is variable, but many cancers, especially the low-risk category, may be considered indolent and may not require immediate treatment. Active surveillance9 with delayed treatment is one of the accepted alternatives to immediate treatment for low-risk cancers.7,10,11 Active surveillance presents several advantages, especially for older men and for those with low life expectancy, but also for those with good life expectancy for whom active surveillance may allow the preservation of quality of life compared with immediate treatment.12–14 One study has shown that when quality of life associated with the clinical discomfort of initial treatment and the psychological discomfort of living under active surveillance were taken into consideration, the quality-adjusted life expectancy (QALE) was greater for active surveillance (11.07 QALE) than for brachytherapy (10.57 QALE), intensity-modulated radiotherapy (10.51 QALE) and radical prostatectomy (10.23 QALE).15 In addition, a second study has shown that in a group of low-risk patients aged 70 years, the initial treatment with radiotherapy had an advantage of about 0.4 QALE compared with watchful waiting,16 but no benefit in the case of radical prostatectomy (9.4 QALE for both radical prostatectomy and watchful waiting). Despite published guideline recommendations,9,17 overtreatment of prostate cancer is common in the United States and elsewhere, with about 70% to 90% of patients undergoing immediate treatment.18,19 In Canada, about 75% of patients with prostate cancer have received immediate treatment from 1995 through 2002.20 It is believed today that more than half of these patients did not require immediate treatment at the time of diagnosis, but the treatments led to cost and morbidity.21,22
The costs of active surveillance with delayed treatment compared with the costs of immediate treatment were recently evaluated from the perspective of the US health care system by Keegan and colleagues.23 The corresponding estimates in the Canadian context of universal health care are unknown. Despite the fact that clinical practice for prostate cancer is similar in the US and Canada, the cost of health care services is likely to differ between the countries. In addition, the US model did not take into consideration mortality and recurrence of prostate cancer, 23 which are elements that could substantially affect the percentage of patients requiring treatment and cost estimates, especially on a long-term basis.
The primary objective of this study was to develop a model to estimate the direct cost associated with active surveillance and immediate treatment for low-risk prostate cancer in Canada. This model accounted for the rate of disease progression requiring delayed treatment, overall mortality, and disease recurrence requiring additional treatment, in the context of the public health care system in Quebec. The second objective was to compare Canadian and US cost estimates.
Methods
Modelling assumptions
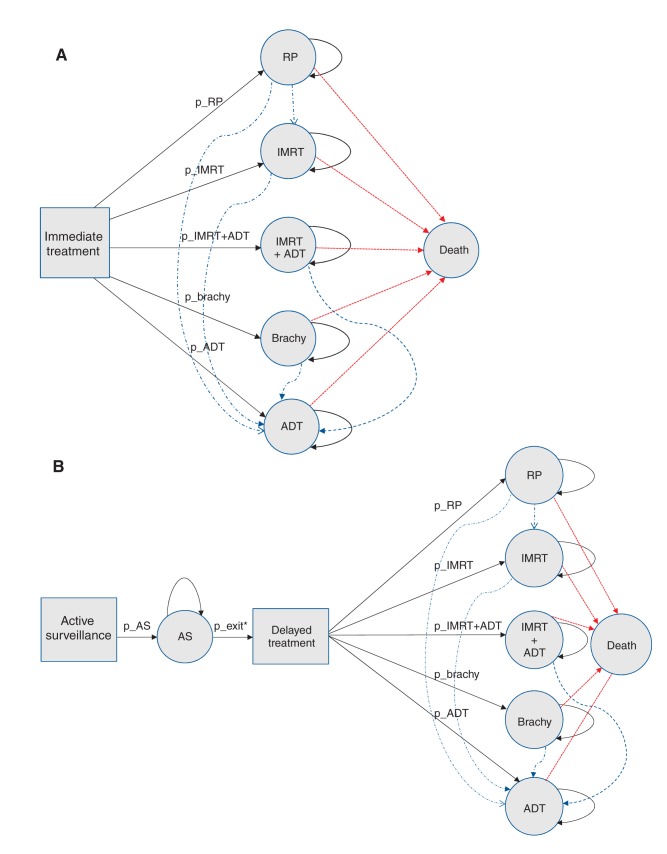
