Abstract
Antioxidants can inhibit atherosclerosis, but it is unclear how inhibition of intimal lipid oxidation relates to atherogenesis. Here we tested the effect of probucol and its metabolite bisphenol on aortic lipid (per)oxidation and atherogenesis in Watanabe heritable hyperlipidemic (WHHL) rabbits. LDL and aortas from rabbits fed probucol contained bisphenol at concentrations comparable to those in bisphenol-treated animals. Bisphenol treatment increased plasma cholesterol slightly, and plasma and aortic α-tocopherol more substantially; these parameters were unaffected by probucol. Bisphenol and probucol treatment both enhanced the resistance of circulating LDL to peroxyl radical–induced lipid peroxidation; this was due to bisphenol, not probucol. Only probucol enhanced LDL’s resistance to Cu2+-induced oxidation. Both bisphenol and probucol treatment strongly inhibited aortic accumulation of hydroperoxides and hydroxides of cholesteryl esters and triglycerides [LO(O)H]. Despite this, however, probucol had a modestly significant effect on the extent of lesion formation; bisphenol had no inhibitory effect. In addition, the extent of atherosclerosis did not correlate with amounts of aortic LO(O)H present, but, as expected, it did correlate with aortic α-tocopherol and cholesterol. Together, these results suggest that aortic accumulation of LO(O)H is not required for, nor is α-tocopherol depleted during, the initiation and progression of atherogenesis in WHHL rabbits.
Introduction
The oxidative modification of LDL within the arterial wall is implicated in the early stages of atherogenesis. According to the oxidation theory (1), oxidized LDL assists in foam cell formation, is cytotoxic, and instigates various proatherogenic processes (1, 2). Lipid peroxidation is one of the earliest processes occurring during LDL oxidation induced by most, but not all, oxidants (3). Increasing antioxidant defense against such damage may therefore attenuate the initial stages of atherogenesis (1). Indeed, some, but not all, lipophilic antioxidants have been shown to inhibit atherogenesis in various animal models (4–11). However, whether this antiatherogenic effect relates to an inhibition of intimal lipid peroxidation in affected vessels is not commonly addressed. Rather, ex vivo “LDL oxidizability” is commonly used to assess antioxidant efficacy.
The presence of oxidized lipids in atherosclerotic lesions is well documented (12), although it remains unclear how lipoprotein-derived lipids become oxidized. Various mechanisms have been suggested (2). Measurement of lag time to Cu2+-induced oxidation of isolated LDL (lag time) is the most commonly used in vitro/ex vivo assay of resistance to lipid oxidation. Lag time reflects the time needed for the major known antioxidant of lipoproteins, α-tocopherol (α-TOH), to be depleted in LDL from untreated animals (13). The large increase in lag time observed after probucol treatment of Watanabe heritable hyperlipidemic (WHHL) rabbits, together with the antiatherosclerotic effect of probucol, forms the basis of the oxidation theory (1). However, the biological relevance of lag time is unclear. Oxidized lipids appear to accumulate in atherosclerotic lesions in the presence of α-TOH (3, 14, 15). In the presence of vitamin E, LDL lipid peroxidation occurs via tocopherol-mediated peroxidation (3, 16). In this process, the fate of α-tocopheroxyl radical (α-TO•, derived from α-TOH oxidation), rather than α-TOH alone, governs the overall activity of vitamin E. Effective protection of LDL lipids is achieved in the presence of α-TOH, together with coantioxidants that reduce α-TO• and export the radical from the lipoprotein particle into the aqueous phase (3, 16, 17). It should also be noted that oxidative processes other than the oxidation of LDL lipids take place in the vessel wall and may affect atherogenesis (2, 18).
The major aim of this study was to test whether prevention of oxidized lipid accumulation in the aorta of WHHL rabbits affects lesion development. We chose to use both probucol and its metabolite bisphenol (Table 1) for several reasons. First, bisphenol inhibits lesion formation and aortic lipid oxidation in mice deficient in the apo E and the LDL receptors (19), although it only mildly increases the lag time of LDL oxidized with Cu2+ (20). Second, bisphenol inhibits tocopherol-mediated peroxidation of LDL lipid, similarly to butylated hydroxytoluene (BHT), which inhibits atherosclerosis in rabbits (21). Third, probucol inhibits lesion formation in rabbits (4), where it is metabolized (22) to bisphenol and its oxidation product, diphenoquinone (Table 1). Lastly, probucol effectively prolongs lag time in LDL exposed to Cu2+ (23), although it does not inhibit tocopherol-mediated peroxidation (17). Thus, the antiatherogenic effect of probucol could conceivably be due to bisphenol. We therefore supplemented WHHL rabbits with probucol (positive control) or bisphenol to test whether inhibition of aortic lipid peroxidation correlates with an antiatherogenic effect in this animal model.
Table 1.
In vitro assessment of coantioxidant activity
Methods
Materials.