We developed a Markov model with Monte Carlo microsimulations to simulate the management of low-risk prostate cancer and cost over the first year, 5 years and 10 years of follow-up, accounting for the rate of death and disease recurrence or progression. We modelled 2 alternative management strategies: active surveillance with delayed treatment, and immediate treatment. The Markov model is a state transition model with a cycle length of 1 year; Figure 1A shows the strategy of immediate treatment, and Figure 1B presents the strategy where patients initially receive active surveillance and can receive delayed treatment. As Figure 1 shows, the patients receiving active surveillance were assumed to receive delayed treatment at a rate of 8.35%, 4.17% and 2.1% per year for the first 2 years, years 3 to 5, and years 6 to 10 of follow-up, respectively. The 5 health states defined in relation to immediate treatment options are as follows: radical prostatectomy, intensity-modulated radiotherapy, brachytherapy, androgen deprivation therapy, and intensity-modulated radiotherapy plus androgen deprivation therapy for 6 months. We derived the models’ assumptions from a Canadian study involving an active surveillance cohort.11
Figure 1:
Markov models with transition to death (red lines) and recurrence requiring additional treatment (blue lines) for (A) immediate treatment and (B) active surveillance. Note: ADT = androgen deprivation therapy, Brachy = brachytherapy, IMRT = intensity-modulated radiotherapy, p_ADT = probability of receiving ADT, p_AS = probability of receiving active surveillance, p_Brachy = probability of receiving brachytherapy, p_IMRT = probability of receiving IMRT, p_IMRT+ADT = probability of receiving IMRT + ADT, p_RP = probability of receiving radical prostatectomy, RP = radical prostatectomy. *p_exit = yearly rate of switch to active treatment, 0.0835 (year 1 and 2 of follow-up), 0.0417 (year 3 to 5 of follow-up) and 0.021 (year 6 to 10 of follow-up).11 Note: The following values were used in the Markov models: p_AS = 1, p_RP = 0.26, p_IMRT = 0.34, p_IMRT+ADT = 0.13, p_Brachy = 0.20, p_ADT = 0.074, 1-year probability of death = 0.038; 1-year probability of recurrence requiring additional treatment = 0.139 for active surveillance and 0.0257 for immediate treatment.11
Cost assignments
All direct costs were assigned in 2012 Canadian dollars and estimated from the 2012 perspective of the public health care system in Quebec. The cost of active surveillance and treatments were categorized into the following: initial cost assigned in the first year, and follow-up cost assigned over the following 5- and 10-year periods. The disease-related method24 has been used to estimate costs of treatments and active surveillance. The cost components and the quantities of resources used were based on specific protocols used at the McGill University Health Centre; however, these protocols are similar to those described by Keegan and colleagues.23 The cost components, unit costs and sources are presented in Table 1.25–29 We estimated the cost of treatments and active surveillance by summing the quantities of resources used and multiplying by the unit cost of each cost component. The active surveillance protocol was derived from Klotz and colleagues.11 This consists of the following: (1) a prostate-specific antigen test performed every 3 months for 2 years and then every 6 months in stable patients; and (2) initial biopsy and confirmatory biopsy performed 6 to 12 months after the initial biopsy, and then every 3 to 4 years. To reflect the time value of money, we used a standard discount rate of 5%.30
Table 1: Cost components and unit costs related to management of prostate cancer (2012 Canadian dollars).