Dulbecco’s phosphate-buffered saline (DPBS) was prepared from nanopure water and stored over Chelex-100 (Bio-Rad Laboratories Inc., Richmond, California, USA) to remove transition metals. Cholesteryl linoleate (C18:2), cholesteryl arachidonate (C20:4) (together referred to as cholesteryl esters [CE]), unesterified cholesterol, formalin saline, BHT, EDTA, D-isoascorbic acid, and ascorbic acid were from Sigma Chemical Co. (St. Louis, Missouri, USA). We obtained [1α, 2α(n)-[3H]C18:2] ([3H]C18:2, 48 Ci/mmol) from Du Pont NEN Research Products (Boston, Massachusetts, USA). Preparation of [3H]C18:2-hydroxide was by oxidation of [3H]C18:2 with rabbit reticulocyte 15-lipoxygenase (a gift from Dagmar Heydeck, Humboldt University, Berlin, Germany) followed by reduction with NaBH4. A gift of α-TOH (purity 96%) was made by Henkel (Sydney, Australia). Eastman Kodak Co. (Sydney, Australia) provided α-tocopherylquinone (α-TQ; purity 99%); probucol came from Jucker Pharma (Stockholm, Sweden); and 2,2′-azobis(2-amidinopropane)hydrochloride (AAPH) and bisphenol (3,3′,5,5′-tetra-tert-butyl-4,4′-bisphenol) were from Polysciences Inc. (Warrington, Pennsylvania, USA). Diphenoquinone (3,3′,5,5′-tetra-tert-butyl-4,4′-diphenoquinone) was prepared from bisphenol (24) and α-tocotrienol, purified as described (14). C18:2-hydroperoxide was prepared (25) and used as standard for hydroperoxides and hydroxides of CE and triglycerides [hereafter referred to as LO(O)H]. Stock K1 rabbit chow (containing 41 mg/kg of vitamin E and 11 mg/kg β-carotene; Lactamin AB, Stockholm, Sweden) was fortified with probucol or bisphenol at 1 and 0.015% (wt/wt), respectively. This dose of probucol is similar to that used in a previous study(4). Dosage of bisphenol was based on a pilot rabbit study indicating that when bisphenol was administered at 0.015% (wt/wt), the plasma bisphenol levels obtained were similar to those obtained using 1% (wt/wt) probucol.
Animals.
Male rabbits (Froxfield Farms, Petersfield, United Kingdom) were caged individually and maintained on standard K1 chow from weaning until 11 weeks of age. Thereafter, rabbits received chow (170 g per day) fortified with placebo (K1), bisphenol, or probucol for an additional 12 weeks, with water supplied ad libitum.
Preparation of plasma and serum.
Blood was drawn from rabbits directly into EDTA or heparin VACUTAINER tubes (Becton Dickinson and Co., Rutherford, New Jersey, USA). Plasma was prepared by centrifugation (1,000 g at 15°C for 15 minutes), and samples were immediately frozen at –70°C. Rabbit sera, produced by allowing blood to clot at 20°C in sterile tubes and then centrifuging it (1,000 g at 4°C for 10 minutes), was also frozen (–70°C). Samples were transported (≤3 days, over dry ice) for lipid and antioxidant analyses.
Perfusion and fixation of vessels.
Animals were anesthetized, the chest opened, and the heart exposed and perfused with ∼1.5 L DPBS containing 100 μM BHT and 1 mM EDTA. The aorta was clamped at the level of the diaphragm, and the proximal section of the abdominal aorta was removed, cleaned, and immediately frozen at –70°C. The heart and remaining vasculature were fixed using 1.5 L of formalin saline, and the thoracic section was removed. Aortas designated for biochemical analyses were not fixed, because pilot studies showed that such treatment caused oxidative modification, as judged by depletion of tissue ascorbate and accumulation of large amounts of LO(O)H compared with nonfixed aortas (data not shown).
Evaluation of atherosclerosis.
En face assessment of lesion size was made using the aortic segment running from the second pair of intercostal artery branches to the diaphragm. Segments were stained with oil red O and photographed. The extent of the oil red O–positive area was evaluated and expressed as a percentage of the total aortic surface (26). Ten cross-sections (2 μm thick, 1 mm apart), centered around the first pair of intercostal artery branches in the descending thoracic aorta, were stained using Weigert’s hematoxylin and van Gieson’s stain. Quantitation of cross-sectional areas of intima and media was obtained by planimetry as described previously (19). Each area was converted to a volume by multiplying by 1 mm, and such volumes were determined for 10 serial sections and expressed as a total volume (over 10 mm). Planimetry was done in a blinded fashion, employing coded samples. Aortic volume is shown as the intima-to-media fractional volume; such normalization reduced variability between aortas. Mean volumes obtained from the same aortas on different occasions varied less than 5%.
Preparation of aortic homogenates.
Clean segments of abdominal aortas were thawed, blotted, and added (25–30 mg wet tissue/mL) to argon-flushed DPBS containing BHT (100 μM) and EDTA (1 mM) to minimize adventitious oxidation (14), as verified here for ascorbate, α-TOH, and C18:2 (not shown). The tissue was minced, and isoascorbate (5 μM) and α-tocotrienol (1 μM) were added as internal standards for ascorbate and vitamin E, respectively. For recovery of lipoprotein-derived oxidized lipids, [3H]C18:2-hydroxide was incorporated into human LDL (27) and added to the vessel prior to homogenization. The samples were homogenized at 4°C for 5 minutes (14). Analysis of spiked homogenate showed 94 ± 1.3% recovery of the label (mean ± range for 2 separate experiments). For ascorbate analyses, raw homogenate (50 μL) was added to metaphosphoric acid (5% vol/vol, 50 μL) and then stored at –70°C until analysis (19). For lipid analyses, the remaining homogenate was extracted and HPLC samples were prepared as described (25).
Preparation of LDL.
LDL was obtained from pooled (n = 3) rabbit plasma (plasma LDL, 1.02 < ρ < 1.05 g/mL) by 45 minutes of density-gradient ultracentrifugation using DPBS (25), or from individual rabbit serum (serum LDL, 1.02 < ρ < 1.063 g/mL) by D2O-gradient ultracentrifugation (28). Plasma LDL was diluted with DPBS to 0.5–0.6 mg protein/mL, kept at 4°C, and used within 30 minutes after removal of potassium bromide and water-soluble antioxidants by size-exclusion chromatography (PD-10; Pharmacia Biotech AB, Uppsala, Sweden) using DPBS as eluent (25). Serum LDL was diluted to 0.15 μM with 10 mM HEPES buffer (140 mM NaCl, pH 7.4) immediately before use.
Oxidation of LDL.
Oxidation of plasma LDL was carried out at 37°C by addition of 2 mM AAPH. Aliquots (75 μL) of the reaction mixture were extracted (25), and lipid parameters were determined by HPLC as described herein. Control plasma LDL was divided into 2 portions prior to oxidation; 1 was supplemented with BHT (10 μM) for comparison of the ex vivo inhibitory activity of bisphenol and probucol versus a known coantioxidant (17). Cu2+-induced oxidation of serum LDL (0.15 μM) was initiated with 5 μM CuSO4; conjugated diene was measured at 250 nm in microtiter plates at 37°C (29). Under these strong oxidizing conditions (Cu2+:LDL = 33), control serum LDL was oxidized without the typically detected lag phase (Cu2+:LDL = 17; ref. 13).
Analysis of lipid and antioxidants.
This was performed by HPLC (25), except for measurement of peroxidized lipids, for which UV234 nm and postcolumn chemiluminescence detection (detection limit 0.4 pmol) were used (25). Probucol and bisphenol coeluted with the solvent front, while diphenoquinone eluted at ∼5.4 minutes; this did not affect quantitation of oxidized lipids. Pilot analyses of homogenates of rabbit aortas indicated the presence of hydroperoxides (LOOH) and hydroxides (LOH) from both CE and triglycerides. HPLC with electrochemical detection was used to measure α-TQ, α-TOH, α-tocotrienol, D-isoascorbate, and ascorbate (14, 25). In sera, analysis of α-TOH was performed by reverse-phase HPLC eluted with MeOH/MeCN/H2O (48:48:4 vol/vol/vol). For plasma LDL oxidation, cholesterol (which remained unoxidized) was used as an internal standard for all lipid-soluble analytes (25). Probucol and its metabolites were analyzed by reverse-phase HPLC: flow rate 1.5 mL/min, with 100% solvent A (MeOH/MeCN/H2O; 10:10:3 vol/vol/vol) for 0–15 minutes monitored at 270 nm, followed by 50% each of solvents A and B (MeOH/MeCN; 1:1 vol/vol) for 15–22 minutes at 242 nm, and then 100% solvent B for 22–28 minutes at 420 nm. Bisphenol, probucol, and diphenoquinone eluted at 9, 17, and 27 minutes, respectively. Compounds were quantified by peak area comparison with standards. Total serum cholesterol and triglycerides were determined enzymatically (Boehringer Mannheim).
Statistics.
Lesion volume followed a normal log distribution. Thus, the effect of drug treatment on lesion volume was compared by repeated-measures ANOVA using log-transformed data. For data sets with values of 0, the Wilcoxon 2-sample test was used. Statistical significance was accepted at P < 0.05.
Results
Table 2 summarizes the concentrations of serum lipids and drugs in rabbits after 12 weeks of intervention. Triglycerides were invariant irrespective of treatment, whereas total cholesterol and α-TOH were increased in both bisphenol- and probucol-treated rabbits compared with control rabbits. Compared with the control, probucol treatment resulted in a marginal decrease in total plasma cholesterol. The increase in plasma cholesterol caused by bisphenol was apparent for the buoyant lipoproteins VLDL and LDL, but not for HDL (data not shown). Concentrations of bisphenol were comparable in the probucol and bisphenol groups, and the ratio of coantioxidant-active (bisphenol) to coantioxidant-inactive (diphenoquinone) drug was also similar for both treatment groups, in support of the study design. Probucol treatment resulted in a marginal decrease in total plasma cholesterol (Table 2).
Table 2.