| Cost components | Cost per unit, $ | Source |
|---|---|---|
|
Component 1 |
||
| Initial office consultation, urology |
77 |
RAMQ‡ list25 |
| Initial office consultation, radiation oncology |
133 |
RAMQ‡ list25 |
|
Component 2 |
||
| Urologist reimbursement for prostate biopsy |
78 |
RAMQ‡ list25 |
| Prostate biopsy (based on 12-sample needle core) | ||
| Pathology |
158 |
MUHC: unpublished data, 2012 |
| Professional and technical fees |
183 |
MUHC: unpublished data, 2012 |
| Prostate analysis after surgery (prostatectomy with obturator lymph nodes) |
||
| Pathology |
204 |
MUHC: unpublished data, 2012 |
| Professional and technical fees |
640 |
MUHC: unpublished data, 2012 |
| PSA test |
11 |
MUHC: unpublished data, 2012 |
|
Component 3 |
||
| Office visits, urology |
59 |
RAMQ‡ list25 |
| Office visits, radiation oncology |
44 |
RAMQ‡ list25 |
| Urologist reimbursement for radical prostatectomy |
922 |
RAMQ‡ list25 |
| Radiation oncologist reimbursement for radiotherapy |
||
| Computed tomography planning and IGRT plan |
1010 |
RAMQ‡ list25 |
| Intensity-modulated radiotherapy session (includes office visit, image fusion and checking) |
81 |
RAMQ‡ list25 |
| Dose planning, ultrasonography guidance and interstitial implant (brachytherapy) |
735 |
RAMQ‡ list25 |
|
Component 4 |
||
| Medication costs (goserelin acetate implant every 3 mo) |
1088 |
RAMQ‡ list26 |
| Nursing (average salary/mo) |
6667 |
MUHC: unpublished data, 2012 |
|
Component 5 |
||
| Surgical procedure* |
4547 |
Ministère de la Santé et des Services sociaux;27 Ministère de la Santé et des Services sociaux: unpublished data, 2012§ |
| Brachytherapy procedure (palladium-103) including seeds cost* |
6700 |
CETS;28 Canadian Patient Cost Database29 |
| Intensity-modulated radiotherapy procedure† |
7402 |
MUHC: unpublished data, 2012 |
|
Component 6 |
||
| Anesthesia for surgery |
650 |
MUHC: unpublished data, 2012 |
| Anesthesia for brachytherapy | 250 | MUHC: unpublished data, 2012 |
Note: CETS = Conseil d’évaluation des technologies de la santé du Québec, IGRT = image-guided radiotherapy, MUHC = McGill University Health Centre, PSA = prostate-specific antigen, RAMQ = Régie de l’assurance maladie du Québec. *This amount includes intervention, nursing care, diagnosis and therapeutic services. †Includes cost of dosimetry (radiotherapist, planning system and information system), physics quality assurance (physicist, physics associates, specialized quality assurance equipment, planning system and information system) and treatment preparation and delivery (radiotherapy, linear accelerator, nurse, information system) equivalent of 38 fractions. This value does not include overheads. ‡The administrator of the public and universal health care insurance program in Quebec. The costs of medical procedures related to treatments and medical visit costs were based on the RAMQ’s billing manual,25 and the costs of medications were based on the RAMQ’s list of medications approved for public reimbursement.26 §Details on collected variables can be found in Performance hospitalière (APR-DRG [J57]).27
Statistical analysis
Cost analyses
With a more than 50% rate of low-risk prostate cancer at diagnosis7,8 and based on the 2011 Canadian incidence of prostate cancer,1 we estimated about 12 750 patients as potential candidates for active surveillance. We simulated the disease management and associated cost of 12 750 incident patients, initially receiving active surveillance or assigned to immediate treatment, over the first year and 5 and 10 years of follow-up by applying the corresponding Markov models (Figure 1). The mean cost per patient is the mean of individual cost estimations obtained with Monte Carlo microsimulations.
Sensitivity analysis
To compare our Canadian cost estimates with US cost estimates, we changed our Markov model to reflect assumptions derived from Keegan and colleagues.23 We considered a discount rate of 3% and 10%. To estimate whether the cost difference between active surveillance and immediate treatment strategies was maintained over a longer period, we modelled a 15-year period of follow-up by assuming mortality after 10 years of follow-up of 8.16% per year. This rate corresponds to the Statistics Canada estimate for the general population aged 75 and older, which is the age category of the simulated incident cases after 10 years of follow-up. Finally, we assumed that 40% and 60% of yearly incident cases are eligible for active surveillance, which yielded a number of 10 200 and 15 300 patients, respectively.
Results
Cost of active surveillance and treatment
Initial and 5-year follow-up costs specific to each treatment are presented in Table 2. Radical prostatectomy and brachytherapy were the treatments with the lowest initial cost ($7428 and $8455, respectively), and the intensity-modulated radiotherapy plus androgen deprivation therapy was the intervention with the highest cost, estimated at $14 444. In contrast, the androgen deprivation therapy was the treatment with the highest cost over the 5-year follow-up period, estimated at $23 202. These estimates were all higher than the initial and 5-year follow-up cost of active surveillance, estimated at $1224 and $1767, respectively.
Table 2: Initial and 5-year cost of treatment and active surveillance (2012 Canadian dollars).
| Cost, $ | ||
|---|---|---|
| Treatment type |
Initial |
5-year follow-up |
| Active surveillance |
1 224 |
1 767 |
| Radical prostatectomy |
7 428 |
929 |
| Intensity-modulated radiotherapy |
12 261 |
618 |
| Intensity-modulated radiotherapy and androgen deprivation therapy* |
14 444 |
618 |
| Brachytherapy |
8 455 |
618 |
| Primary androgen deprivation therapy | 5 136 | 23 202 |
*For 6 months.