Serum lipids in WHHL rabbits measured after 12 weeks of intervention
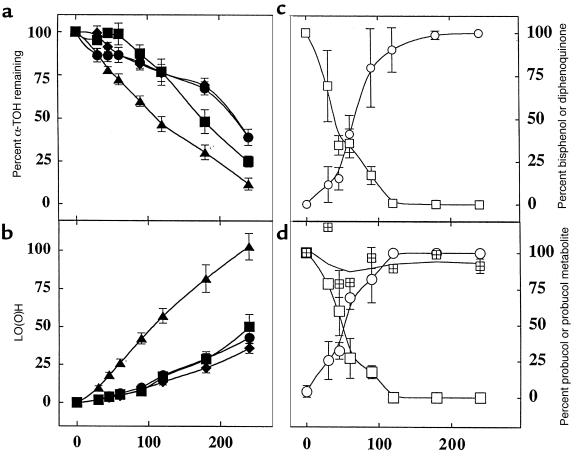
To test the effect of probucol and bisphenol treatment on ex vivo oxidizability of circulating LDL, 2 different conditions were employed. First, relatively mild oxidizing conditions employing AAPH were used, so that antioxidant activity (17) could be assessed during the α-TOH–containing period of LDL oxidation. In LDL from BHT-free control rabbits, LO(O)H accumulated over time and in a chain reaction (Figure 1) as α-TOH was consumed, consistent with tocopherol-mediated peroxidation (16). Consumption of α-TOH (Figure 1a) and accumulation of LO(O)H (Figure 1b) were inhibited in ex vivo LDL from BHT-supplemented animals, as reported previously for human LDL (17). Similar inhibition was obtained with LDL from bisphenol- and probucol-treated animals (Figure 1), indicating that the coantioxidant capacity of LDL was comparably enhanced by both treatments. Inhibition of α-TOH consumption during the first 60 minutes of oxidation was significantly greater for bisphenol than for either BHT or probucol, for unknown reasons.
Figure 1.
LDL from WHHL rabbits treated with probucol or bisphenol is resistant to ex vivo lipid peroxidation induced by AAPH. Pooled LDL (0.5-0.6 mg protein/mL) from rabbits fed probucol (filled circles), bisphenol (filled squares), control diets (filled triangles), or control LDL + BHT (10 μM) (filled diamonds) was treated at 37°C with 2 mM AAPH. Aliquots were removed and analyzed for α-TOH (a) and LO(O)H (b). LDL from bisphenol-treated (c) and probucol-treated (d) rabbits was also analyzed for bisphenol (open squares), diphenoquinone (open circles), and probucol (cross-hatched squares). Data represent mean ± SD of 4 independent studies. Initial concentrations (i.e., 100%) of α-TOH were 13 ± 2, 13 ± 3, 15 ± 2, and 15 ± 6 for probucol-treated, bisphenol-treated, control, and BHT-treated LDL, respectively. Initial levels of bisphenol and diphenoquinone were 10 ± 2 and 8 ± 1 μM and 12 ± 2 and 10 ± 3 μM for LDL from bisphenol- and probucol-treated rabbits, respectively. Initial levels of diphenoquinone were 1.6 ± 0.7 and 1.0 ± 0.1 μM for bisphenol and probucol samples, respectively. Probucol was expressed as percent peak area relative to that before oxidation. BHT-free control LDL oxidized to LO(O)H in a chain reaction, with chain length υ∼15 (see ref. 16 for determination of υ).
During AAPH-induced oxidation, bisphenol was oxidized stoichiometrically to diphenoquinone, regardless of whether the LDL used was from probucol- or bisphenol-treated rabbits (Figure 1, c and d). Importantly, LO(O)H accumulation was inhibited (0.07 vs. 0.42 μM/min for bisphenol-containing vs. control LDL) until bisphenol was depleted to <20% of the initial level (Figure 1, a–c). At that time, the rate of LO(O)H accumulation increased (≥0.21 μM/min), although it did not reach that in the absence of the drug, at least during the time period monitored. The reasons for this were not further investigated. By contrast, probucol was not oxidized during the period monitored (Figure 1d), confirming that bisphenol, not probucol, inhibited LDL lipid oxidation in the presence of α-TOH. The observation that bisphenol (and BHT), but not probucol, inhibited AAPH-induced LDL oxidation can be explained readily by tocopherol-mediated peroxidation, and the fact that bisphenol and BHT, but not probucol, are coantioxidants (16, 17).
We next oxidized LDL with Cu2+ at a Cu2+:LDL ratio of 33 (see Methods). Under these comparatively harsh oxidizing conditions, serum LDL from both bisphenol- and probucol-treated animals showed enhanced antioxidant capacities. However, the extent of this varied substantially between the 2 treatment groups (lag time of 1.6 ± 0.2 and 13.3 ± 2.7 hours for bisphenol and probucol, respectively, vs. 0 hours for BHT-free control LDL), consistent with a previous report (20).
In agreement with previous studies, probucol treatment decreased atherosclerosis in thoracic aortas by 30 and 55%, as assessed by en face and intimal volume measurements, respectively (Table 3). By contrast, bisphenol treatment had no effect on lesion formation, independent of the mode of assessment (Table 3). In a separate control group, lesions in the thoracic aortas correlated positively with abdominal lesions (lesionthoracic = 0.38 × lesionabdominal + 0.04; P = 0.01). Hence, we compared the effects of bisphenol and probucol treatments on biochemical parameters in abdominal aortas.
Table 3.