Treatment course and associated costs
At the end of 5 years of follow-up, with the use of the Markov model we estimated 2805 (22.0%) patients having received delayed treatment, 442 (3.5%) having received delayed treatment and having died, 2205 (17.3%) having died while receiving active surveillance and 7298 (57.2%) still receiving active surveillance. The corresponding rates after 10 years were as follows: 2938 (23.0%) of patients having received delayed treatment, 984 (7.7%) having received delayed treatment and having died, 3454 (27.1%) having died while receiving active surveillance and 5374 (42.1%) still receiving active surveillance.
The cost per patient over the first year and 5 years of follow-up was estimated at $13 735 (95% CI $13 615–$13 855) for immediate treatment and $6200 (95% CI $6083–$6317) for active surveillance (Table 3). The cost associated with recurrence accounted for $1589 and $1558, respectively, of these costs. With a 5% discount rate, these figures were $13 166 (95% CI $12 966–$13 165) and $5515 (95% CI $5413–$5619), respectively, corresponding to a relative reduction of 57.8%. The 10-year follow-up period showed that the absolute cost benefits observed in the previous period were maintained at the end of this period.
Table 3: Cost estimates for active surveillance and immediate treatment over the first year and 5 to 10 years of follow-up.
| Period of follow-up; strategy | % |
Mean cost per patient (95% CI)†, $ | Cost difference (immediate treatment v. active surveillance) |
|||
|---|---|---|---|---|---|---|
| Mortality* | Recurrence* | Discount* | Absolute, $ | Relative, % | ||
|
First year and 5-year follow-up | ||||||
| Active surveillance |
3.8 |
13.9 |
— |
6 200 (6 083–6 317) |
7 535 |
54.9 |
| 3.8 |
13.9 |
5 |
5 515 (5 413–5 619) |
7 551 |
57.8 |
|
| Immediate treatment |
3.8 |
2.57 |
— |
13 735 (13 615–13 855) |
||
| 3.8 |
2.57 |
5 |
13 166 (12 966–13 165) |
|||
|
First year and 10-year follow-up | ||||||
| Active surveillance |
3.8 |
13.9 |
— |
10 600 (10 377–10 822) |
7 808 |
42.4 |
| 3.8 |
13.9 |
5 |
8 484 (8 313–8 654) |
7 755 |
47.8 |
|
| Immediate treatment | 3.8 |
2.57 |
— |
18 408 (18 162–18 653) |
||
| 3.8 | 2.57 | 5 | 16 239 (16 060–16 418) | |||
Note: CI = confidence interval. *Yearly rate. †The 95% CI of the mean cost was obtained by the simulation of 1000 samples of equal sample size of 12 750 patients.
We estimated that patients continuing to receive active surveillance over the 5-year period of follow-up would incur a mean cost of $2764 compared with $16 257 in the group of patients receiving delayed treatment. Depending on the type of treatment, this cost varied from $12 821 (95% CI $12 452–$13 190) per patient having received surgery or $14 512 (95% CI $14 046–$14 973) per patient having received brachytherapy, to $20 377 (95% CI $19 826–$20 927) per patient having received intensity-modulated radiotherapy plus androgen deprivation therapy. The estimated cost associated with the recurrence accounted for 25%, 28% and 19%, respectively, of these costs. Furthermore, delaying androgen deprivation therapy would allow important savings over immediate treatment, estimated at $4000 per patient per year.
Furthermore, over the 10 years of follow-up, the lowest mean cost was still observed in the group of patients having received surgery ($20 935, 95% CI $20 117–$21 753) or brachytherapy ($23 401, 95% CI $22 421–$24 382). However, highest cost was estimated for patients having received androgen deprivation therapy ($30 524, 95% CI $28 813–$32 236).
Total cost estimation
The overall cost savings attributable to active surveillance over the first year and 5 years of follow-up was estimated at $96.1 million (Table 4). This value is explained by a total savings of $104.4 million obtained by avoiding treatment in 17.3% of patients who never required treatment and died from causes other than prostate cancer, and 57.2% of patients still receiving active surveillance, and a supplementary cost of $8.2 million (equivalent to $500/patient per year) for delaying treatment for 25.5% of patients. When the rate of patients eligible for active surveillance was set to 40% and 60%, estimated total costs were $76.9 million, and $115.3 million, respectively (data not shown). Similar values were observed over 10 years of follow-up, with an overall cost savings of $99.5 million (Table 4).