Aortic lesions in thoracic aortas from rabbits receiving control, probucol-fortified, or bisphenol-fortified diet
Aortic lipids and antioxidants varied between groups (Table 4). Cholesterol and CE were lower in probucol-treated rabbits than in control rabbits. Similar to plasma, aortic α-TOH was increased in the bisphenol group compared with control and probucol groups. The total metabolite concentration and the ratio of bisphenol to diphenoquinone were slightly higher in the bisphenol group than in the probucol group. Treatment of rabbits with either probucol or bisphenol effectively prevented aortic lipid oxidation, as judged by significantly lower levels of both LOOH and LOH (Figure 2a and Table 4) in the drug-treated versus control animals. Aortic contents of LOOH and LOH were comparable. This contrasts with levels in advanced human plaque (12, 14) and lesions in apo E receptor–deficient, LDL receptor–deficient (19), and apo E–deficient mice (15), in all of which LOH predominates. LO(O)H were detected in 9 of 11 aortas from control rabbits, albeit at markedly different concentrations (Figure 2b and Table 4). The large error in aortic LO(O)H content in the bisphenol group was due to detection of LO(O)H (125 and 135 pmol/mg protein LOOH and LOH, respectively) in only 1 of 11 aortas; none of the probucol samples contained detectable LO(O)H (Table 4). Aortas from probucol- and bisphenol-treated animals also contained significantly less α-TQ (Table 4), another independent marker of lipid oxidation (14).
Table 4.
Lipid and antioxidants in homogenates of aortas from WHHL rabbits after 12 weeks of intervention
Figure 2.
Probucol and bisphenol inhibit aortic lipid oxidation, while aortic levels of LO(O)H correlate poorly with atherosclerotic lesion size. (a) Aortas from control (A), probucol-treated (B), and bisphenol-treated (C) rabbits were homogenized, and the organic extracts were analyzed by HPLC with chemiluminescence detection. Representative results are shown with hydroperoxides of triglycerides and CE eluting at 5–7 minutes and ∼8 minutes, respectively. (b) Fractional intima-to-media volume derived from control (open circles; n = 11), bisphenol (open squares; n = 11), and probucol (open diamonds; n = 12) groups vs. aortic LO(O)H. The coefficient of variation determined by linear regression is r = 0.11.
The above results demonstrate that both probucol and bisphenol effectively prevented accumulation of aortic LO(O)H, yet bisphenol had no relevance to atherogenesis, implying a dissociation of these 2 processes in WHHL rabbits. Consistent with this, linear regression analyses showed no correlation between aortic LO(O)H and lesion size, either when all data were combined (Figure 2b) or when data for each group were analyzed separately (–0.25 < r < 0.31; data not shown). However, LO(O)H showed a moderate positive correlation with aortic α-TOH in the control group (r = 0.51, n = 11; data not shown). Lesion size also correlated positively and most strongly with aortic α-TOH (r = 0.83), followed by tissue levels of drugs (r = 0.78; data not shown) and aortic total cholesterol (r = 0.71; data not shown). Furthermore, tissue α-TOH correlated strongly and positively with aortic cholesterol (r = 0.84; data not shown). By contrast, none of the measured plasma parameters correlated with lesion size in any treatment group (–0.30 < r < 0.30), except serum α-TOH, which exhibited a moderate positive correlation when all groups were combined for analysis (r = 0.55, n = 34). Specifically, there was no statistically significant correlation between total plasma cholesterol and lesion size, regardless of whether treatment groups were evaluated separately or together. The results suggest that the effects of bisphenol and probucol treatment on atherogenesis are not due to effects on lipid oxidation.
Discussion
This study shows that administration of either probucol or bisphenol to WHHL rabbits prevents the aortic accumulation of LO(O)H. Despite this apparently effective inhibition of lipid oxidation in the artery wall in both treatment groups, only probucol had a significant, albeit modest, inhibitory effect on lesion formation. In agreement with this, there was no significant correlation between atherosclerosis and the extent of aortic accumulation of LO(O)H. Together, these findings clearly dissociate the 2 processes and suggest that aortic lipid oxidation may not be required for atherogenesis to occur in WHHL rabbits.
Our observation that bisphenol inhibited aortic LO(O)H accumulation, yet failed to affect lesion formation, also suggests that inhibition of aortic lipid oxidation is not the sole requirement for a compound to be antiatherogenic in WHHL rabbits. All 3 parameters used to assess the effect of bisphenol on atherosclerosis (cross-sectional and en face lesion assessment, and measurement of aortic nonoxidized lipids) yielded consistent results. However, bisphenol significantly increased plasma cholesterol, which could have counteracted a putative antiatherogenic action. Indeed, the antiatherogenic effect of antioxidants can be outweighed by hypercholesterolemia (30); in this study, the antiatherogenic activity of probucol was associated with a marginal cholesterol-lowering effect, although this did not reach statistical significance. To clarify further the results obtained here, it would be interesting to reinvestigate the antiatherogenic effect of bisphenol, keeping plasma cholesterol levels equal in the control and treated groups. However, it should be noted that plasma cholesterol did not correlate significantly with lesion volume in this study.
We measured LO(O)H in extracts of homogenates of abdominal aortas. Therefore, the results do not distinguish between oxidized lipids of intimal lipoproteins, other extracellular deposits, or lipids present within foam cells. The procedures used did effectively recover lipoprotein-derived LO(O)H (see Methods), so we would have detected it if it had been present in the vessel walls of the drug-treated animals. Therefore, our results suggest that like in control animals, lesions developed normally in bisphenol-treated rabbits, except that there was no measurable accumulation of lipoprotein-derived LO(O)H in the vessel wall.
It is not clear at present whether the observed normal lesion development in the apparent absence of lipoprotein-derived LO(O)H in bisphenol-treated rabbits means that intimal lipoprotein oxidation is not required for atherogenesis in this model. Lipoprotein oxidation is associated with the formation of several products, including protein oxidation products, which have varying profiles depending on the oxidant involved and the stage of oxidation. Although neither of these variables has ever been described in the arterial wall for any study, the hydroperoxides and hydroxides of CE and triglycerides [i.e., LO(O)H] that we measured are the primary and major lipid oxidation products formed in lipoproteins that undergo in vitro oxidation (3, 16). LO(O)H also represents the major oxidized lipids found in advanced human atherosclerotic lesions (14) and the lipoproteins isolated from such lesions (31). In addition, the amounts of LO(O)H increase with increasing lesion size and severity in aortas of both humans (J.M. Upston et al., unpublished observations) and apo E–deficient mice (15). Furthermore, where examined, LO(O)H appear to be associated with aortic lipoproteins (15, 31). Together, these findings suggest that lesions can develop in WHHL rabbits without measurable accumulation of the major products of lipoprotein lipid oxidation. An interesting possibility with potential clinical importance is that atherosclerotic lesions devoid of LO(O)H may be more stable, less prone to rupture, and thus less dangerous than their LO(O)H-containing counterparts.
The results of this study do not exclude the possibility that bisphenol treatment failed to prevent the formation of minor lipid or protein oxidation products that contribute to atherogenesis and that we did not measure. For example, hydroxynonenal- and malondialdehyde-lysine adducts are present in human and rabbit lesions (32) and are used as markers of lipid oxidation. Similarly, breakdown products derived from oxidized phospholipids that can be formed during in vitro LDL oxidation are present in lesions of cholesterol-fed rabbits, and have several potential proatherogenic properties (33). Formation of these secondary lipid oxidation products is dependent on the fragmentation of LOOH that commonly occurs after the depletion of α-TOH (13). Interestingly however, α-TOH was not depleted in the aortas of bisphenol-treated rabbits, so extensive LOOH fragmentation does not appear likely. Also, bisphenol inhibited the accumulation of α-TQ, indicating that inhibition of aortic lipid oxidation extended to markers other than LO(O)H. It will be important for future studies to test whether bisphenol affects secondary lipid oxidation and/or protein oxidation, and, if so, how this relates to atherogenesis.
Regardless of whether aortic lipoprotein oxidation contributes to atherogenesis in WHHL rabbits, the present study revealed a strong positive correlation between aortic levels of α-TOH and lesion size. This is not surprising, given that LDL is the major transport vehicle for both intimal lipid and α-TOH (34), consistent with the positive correlation between tissue cholesterol and α-TOH. Supplementation with bisphenol, but not probucol, caused serum and aortic α-TOH levels to increase. Serum levels of α-TOH paralleled those of total cholesterol in both groups, suggesting that bisphenol treatment increased α-TOH by increasing plasma lipoproteins. In any case, the results show that α-TOH does not become depleted as atherosclerotic lesions develop in WHHL rabbits, consistent with the situation in human aortas (ref. 14; and J.M. Upston et al., unpublished observations) and in animals that are deficient in apo E (15) or apo E and LDL receptor (19).
Ex vivo oxidation of plasma LDL is commonly used to assess in vivo efficacy of antioxidants. Here we show that the commonly applied Cu2+/LDL oxidizability test (13) is not a good predictor of in vivo protection against LO(O)H accumulation. Compared with probucol, bisphenol only moderately increased Cu2+-induced oxidation lag time, whereas it inhibited aortic accumulation of LO(O)H as effectively as probucol did. Our results suggest further that the conditions used to measure coantioxidant activity (17) may be suitable to assess in vivo lipid peroxidation inhibition, although they are clearly not suitable as a surrogate for lesion development. On the other hand, the lag times obtained in the Cu2+/LDL oxidizability tests for bisphenol and probucol reflected lesion size in both groups. This implies that this test is a useful surrogate for lesion development. If so, however, the reason for this remains unclear. In addition, such an interpretation would imply that, as already stated, aortic lipoprotein lipid oxidation may not cause atherosclerosis, because bisphenol effectively prevented this process yet failed to substantially increase the lag time or decrease atherosclerosis. It has been argued that measurement of plasma LDL lag time is not useful for assessing antiatherogenic activity (7, 10, 35), and that different in vitro oxidizing conditions can give different results (ref. 16 and this study). In fact, none of the plasma parameters determined herein showed strong correlation with lesion formation, indicating that these parameters do not reflect the situation in the vessel wall.
A disadvantage of performing biochemical analyses on aortas is that they cannot be performed on the same tissue used for histological lesion assessment. Thus, our interpretation of a dissociation of aortic lipid peroxidation and atherogenesis assumes that lesion formation in the descending thoracic aorta correlates with that in the abdominal aorta. Our data showing a linear correlation between lesion volume in abdominal versus thoracic aortas supports this interpretation.