Table 4: Total cost for active surveillance and immediate treatment and possible cost savings corresponding to an annual cohort of 12 750 patients with prostate cancer stratified by patients’ treatment status.
| Treatment status | % (no.) of patients | Mean cost per patient, $ |
Total cost, $ |
Cost difference, $ |
||||
|---|---|---|---|---|---|---|---|---|
| Active surveillance | Immediate treatment | Active surveillance | Immediate treatment | Per patient | Total | |||
|
First year and 5-year follow-up |
||||||||
| Patients requiring treatment |
25.5 |
(3 247) |
16 257 |
13 735 |
52 786 479 |
44 597 545 |
2 522 |
8 188 934* |
| Patients not requiring treatment |
74.5 |
(9 503) |
2 753 |
13 735 |
26 161 759 |
130 523 705 |
–10 982 |
–104 361 946† |
| Total |
100.0 |
(12 750) |
6 200 |
13 735 |
79 050 000 |
175 121 250 |
–7 535 |
–96 071 250‡ |
|
First year and 10-year follow-up |
||||||||
| Patients requiring treatment |
30.8 |
(3 922) |
25 552 |
18 407 |
100 214 944 |
72 192 254 |
7 145 |
28 022 690* |
| Patients not requiring treatment |
69.2 |
(8 828) |
3 959 |
18 407 |
34 950 052 |
162 496 996 |
–14 448 |
–127 546 944† |
| Total | 100.0 | (12 750) | 10 600 | 18 407 | 135 150 000 | 234 689 250 | –7 807 | –99 539 250‡ |
*Additional cost attributable to delayed treatment. †Cost savings attributable to active surveillance. ‡Total cost savings obtained through active surveillance.
Sensitivity analysis
The results of the sensitivity analysis are presented in Table 5. First, the model integrating assumptions from Keegan and colleagues23 has simulated that 30% of patients receiving active surveillance have received delayed treatment after 5 years of follow-up, and 45% after 10 years of follow-up. The corresponding cost over the first year and a 5-year period of follow-up was $12 712 (95% CI $12 161–$12 381) per patient receiving immediate treatment, and $5855 (95% CI $5780–$5930) per patient receiving active surveillance. Thus, the mean cost savings attributable to the active surveillance strategy was $6416, corresponding to a relative reduction of 52.3% from the cost of immediate treatment. In addition, similar trends have been observed between US cost estimates23 and Canadian estimates with respect to the number of years of active surveillance (Appendix 1). With the exception of androgen deprivation therapy, delayed treatment is associated with an estimated increase of treatment cost between 3.3% and 6.7% per year (data not shown). Second, the sensitivity analyses performed with various discount rates have shown high consistency with the primary results. Finally, the model simulating 15 years of follow-up counts 2297 (18.0%) patients having received delayed treatment, 2102 (16.5%) having received delayed treatment and having died, 5259 (41.2%) having died while receiving active surveillance, and 3092 (24.3%) still receiving active surveillance at the end of this period. Furthermore, active surveillance was associated with a cost reduction of $7000 to $8000 per patient when compared with immediate treatment.
Table 5: Sensitivity analysis.