Our observation that bisphenol effectively prevented intimal lipid oxidation in WHHL rabbits (this study), apo E–deficient mice, and apo E–, LDL receptor–deficient mice (19) further supports the notion (17) that coantioxidants are potentially effective inhibitors of in vivo lipoprotein lipid oxidation. More importantly however, the observed dissociation of aortic lipid oxidation from atherogenesis in WHHL rabbits adds to the complexity of the assessment of antioxidants as antiatherogenic drugs (Table 5). In support of the general idea that antioxidants are antiatherogenic agents (1, 2, 18), most antioxidants inhibit atherosclerosis in various animals, although there are exceptions to this. Perhaps most strikingly, probucol enhances lesion formation in the aortic root in mice (9–11, 36–38). The present study, along with a previous study with BM15.0639 (39) and the results of Fruebis et al. (10), suggests that antioxidants that inhibit tocopherol-mediated peroxidation also fail to generally prevent atherosclerosis (Table 5). The reasons for this are not known at present. Oxidation parameters other than LO(O)H may be relevant (see above), although there is currently no direct evidence for this. Also, it is increasingly clear that the concept of oxidative stress in the vascular wall is more complex than simple LDL oxidation, and likely extends to other lipoproteins and cellular processes (18). It would therefore be interesting to test how antioxidants in general, and bisphenol (and BM15.0639) in particular, affect some of these processes. Probucol might also affect inflammatory processes in the vessel, such as the production of cytokines by macrophages (40), and this could contribute to its antiatherosclerotic effect. Whether other antioxidants affect such processes is not known, and would be of interest in future investigations.
Table 5.
Results from intervention studies in animals and humans
In conclusion, this study indicates that intimal lipid oxidation does not appear to be required for early atherogenesis in WHHL rabbits. Although this appears not to support the central component of the oxidation theory in this particular animal model, future studies are needed to examine whether this finding can be extended to other oxidation parameters and/or models of atherosclerosis.
Acknowledgments
We thank M. Englund for assistance, and J.F. Keaney, W. Jessup, and J.M. Upston for stimulating discussions and for critical reading of the manuscript. This work was supported by National Health & Medical Research Council grant 970998 (to R. Stocker).
References
- 1.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 3.Upston JM, Terentis AC, Stocker R. Tocopherol-mediated peroxidation (TMP) of lipoproteins: implications for vitamin E as a potential antiatherogenic supplement. FASEB J. 1999;13:977–994. doi: 10.1096/fasebj.13.9.977. [DOI] [PubMed] [Google Scholar]
- 4.Kita T, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparrow CP, et al. Low density lipoprotein is protected from oxidation and the progression of atherosclerosis is slowed in cholesterol-fed rabbits by the antioxidant N,N′-diphenyl-phenylenediamine. J Clin Invest. 1992;89:1885–1891. doi: 10.1172/JCI115793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasahara M, et al. Inhibition of hypercholesterolemia-induced atherosclerosis in the nonhuman primate by probucol. I. Is the extent of atherosclerosis related to resistance of LDL to oxidation? J Clin Invest. 1994;94:155–164. doi: 10.1172/JCI117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaish A, Daugherty A, O’Sullivan F, Schonfeld G, Heinecke JW. Beta-carotene inhibits atherosclerosis in hypercholesterolemic rabbits. J Clin Invest. 1995;96:2075–2082. doi: 10.1172/JCI118256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fruebis J, Carew TE, Palinski W. Effect of vitamin E on atherogenesis in LDL receptor-deficient rabbits. Atherosclerosis. 1995;117:217–224. doi: 10.1016/0021-9150(95)05574-g. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SH, et al. Paradoxical enhancement of atherosclerosis by probucol treatment in apolipoprotein E-deficient mice. J Clin Invest. 1997;99:2858–2866. doi: 10.1172/JCI119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruebis J, Steinberg D, Dresel HA, Carew TA. A comparison of the antiatherogenic effects of probucol and a structural analogue of probucol in low density lipoprotein receptor-deficient rabbits. J Clin Invest. 1994;94:392–398. doi: 10.1172/JCI117334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird DA, et al. The effect of probucol on LDL oxidation and atherosclerosis in LDL receptor-deficient mice. J Lipid Res. 1998;39:1079–1090. [PubMed] [Google Scholar]
- 12.Carpenter KL, et al. Lipids and oxidised lipids in human atherosclerotic lesions at different stages of development. Biochim Biophys Acta. 1995;1256:141–150. doi: 10.1016/0005-2760(94)00247-v. [DOI] [PubMed] [Google Scholar]
- 13.Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6:67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- 14.Suarna C, Dean RT, May J, Stocker R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of α-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 15.Letters JM, et al. Changes to lipids and antioxidants in plasma and aortae of apoE-deficient mice. J Lipid Res. 1999;40:1104–1112. [PubMed] [Google Scholar]
- 16.Bowry VW, Stocker R. Tocopherol-mediated peroxidation. The pro-oxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc. 1993;115:6029–6044. [Google Scholar]