| Period of follow-up; strategy | % |
Mean cost per patient (95% CI),† $ |
Cost difference (immediate treatment v. active surveillance) |
|||
|---|---|---|---|---|---|---|
| Mortality* | Recurrence* | Discount* | Absolute, $ | Relative, % | ||
|
Model integrating assumptions derived from Keegan et al.23‡ |
||||||
| First year and 5-year follow-up |
||||||
| Active surveillance |
0 |
0 |
— |
5 855 (5 780–5 930) |
6 416 |
52.3 |
| 0 |
0 |
5 |
5 474 (5 408–5 539) |
6 237 |
53.3 |
|
| Immediate treatment |
0 |
0 |
— |
12 271 (12 161–12 381) |
||
| 0 |
0 |
5 |
11 711 (11 625–11 798) |
|||
| First year and 10-year follow-up |
||||||
| Active surveillance |
0 |
0 |
— |
9 201 (9 069–9 334) |
5 843 |
38.8 |
| 0 |
0 |
5 |
7 673 (7 584–7 763) |
6 165 |
44.6 |
|
| Immediate treatment |
0 |
0 |
— |
15 044 (14 806–15 282) |
||
| 0 |
0 |
5 |
13 838 (13 679–13 997) |
|||
|
Model with discount rates of 3% and 10% |
||||||
| First year and 5-year follow-up |
||||||
| Active surveillance |
3.8 |
13.9 |
3 |
5 810 (5 701–5 920) |
7 496 |
56.3 |
| 3.8 |
13.9 |
10 |
5 134 (5 039–5 230) |
7 367 |
58.9 |
|
| Immediate treatment |
3.8 |
2.57 |
3 |
13 306 (13 199–13 413) |
||
| 3.8 |
2.57 |
10 |
12 501 (12 417–12 586) |
|||
| First year and 10-year follow-up |
||||||
| Active surveillance |
3.8 |
13.9 |
3 |
9 438 (9 246–9 629) |
7 601 |
44.6 |
| 3.8 |
13.9 |
10 |
7 357 (7 214–7 499) |
7 239 |
49.6 |
|
| Immediate treatment |
3.8 |
2.57 |
3 |
17 039 (16 836–17 242) |
||
| 3.8 |
2.57 |
10 |
14 596 (14 461–14 731) |
|||
|
Model with 15 years of follow-up |
||||||
| Active surveillance |
3.8§; 8.16¶ |
13.9 |
— |
14 806 (14 480–15 132) |
8 063 |
35.3 |
| 3.8§; 8.16¶ |
13.9 |
5 |
11 082 (10 853–11 311) |
7 013 |
38.8 |
|
| Immediate treatment |
3.8§; 8.16¶ |
2.57 |
— |
22 869 (22 511–23 227) |
||
| 3.8§; 8.16¶ | 2.57 | 5 | 18 095 (17 864–18 324) | |||
Note: CI = confidence interval. *Yearly rate. †The 95% CI of the mean cost was obtained by the simulation of 1000 samples of equal sample size of 12 750 patients. ‡The assumptions are as follows: 1) 2 additional biopsies in the first 5-year period of follow-up; 2) rate of death and rate of recurrence, both set to 0; 3) probability of receiving each specific treatment assumed to be 0.4 for radical prostatectomy, 0.25 for intensity-modulated radiotherapy, 0.1 for intensity-modulated radiotherapy plus androgen deprivation therapy, 0.15 for brachytherapy and 0.1 for androgen deprivation therapy; and 4) probability of receiving delayed treatment of 7% per year, in the first 5 years of follow-up, and 4.5% per year in the following 5-year period. §Years 1 to 10. ¶Years 11 to 15.
Interpretation
Our study demonstrates that for eligible patients, active surveillance could offer economic benefits to the health care system. At the national level, the overall cost savings of an annual cohort of incident prostate cancers managed with active surveillance over a first year and 5 years of follow-up was substantial. The cost savings are explained by the low cost of active surveillance in Quebec, the avoidance of the high cost of treatment for about three-quarters of eligible patients, and a minimal additional cost related to delayed treatment for one-quarter of the patients, estimated at $500 per patient per year. In addition, these cost benefits could be maintained over a longer period of 10 to 15 years of follow-up. At the end of the 15-year period, we estimated that one-quarter of patients would still be receiving active surveillance. These patients would likely be 80 years or older, and would likely cease active surveillance or no longer be eligible for treatment.
The US cost estimates23 are higher than Canadian estimates but similar trends were observed for specific treatments in relation to the number of years of active surveillance, and treatment delay, respectively (Appendix 1). Whereas the mean cost of surgery, brachytherapy, intensity-modulated radiotherapy, and intensity-modulated radiotherapy plus androgen deprivation therapy is slightly increased with each additional year of active surveillance, the mean cost of androgen deprivation therapy is considerably decreased with the delay of treatment initiation. In addition, Keegan and colleagues23 suggested that the accrued cost of patients receiving active surveillance undergoing delayed treatment are substantial, and have highlighted the importance of rapidly identifying those patients likely to need immediate treatment. Our results confirm that delayed treatment is associated with additional costs in patients requiring immediate treatment, but this cost is minimal. The cost of treatments will increase between 3.3% and 6.7% per year; however, this increase will be entirely balanced by the 5% yearly discount rate of a delayed expense. In addition, when no additional clinical benefits are expected with an early initiation of androgen deprivation therapy, clinicians can be reassured that there is no economic reason for it.
Four other studies have evaluated the cost of active surveillance and the cost of immediate treatment (i.e., radical prostatectomy, brachytherapy, intensity-modulated radiotherapy) with all but one reflecting US costs, which are considerably higher than Canadian costs.31–34 In addition, high variation has been observed across the US studies, mainly for the initial cost of treatments. Therefore, these estimates are higher in the study by Keegan and colleagues23 and correspond to 1.5 times the cost of brachytherapy and to 5.5 times the cost of active surveillance found by Eldefrawy and colleagues.31 Moreover, the only non-US evaluation of active surveillance cost was performed in Sweden and was compared with the cost of radical prostatectomy.34 These results showed that during a median follow-up of 12 years, the overall cost in the radical prostatectomy group was 34% higher than in the active surveillance group, corresponding to a difference of €6123.34
Limitations
Our study presents some limitations. First, this economic evaluation was mainly based on costs derived from the Régie de l’assurance maladie du Québec’s lists (billing manual and list of medications approved for public reimbursement). 25,26 Although medication costs, professional fees and laboratory costs are generally similar across Canadian provinces, the medical fees and honoraria may be sometimes lower in Quebec. However, we presumed that these differences were minor and would not affect the generalizability of our results to other Canadian provinces. Second, although our model expands on the work of Keegan and colleagues23 by accounting for risk of death and recurrence requiring additional treatment, this model does not account for costs associated with adverse effects or complications related to treatments. Third, we considered treatment distribution to be constant over time. Even if the probability of receiving radiotherapy can increase with age, we believe the variation will be minimal in a population aged 65 years and older, and its impact on cost estimates will not be substantial. However, we have evaluated the effect of cost savings from active surveillance in the case when only 10% of patients could receive radical prostatectomy after 10 years of follow-up (41.6% intensity-modulated radiotherapy, 16% intensity-modulated radiotherapy plus androgen deprivation therapy, 25% brachytherapy and 7.4% androgen deprivation therapy). The corresponding cost benefits of active surveillance were even more important. The decrease in the percentage of patients receiving both radical prostatectomy and intensity-modulated radiotherapy (as primary treatment followed by salvage therapy) is likely to be the main factor explaining this situation.
Conclusion
Ten years after diagnosis of prostate cancer, active surveillance with delayed treatment had a lower cost than immediate treatment. With health care costs growing rapidly and access to innovative medicines being limited or restricted by public funding, it is desirable to find ways to increase efficiency. Furthermore, optimizing the management of low-risk prostate cancer could result in cost reallocation and maximization of health care services offered to patients with prostate cancer. The results of our study add to the economic rationale advocating active surveillance for eligible men with low-risk prostate cancer and highlights estimated cost savings specific to the Canadian health system.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/2/2/E60/suppl/DC1
Supplementary Material
Acknowledgements
The authors thank the Coté Sharp Family Foundation for its financial contribution to the Program in Health Economics of Prostate Cancers at McGill University’s Division of Urology.
References
- 1.Canadian Cancer Statistics 2011 Ottawa (ON): Statistics Canada. Available: www.cancer-asian.com/images/news/Canadian_Cancer%20Statistics_2011_English.pdf (accessed 2012 June).
- 2.Cancer trends progress report — 2010/2011 update Bethseda (MD): National Cancer Institute; 2006.
- 3.Fourcade RO, Benedict A, Black LK, et al. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy, Spain and the UK. BJU Int 2010;105:49-56 [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, Black LK. The economic burden of prostate cancer. BJU Int 2011;108:806-13 [DOI] [PubMed] [Google Scholar]
- 5.Grover SA, Coupal L, Zowall H, et al. The economic burden of prostate cancer in Canada: forecasts from the Montreal Prostate Cancer Model. CMAJ 2000;162:987-92 [PMC free article] [PubMed] [Google Scholar]
- 6.Economic burden of illness in Canada, 1998 Ottawa (ON): Health Canada; 2002. Available: http://publications.gc.ca/collections/Collection/H21-136-1998E.pdf (accessed 2012 June).