- 17.Bowry VW, Mohr D, Cleary J, Stocker R. Prevention of tocopherol-mediated peroxidation of ubiquinol-10-free human low density lipoprotein. J Biol Chem. 1995;270:5756–5763. doi: 10.1074/jbc.270.11.5756. [DOI] [PubMed] [Google Scholar]
- 18.Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 19.Witting PK, et al. Inhibition by a co-antioxidant of aortic lipoprotein lipid peroxidation and atherosclerosis in apolipoprotein E and low density lipoprotein receptor gene double knockout mice. FASEB J. 1999;13:667–675. doi: 10.1096/fasebj.13.6.667. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary VJ, Tilling L, Fleetwood G, Stone D, Darley-Usmar V. The resistance of low density lipoprotein to oxidation promoted by copper and its use as an index of antioxidant therapy. Atherosclerosis. 1996;119:169–179. doi: 10.1016/0021-9150(95)05644-0. [DOI] [PubMed] [Google Scholar]
- 21.Freyschuss A, et al. Antioxidant treatment inhibits the development of intimal thickening after balloon injury of the aorta in hypercholesterolemic rabbits. J Clin Invest. 1993;91:1282–1288. doi: 10.1172/JCI116326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnhart RL, Busch SJ, Jackson RL. Concentration-dependent antioxidant activity of probucol in low density lipoproteins in vitro: probucol degradation precedes lipoprotein oxidation. J Lipid Res. 1989;30:1703–1710. [PubMed] [Google Scholar]
- 23.Parthasarathy S, Young SG, Witztum JL, Pittman RC, Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986;77:641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelter A, Elgendy S. Phenolic oxidation with (diacetoxyiodo)benzene. Tetrahedron Lett. 1988;29:677–680. [Google Scholar]
- 25.Sattler W, Mohr D, Stocker R. Rapid isolation of lipoproteins and assessment of their peroxidation by HPLC postcolumn chemiluminescence. Methods Enzymol. 1994;233:469–489. doi: 10.1016/s0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- 26.Mao SJ, Yates MT, Parker RA, Chi EM, Jackson RL. Attenuation of atherosclerosis in a modified strain of hypercholesterolemic Watanabe rabbits with use of a probucol analogue (MDL 29,311) that does not lower serum cholesterol. Arterioscler Thromb. 1991;11:1266–1275. doi: 10.1161/01.atv.11.5.1266. [DOI] [PubMed] [Google Scholar]
- 27.Christison JK, Rye K-A, Stocker R. Exchange of oxidized cholesteryllinoleate between LDL and HDL mediated by cholesteryl ester transfer protein. J Lipid Res. 1995;36:2017–2026. [PubMed] [Google Scholar]
- 28.Hallberg C, et al. Lipoprotein fractionation in deuterium oxide gradients: a procedure for evaluation of antioxidant binding and susceptibility to oxidation. J Lipid Res. 1994;35:1–9. [PubMed] [Google Scholar]
- 29.Camejo G, Wallin B, Enojärvi M. Analysis of oxidation and antioxidants using microtitre plates. Methods Mol Biol. 1988;45:44–49. doi: 10.1385/0-89603-472-0:377. [DOI] [PubMed] [Google Scholar]
- 30.Parker RA, Sabrah T, Cap M, Gill BT. Relation of vascular oxidative stress, α-tocopherol, and hypercholesterolemia to early atherosclerosis in hamsters. Arterioscler Thromb Vasc Biol. 1995;15:349–358. doi: 10.1161/01.atv.15.3.349. [DOI] [PubMed] [Google Scholar]
- 31.Niu, X., Zammit, V., Upston, J.M., Dean, R.T., and Stocker, R. 1999. Co-existence of oxidized lipids and α-tocopherol in all lipoprotein fractions isolated from advanced human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. In press. [DOI] [PubMed]
- 32.Ylä-Herttuala S, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson AD, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 34.Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res. 1993;34:343–358. [PubMed] [Google Scholar]
- 35.Fruebis J, Bird DA, Pattison J, Palinski W. Extent of antioxidant protection of plasma LDL is not a predictor of the antiatherogenic effect of antioxidants. J Lipid Res. 1997;38:2455–2464. [PubMed] [Google Scholar]
- 36.Benson GM, et al. Effect of probucol on serum lipids, atherosclerosis and toxicology in fat-fed LDL receptor deficient mice. Atherosclerosis. 1998;141:237–247. doi: 10.1016/s0021-9150(98)00177-4. [DOI] [PubMed] [Google Scholar]
- 37.Cynshi O, et al. Antiatherogenic effects of the antioxidant BO-653 in three different animal models. Proc Natl Acad Sci USA. 1998;95:10123–10128. doi: 10.1073/pnas.95.17.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moghadasian MH, McManus BM, Godin DV, Rodrigues B, Frohlich JJ. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E–deficient mice: possible mechanisms of action. Circulation. 1999;99:1733–1739. doi: 10.1161/01.cir.99.13.1733. [DOI] [PubMed] [Google Scholar]
- 39.Witting PK, Westerlund C, Stocker R. A rapid and simple screening test for potential inhibitors of tocopherol-mediated peroxidation of LDL lipids. J Lipid Res. 1996;37:853–867. [PubMed] [Google Scholar]
- 40.Ku G, Doherty NS, Schmidt LF, Jackson RL, Dinerstein RJ. Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties. FASEB J. 1990;4:1645–1653. doi: 10.1096/fasebj.4.6.2318380. [DOI] [PubMed] [Google Scholar]
- 41.Schwenke DC, Behr SR. Vitamin E combined with selenium inhibits atherosclerosis in hypercholesterolemic rabbits independently of effects on plasma cholesterol concentrations. Circ Res. 1998;83:366–377. doi: 10.1161/01.res.83.4.366. [DOI] [PubMed] [Google Scholar]
- 42.Björkhem I, et al. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991;11:15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- 43.Tangirala RK, et al. Effect of the antioxidant N,N′-diphenyl 1,4-phenylenediamine (DPPD) on atherosclerosis in apoE-deficient mice. J Lipid Res. 1995;15:1625–1630. doi: 10.1161/01.atv.15.10.1625. [DOI] [PubMed] [Google Scholar]