- 7.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andriole GL, Crawford ED, Grubb RL, III, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohler J, Bahnson RR, Busby JE. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw 2010;8:162-200 [DOI] [PubMed] [Google Scholar]
- 10.Wu JN, Dall’Era MA. Active surveillance for localized prostate cancer--current practices and recommendations. ScientificWorldJournal 2010;10:2352-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31 [DOI] [PubMed] [Google Scholar]
- 12.Carlsson S, Aus G, Bergdahl S, et al. The excess burden of side-effects from treatment in men allocated to screening for prostate cancer. The Goteborg randomised population-based prostate cancer screening trial. Eur J Cancer 2011;47:545-53 [DOI] [PubMed] [Google Scholar]
- 13.Johansson E, Bill-Axelson A, Holmberg L, et al. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: the Randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol 2009;55:422-30 [DOI] [PubMed] [Google Scholar]
- 14.Thong MS, Mols F, Kil PJ, et al. Prostate cancer survivors who would be eligible for active surveillance but were either treated with radiotherapy or managed expectantly: comparisons on long-term quality of life and symptom burden. BJU Int 2010;105:652-8 [DOI] [PubMed] [Google Scholar]
- 15.Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA 2010;304:2373-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommers BD, Beard CJ, D’Amico AV, et al. Decision analysis using individual patient preferences to determine optimal treatment for localized prostate cancer. Cancer 2007;110:2210-7 [DOI] [PubMed] [Google Scholar]
- 17.Prostate cancer: diagnosis and treatment NICE clinical guideline 58. London (UK): National Institute for Health and Clinical Excellence;2008. [Google Scholar]
- 18.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 2010;28:1117-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford ED, Black L, Eaddy M, et al. A retrospective analysis illustrating the substantial clinical and economic burden of prostate cancer. Prostate Cancer Prostatic Dis 2010;13:162-7 [DOI] [PubMed] [Google Scholar]
- 20.Krahn MD, Zagorski B, Laporte A, et al. Healthcare costs associated with prostate cancer: estimates from a population-based study. BJU Int 2010;105:338-46 [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen PJ, Connolly D, Gavin A, et al. Prostate cancer mortality in screen and clinically detected prostate cancer: estimating the screening benefit. Eur J Cancer 2010;46:377-83 [DOI] [PubMed] [Google Scholar]
- 22.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keegan KA, Dall'Era MA, Durbin-Johnson B, et al. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer 2012;118:3512-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akobundu E, Ju J, Blatt L, et al. Cost-of-illness studies: a review of current methods. Pharmacoeconomics 2006;24:869-90 [DOI] [PubMed] [Google Scholar]
- 25.Manuel des médecins spécialistes. Québec (QC): Régie de l’assurance maladie du Québec; 2012. Available: www.ramq.gouv.qc.ca/fr/professionnels/medecins-specialistes/manuels/Pages/facturation.aspx (accessed 2012 June).
- 26.Manuel des pharmaciens. Québec (QC): Régie de l’assurance maladie du Québec; 2012. Available: www.ramq.gouv.qc.ca/fr/professionnels/pharmaciens/manuels/Pages/manuel-pharmaciens.aspx (accessed 2012 June).
- 27.Ministère de la Santé et des Services sociaux. Performance hospitalière (APR-DRG (J57)). Available: www.informa.msss.gouv.qc.ca/Details.aspx?Id=SD+emlWTaZk=&Source=/dlVmYIVYBQ= (accessed 2012 June).
- 28.Brachytherapy and prostate cancer. Montréal (QC): Conseil d’évaluation des technologies de la santé du Québec (CETS); 2000.
- 29.Canadian Patient Cost Database (CPCD) metadata. Ottawa (ON): Canadian Institute for Health Information; 2011. Available: www.cihi.ca/CIHI-ext-portal/internet/en/document/spending+and+health+workforce/spending/spending+by+geography/cpcd_metadata (accessed 2012 June).
- 30.Drummond MF, Sculpher MJ, Torrance G. Methods for the economic evaluation of health care programmes, third edition New York (NY): Oxford University Press; 2005. [Google Scholar]
- 31.Eldefrawy A, Katkoori D, Abramowitz M, et al. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol 2013;31:576-80 [DOI] [PubMed] [Google Scholar]
- 32.Corcoran AT, Peele PB, Benoit RM. Cost comparison between watchful waiting with active surveillance and active treatment of clinically localized prostate cancer. Urology 2010;76:703-7 [DOI] [PubMed] [Google Scholar]
- 33.Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer 2007;109:518-27 [DOI] [PubMed] [Google Scholar]
- 34.Andersson SO, Andren O, Lyth J, et al. Managing localized prostate cancer by radical prostatectomy or watchful waiting: cost analysis of a randomized trial (SPCG-4). Scand J Urol Nephrol 2011;45:177-83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.